Gefitinib Plus Interleukin-2 in Advanced Non-Small Cell Lung Cancer Patients Previously Treated with Chemotherapy
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients Selection
2.2. Study Design and Treatment Plan
| Day of Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| IL-2 bid | x | x | |||||
| IL-2/die | x | x | x |
2.3. Assessments
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
| PARAMETER | Gefitinib Only No. of Patients | Gefitinib + IL-2 No. of Patients | AllPatients | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Number of patients | 39 (56%) | 31 (44%) | 70 | |
| Males | 28 (72%) | 17 (55%) | 45 (64%) | |
| Females | 11 (28%) | 14 (45%) | 25 (36%) | |
| Age, years | ||||
| Median | 71 | 69 | 70 | |
| Range | 39–84 | 44–80 | 39–84 | |
| ECOG PS | ||||
| 0 | 14 (36%) | 17 (55%) | 31 (44%) | |
| 1 | 17 (44%) | 13 (42%) | 30 (43%) | |
| ≥ 2 | 8 (20%) | 1 (3%) | 9 (13%) | |
| Smoking History | ||||
| Yes | 35 (90%) | 23 (74%) | 58 (83%) | |
| No | 3 (8%) | 7 (23%) | 10 (14%) | |
| Unknown | 1 (2%) | 1 (3%) | 2 (3%) | |
| Histology | ||||
| Adenocarcinoma | 24 (62%) | 24 (77%) | 48 (69%) | |
| Squamous cell carcinoma | 5 (13%) | 3 (10%) | 8 (11%) | |
| Bronchoalveolar-cell | 2 (5%) | 2 (6%) | 4 (6%) | |
| Undifferentiated | 8 (20%) | 2 (6%) | 10 (14%) | |
| Number of disease sites | ||||
| 1–2 | 12 (31%) | 9 (29%) | 21 (30%) | |
| ≥3 (range 3–8) | 27 (69%) | 22 (71%) | 49 (70%) | |
| Lung disease | ||||
| Yes | 37 (95%) | 30 (97%) | 67 (96%) | |
| No | 2 (5%) | 1 (3%) | 3 (4%) | |
| Liver metastasis | ||||
| Yes | 10 (26%) | 1 (3%) | 11 (16%) | |
| No | 29 (74%) | 30 (97%) | 59 (84%) | |
| Bone metastasis | ||||
| Yes | 12 (31%) | 12 (39%) | 24 (34%) | |
| No | 27 (69%) | 19 (61%) | 46 (66%) | |
