Monoclonal Antibodies Specific for STAT3β Reveal Its Contribution to Constitutive STAT3 Phosphorylation in Breast Cancer
Abstract
:1. Introduction
2. Results
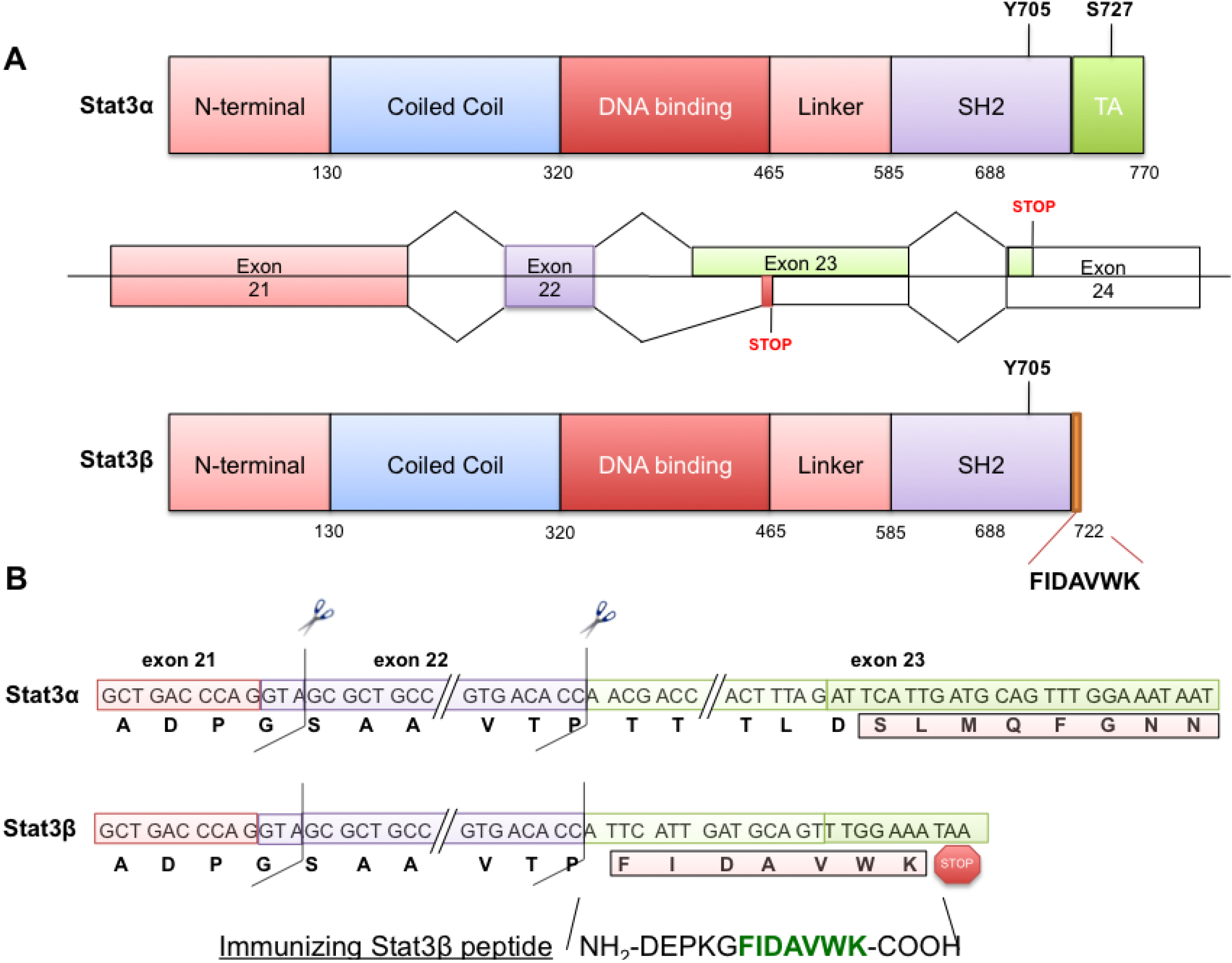
2.1. Stat3β Immunogen Design and Mouse Immunization
2.2. Antisera from Mice