| Node metastasis | ||||
| Yes | 26 (66%) | 18 (58%) | 44 (63%) | |
| No | 13 (33%) | 13 (42%) | 26 (37%) | |
| Adrenal metastasis | ||||
| Yes | 5 (13%) | 4 (13%) | 9 (13%) | |
| No | 34 (87%) | 27 (87%) | 61 (87%) | |
| Brain metastasis | ||||
| Yes | 8 (21%) | 5 (16%) | 14 (20%) | |
| No | 31(79%) | 26 (84%) | 56 (80%) | |
| Previous Chemotherapy for metastatic disease | ||||
| One line | 31 (79%) | 22 (71%) | 53 (76%) | |
| Two lines | 6 (15%) | 7 (23%) | 13 (18%) | |
| Three lines | 2 (5%) | 2 (6%) | 4 (6%) | |
| Previous platinum therapy | ||||
| Yes | 34 (87%) | 30 (97%) | 64 (91%) | |
| No | 5 (13%) | 1 (3%) | 6 (9%) | |
| Further therapy after progressive disease on treatment study | ||||
| Yes | 9 (23%) | 13 (42%) | 22 (31%) | |
| No | 30 (77%) | 18 (58%) | 48 (69%) |
3.2. Responses, Effectiveness and Survival
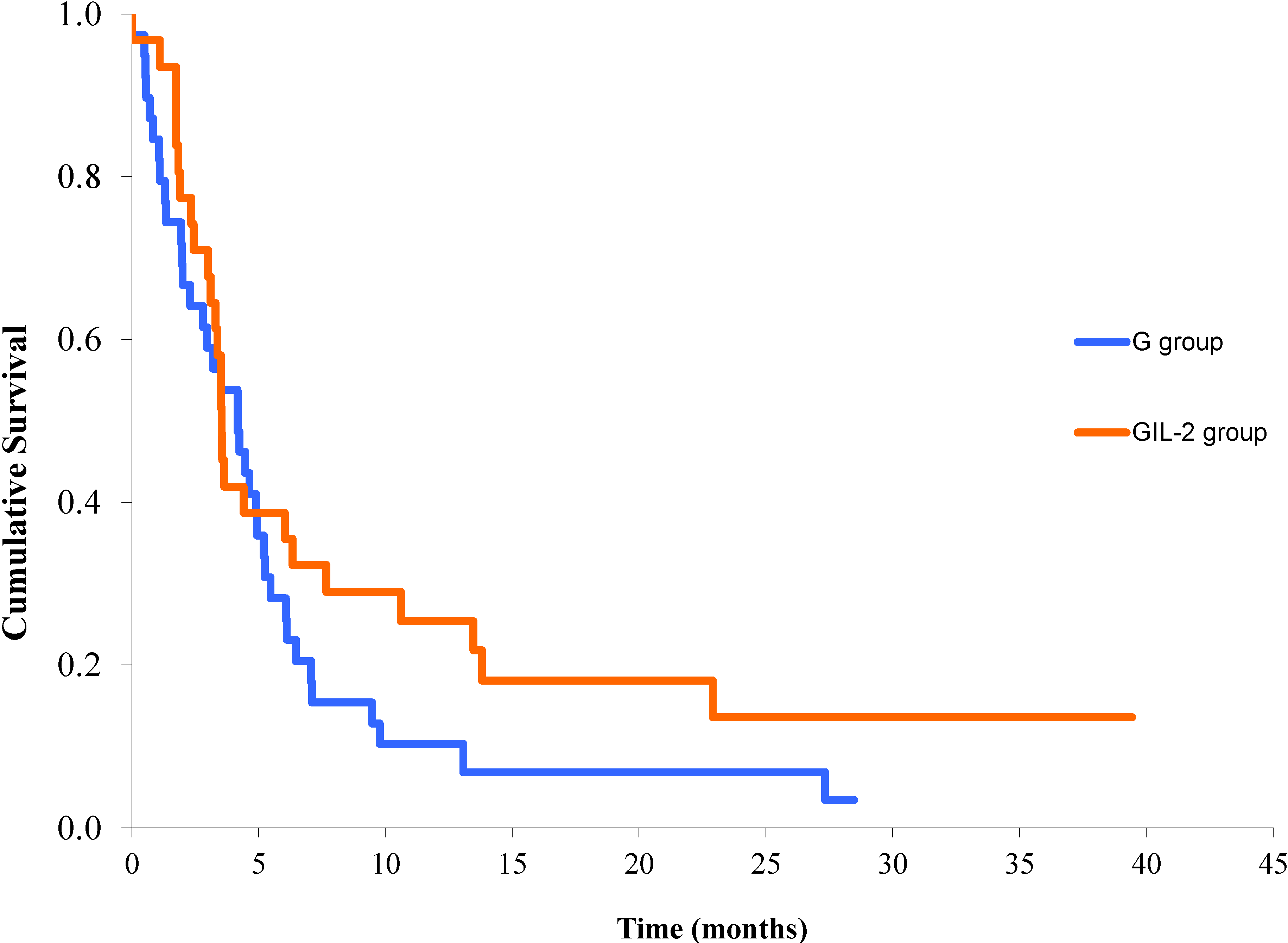
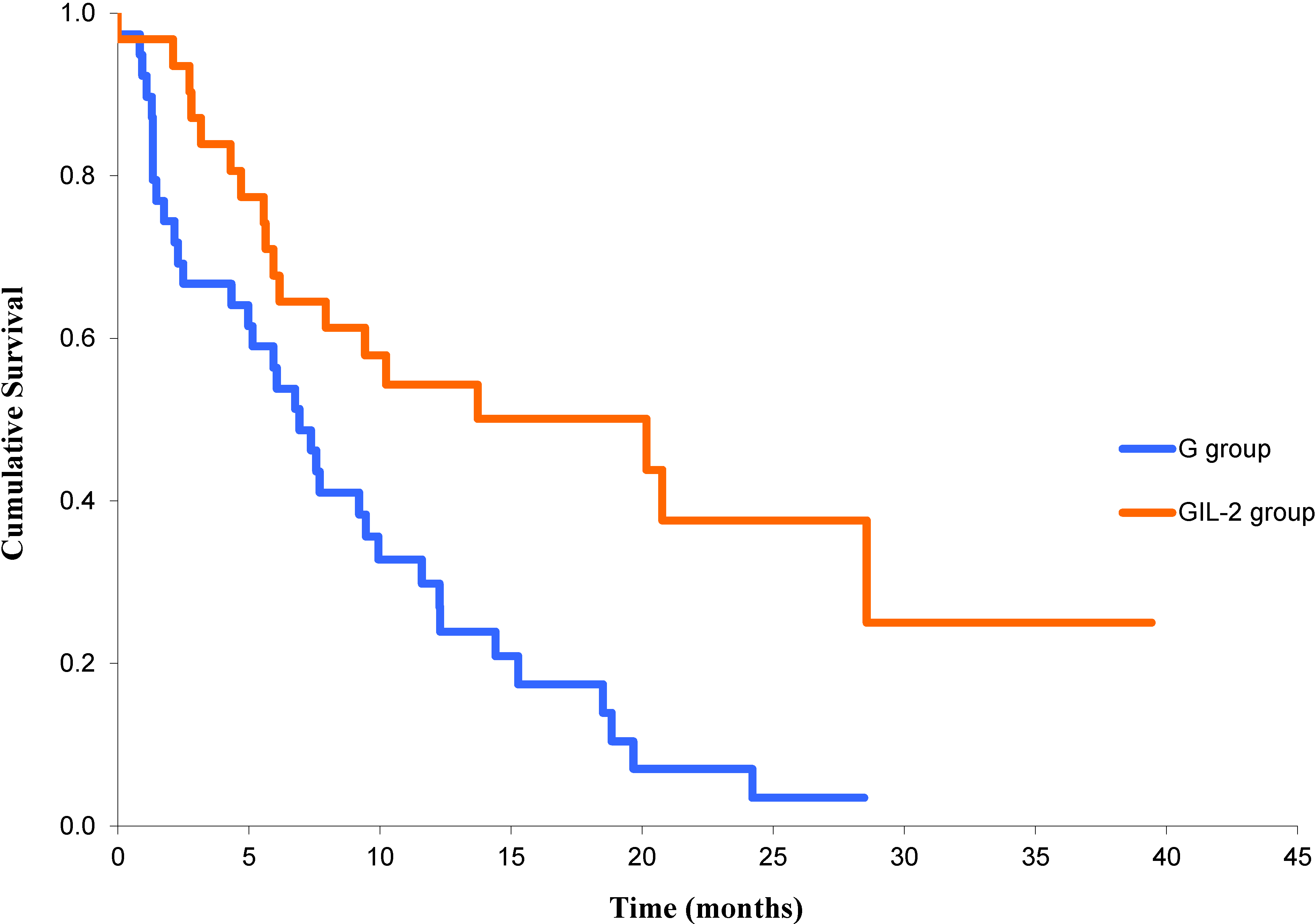
3.3. Toxicity
| Toxicity | Events(No. of Patients, %) | WHO grade (No. of Patients, %) | |||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Rash | 31 (44.3%) | 8 (11.4%) | 18 (25.7%) | 4 (5.7%) | 1 (1.4%) |
| Asthenia | 18 (25.7%) | 8 (11.4%) | 7 (10%) | 3 (4.3%) | 0 |
| Anorexia | 15 (21.4%) | 9 (12.9%) | 5 (7.1%) | 1 (1.4%) | 0 |
| Diarrhea | 12 (17.1%) | 7 (10%) | 0 | 5 (7.1%) | 0 |
| Dyspnea | 8 (11.4%) | 6 (8.6%) | 2 (2.9%) | 0 | 0 |
| Transaminase alteration | 4 (5.7%) | 3 (4.3%) | 0 | 1 (1.4%) | 0 |
| Fever | 18 (25.7%) | 4 (5.7%) | 14 (20%) | 0 | |
| Fatigue | 7 (10%) | 0 | 7 (10%) | 0 | |
| Arthralgias | 4 (5.7%) | 0 | 4 (5.7%) | 0 | |
4. Discussion and Conclusions
Author Contributions
Conflicts of Interest
References
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar]
- Kim, E.S.; Hirsh, V.; Mok, T.; Socinski, M.A.; Gervais, R.; Wu, Y.L.; Li, L.Y.; Watkins, C.L.; Sellers, M.V.; Lowe, E.S.; Sun, Y.; et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet 2008, 372, 1809–1818. [Google Scholar]
- Douillard, J.Y.; Shepherd, F.A.; Hirsh, V.; Mok, T.; Socinski, M.A.; Gervais, R.; Liao, M.L.; Bischoff, H.; Reck, M.; Sellers, M.V.; et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: Data from the randomized phase III INTEREST trial. J. Clin. Oncol. 2010, 28, 744–752. [Google Scholar]
- Thatcher, N.; Chang, A.; Parikh, P.; Rodrigues Pereira, J.; Ciuleanu, T.; von Pawel, J.; Thongprasert, S.; Tan, E.H.; Pemberton, K.; Archer, V.; et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa survival evaluation in lung cancer). Lancet 2005, 366, 1527–1537. [Google Scholar]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar]
- Ardizzoni, A.; Bonavia, M.; Viale, M.; Baldini, E.; Mereu, C.; Verna, A.; Ferrini, S.; Cinquegrana, A.; Molinari, S.; Mariani, G.L. Biologic and clinical effects of continuous infusion interleukin-2 in patients with non-small cell lung cancer. Cancer 1994, 73, 1353–1360. [Google Scholar]
- Schiller, J.H.; Morgan-Ihrig, C.; Levitt, M.L. Concomitant administration of interleukin-2 plus tumor necrosis factor in advanced non-small cell lung cancer. Am. J. Clin. Oncol. 1995, 18, 47–51. [Google Scholar]
- Lissoni, P.; Meregalli, S.; Fossati, V.; Paolorossi, F.; Barni, S.; Tancini, G.; Frigerio, F. A randomized study of immunotherapy with low-dose subcutaneous interleukin-2 plus melatonin vs. chemotherapy with cisplatin and etoposide as first-line therapy for advanced non-small cell lung cancer. Tumori 1994, 80, 464–467. [Google Scholar]