Immunized with CT7 Peptide Specifically Detect Stat3β by Immunoblotting
2.3. Generation and Subcloning of Hybridomas
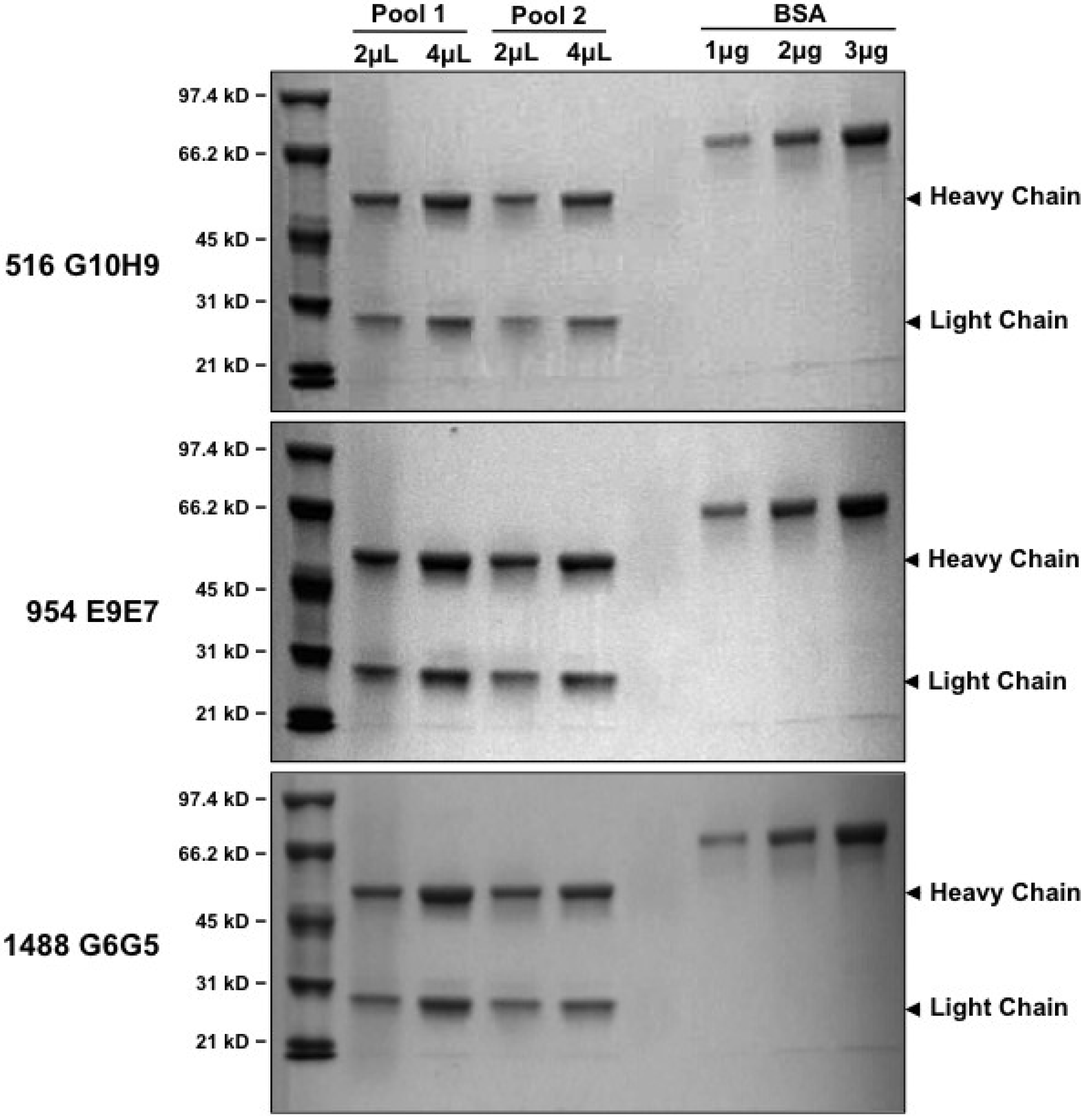