- Tester, W.J.; Kim, K.M.; Krigel, R.L.; Bonomi, P.D.; Glick, J.H.; Asbury, R.F.; Kirkwood, J.M.; Blum, R.H.; Schiller, J.H. A randomized Phase II study of interleukin-2 with and without beta-interferon for patients with advanced non-small cell lung cancer: An Eastern Cooperative Oncology Group study (PZ586). Lung Cancer 1999, 25, 199–206. [Google Scholar]
- De Vita, F.; Turitto, G.; di Grazia, M.; Frattolillo, A.; Catalano, G. Analysis of interleukin-2/interleukin-2 receptor system in advanced non-small-cell lung cancer. Tumori 1998, 84, 33–38. [Google Scholar]
- Chen, Y.M.; Yang, W.K.; Whang-Peng, J.; Tsai, W.Y.; Hung, Y.M.; Yang, D.M.; Lin, W.C.; Perng, R.P.; Ting, C.C. Restoration of the immunocompetence by IL-2 activation and TCR-CD3 engagement of the in vivo anergized tumor-specific CTL from lung cancer patients. J. Immunother. 1997, 20, 354–364. [Google Scholar]
- Brahmer, J.R. Harnessing the immune system for the treatment of non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 1021–1028. [Google Scholar]
- McCarthy, F.; Roshani, R.; Steele, J.; Hagemann, T. Current clinical immunotherapy targets in advanced nonsmall cell lung cancer (NSCLC). J. Leukoc. Biol. 2013, 94, 1201–1206. [Google Scholar]
- Reck, M. What future opportunities may immuno-oncology provide for improving the treatment of patients with lung cancer? Ann. Oncol. 2012, 8, 28–34. [Google Scholar]
- Shepherd, F.A.; Douillard, J.Y.; Blumenschein, G.R., Jr. Immunotherapy for non-small cell lung cancer: Novel approaches to improve patient outcome. J. Thorac. Oncol. 2011, 6, 1763–1773. [Google Scholar]
- Quoix, E. Phase IIB/III of TG4010 immunotherapy in patients with stage IV non-small cell lung cancer (TIME). Available online: http://www.clinicaltrials.gov/ct2/show/NCT01383148?term=NCT01383148&rank=1 (accessed on 18 September 2014).
- Iwai, K.; Soejima, K.; Kudoh, S.; Umezato, Y.; Kaneko, T.; Yoshimori, K.; Tokuda, H.; Yamaguchi, T.; Mizoo, A.; Setoguchi, Y.; et al. Extended survival observed in adoptive activated T lymphocyte immunotherapy for advanced lung cancer: Results of a multicenter historical cohort study. Cancer Immunol. Immunother. 2012, 61, 1781–1790. [Google Scholar]
- Gupta, P.; Emdad, L.; Lebedeva, I.V.; Sarkar, D.; Dent, P.; Curiel, D.T.; Settleman, J.; Fisher, P.B. Targeted combinatorial therapy of non-small cell lung carcinoma using a GST-fusion protein of full-length or truncated MDA-7/IL-24 with Tarceva. J. Cell Physiol. 2008, 215, 827–836. [Google Scholar]
- Emdad, L.; Lebedeva, I.V.; Su, Z.Z.; Gupta, P.; Sarkar, D.; Settleman, J.; Fisher, P.B. Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis-induction and reverses resistance to a single therapy. J. Cell Physiol. 2007, 210, 549–559. [Google Scholar]
- Kanazawa, S.; Muramatsu, M.; Kinoshita, Y.; Yamaguchi, K.; Nomura, S. Gefitinib has the potential of activating cell immunity against malignant cells. J. Clin. Oncol. 2005, 23, 3865–3856. [Google Scholar]
- Kanazawa, S.; Yamaguchi, K.; Kinoshita, Y.; Komiyama, Y.; Muramatsu, M.; Nomura, S. Elevation of soluble interleukin-2 receptor in patients with non-small cell lung cancer treated with gefitinib. J. Cancer Res. Clin. Oncol. 2006, 132, 719–725. [Google Scholar]
- Umekawa, K.; Kimura, T.; Kudoh, S.; Suzumura, T.; Oka, T.; Nagata, M.; Mitsuoka, S.; Matsuura, K.; Nakai, T.; Yoshimura, N.; et al. Plasma RANTES, IL-10, and IL-8 levels in non-small-cell lung cancer patients treated with EGFR-TKIs. BMC Res. Notes 2013, 6, e139. [Google Scholar]
- Yamamoto, N.; Honma, M.; Suzuki, H. Off-target serine/threonine kinase 10 inhibition by erlotinib enhances lymphocytic activity leading to severe skin disorders. Mol. Pharmacol. 2011, 80, 466–475. [Google Scholar]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar]
- Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed on 24 June 2014).
- Grande, C.; Firvida, J.L.; Navas, V.; Casal, J. Interleukin-2 for the treatment of solid tumors other than melanoma and renal cell carcinoma. Anticancer Drugs 2006, 17, 1–12. [Google Scholar]
- Masotti, A.; Morandini, G.; Ortolani, R.; Fumagalli, L. Phase-II randomized study of pre-operative IL-2 administration in operable NSCLC. Lung Cancer 1998, 20, 191–202. [Google Scholar]
- Recchia, F.; Saggio, G.; Nuzzo, A.; Biondi, E.; Di Blasio, A.; Cesta, A.; Candeloro, G.; Alesse, E.; Rea, S. Multicenter phase 2 study of interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced non-small-cell lung cancer. J. Immunother. 2006, 29, 87–94. [Google Scholar]
- Mantovani, G.; Madeddu, C.; Gramignano, G.; Lusso, M.R.; Mocci, M.; Massa, E.; Ferreli, L.; Astara, G.; Macciò, A.; Serpe, R. Subcutaneous interleukin-2 in combination with medroxyprogesterone acetate and antioxidants in advanced cancer responders to previous chemotherapy: Phase II study evaluating clinical, quality of life, and laboratory parameters. J. Exp. Ther. Oncol. 2003, 3, 205–219. [Google Scholar]
- Yang, S.C.; Owen-Schaub, L.; Grimm, E.A.; Roth, J.A. Induction of lymphokine-activated killer cytotoxicity with interleukin-2 and tumor necrosis factor-alpha against primary lung cancer targets. Cancer Immunol. Immunother. 1989, 29, 193–198. [Google Scholar]
- Yang, S.C.; Grimm, E.A.; Parkinson, D.R.; Carinhas, J.; Fry, K.D.; Mendiguren-Rodriguez, A.; Licciardello, J.; Owen-Schaub, L.B.; Hong, W.K.; Roth, J.A. Clinical and immunomodulatory effects of combination immunotherapy with low-dose interleukin 2 and tumor necrosis factor alpha in patients with advanced non-small cell lung cancer: A phase I trial. Cancer Res. 1991, 51, 3669–3676. [Google Scholar]
- Valone, F.H.; Gandara, D.R.; Deisseroth, A.B.; Perez, E.A.; Rayner, A.; Aronson, F.R.; Luce, J.; Paradise, C. Interleukin-2, cisplatin, and 5-fluorouracil for patients with non-small cell lung and head/neck carcinomas. J. Immunother. 1991, 10, 207–213. [Google Scholar]
- Jansen, R.L.; Slingerland, R.; Goey, S.H.; Franks, C.R.; Bolhuis, R.L.; Stoter, G. Interleukin-2 and interferon-alpha in the treatment of patients with advanced non-small-cell lung cancer. J. Immunother. 1992, 12, 70–73. [Google Scholar]
- Lissoni, P.; Tisi, E.; Barni, S.; Ardizzoia, A.; Rovelli, F.; Rescaldani, R.; Ballabio, D.; Benenti, C.; Angeli, M.; Tancini, G. Biological and clinical results of a neuroimmunotherapy with interleukin-2 and the pineal hormone melatonin as a first line treatment in advanced non-small cell lung cancer. Br. J. Cancer 1992, 66, 155–158. [Google Scholar]
- Mantovani, G.; Macciò, A.; Mulas, C.; Massa, E.; Madeddu, C.; Mura, L.; Contu, P.; Versace, R. Dose-intense phase II study of weekly cisplatin and epidoxorubicin plus medroxyprogesterone acetate and recombinant interleukin 2 in stage IIIB-IV non-small cell lung cancer. Oncol. Rep. 2002, 9, 661–670. [Google Scholar]
- Petrelli, F.; Borgonovo, K.; Cabiddu, M.; Lonati, V.; Barni, S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: A literature-based meta-analysis of 24 trials. Lung Cancer 2012, 78, 8–15. [Google Scholar]
- Liu, H.B.; Wu, Y.; Lv, T.F.; Yao, Y.W.; Xiao, Y.Y.; Yuan, D.M.; Song, Y. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: A systematic review and meta-analysis. PLoS One 2013, 8, e55128. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bersanelli, M.; Buti, S.; Camisa, R.; Brighenti, M.; Lazzarelli, S.; Mazza, G.; Passalacqua, R. Gefitinib Plus Interleukin-2 in Advanced Non-Small Cell Lung Cancer Patients Previously Treated with Chemotherapy. Cancers 2014, 6, 2035-2048. https://doi.org/10.3390/cancers6042035
Bersanelli M, Buti S, Camisa R, Brighenti M, Lazzarelli S, Mazza G, Passalacqua R. Gefitinib Plus Interleukin-2 in Advanced Non-Small Cell Lung Cancer Patients Previously Treated with Chemotherapy. Cancers. 2014; 6(4):2035-2048. https://doi.org/10.3390/cancers6042035
Chicago/Turabian StyleBersanelli, Melissa, Sebastiano Buti, Roberta Camisa, Matteo Brighenti, Silvia Lazzarelli, Giancarlo Mazza, and Rodolfo Passalacqua. 2014. "Gefitinib Plus Interleukin-2 in Advanced Non-Small Cell Lung Cancer Patients Previously Treated with Chemotherapy" Cancers 6, no. 4: 2035-2048. https://doi.org/10.3390/cancers6042035
APA StyleBersanelli, M., Buti, S., Camisa, R., Brighenti, M., Lazzarelli, S., Mazza, G., & Passalacqua, R. (2014). Gefitinib Plus Interleukin-2 in Advanced Non-Small Cell Lung Cancer Patients Previously Treated with Chemotherapy. Cancers, 6(4), 2035-2048. https://doi.org/10.3390/cancers6042035