2.4. IgG Purification
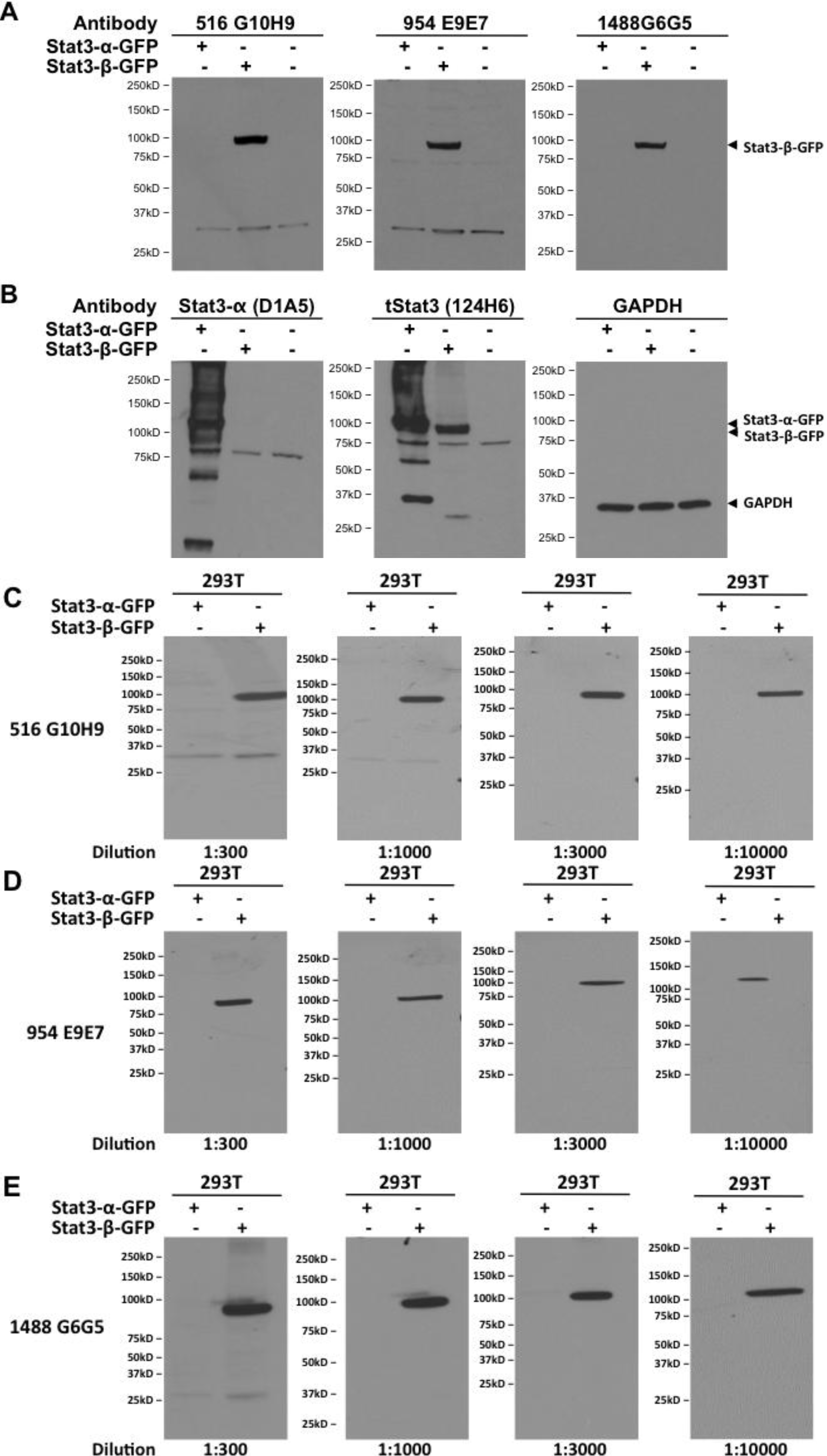
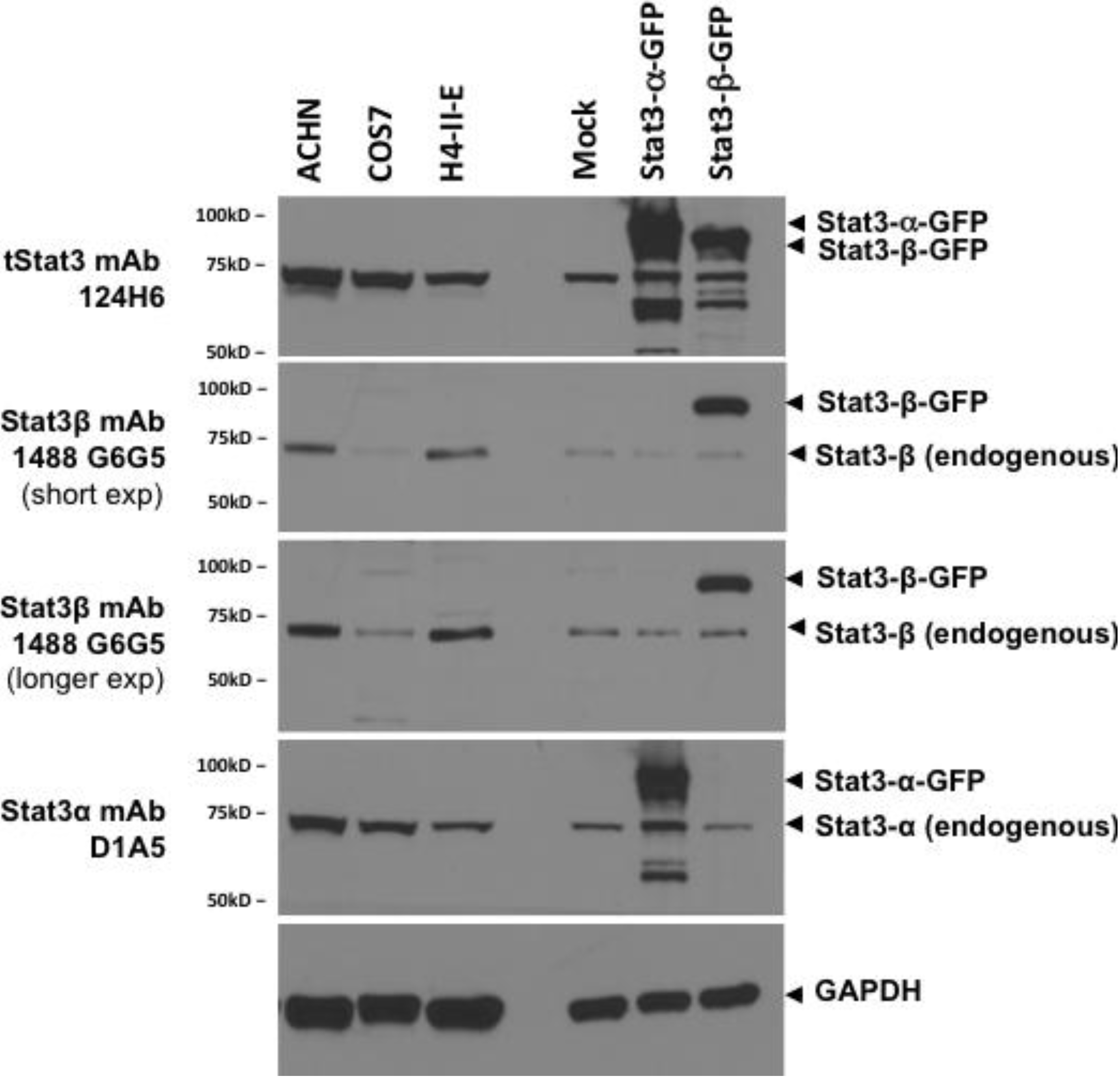
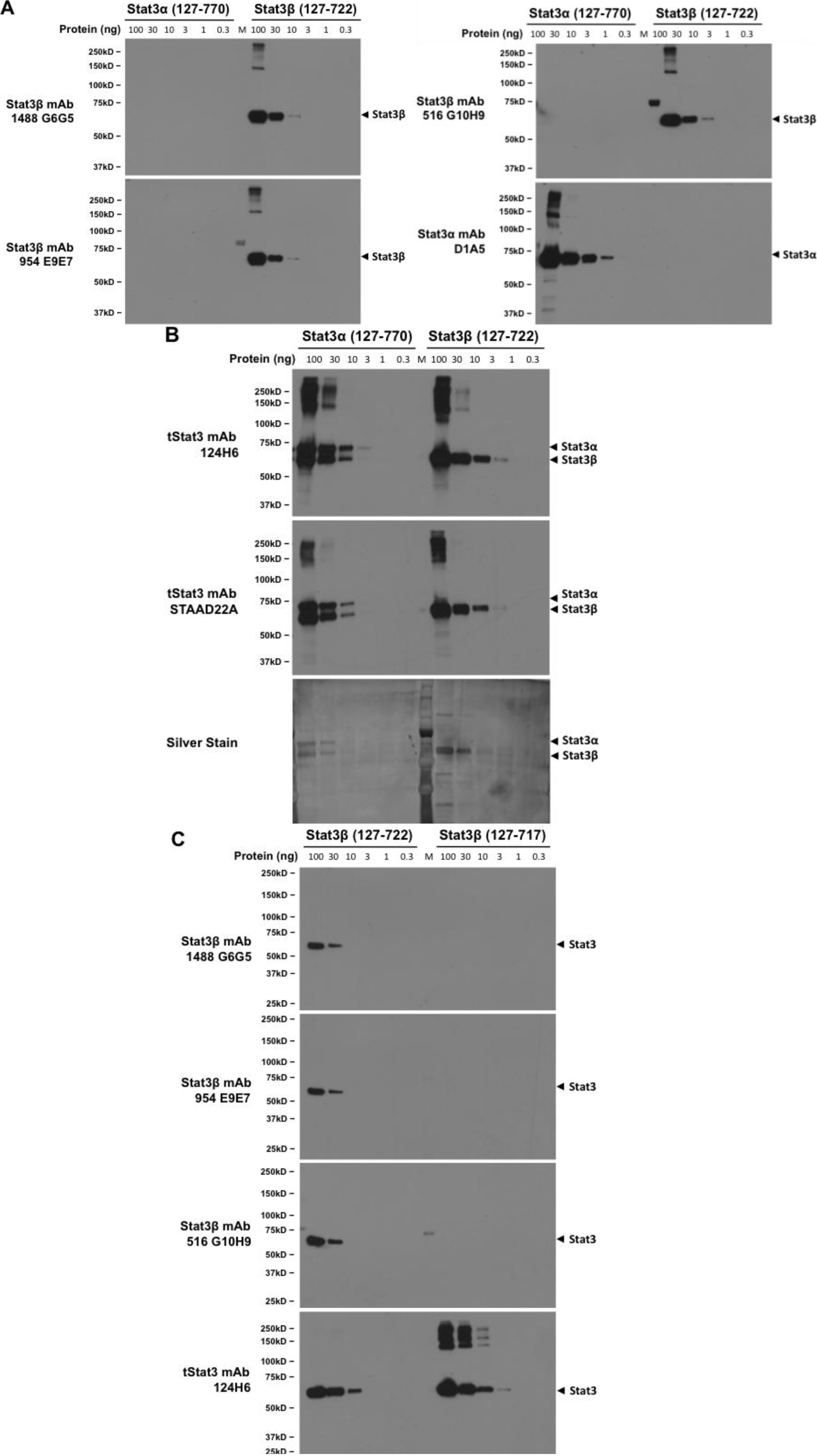
2.5. Dependence of Stat3β-Specific Monoclonal Antibodies to Detect Recombinant Stat3β upon Presence of the CT7 Epitope
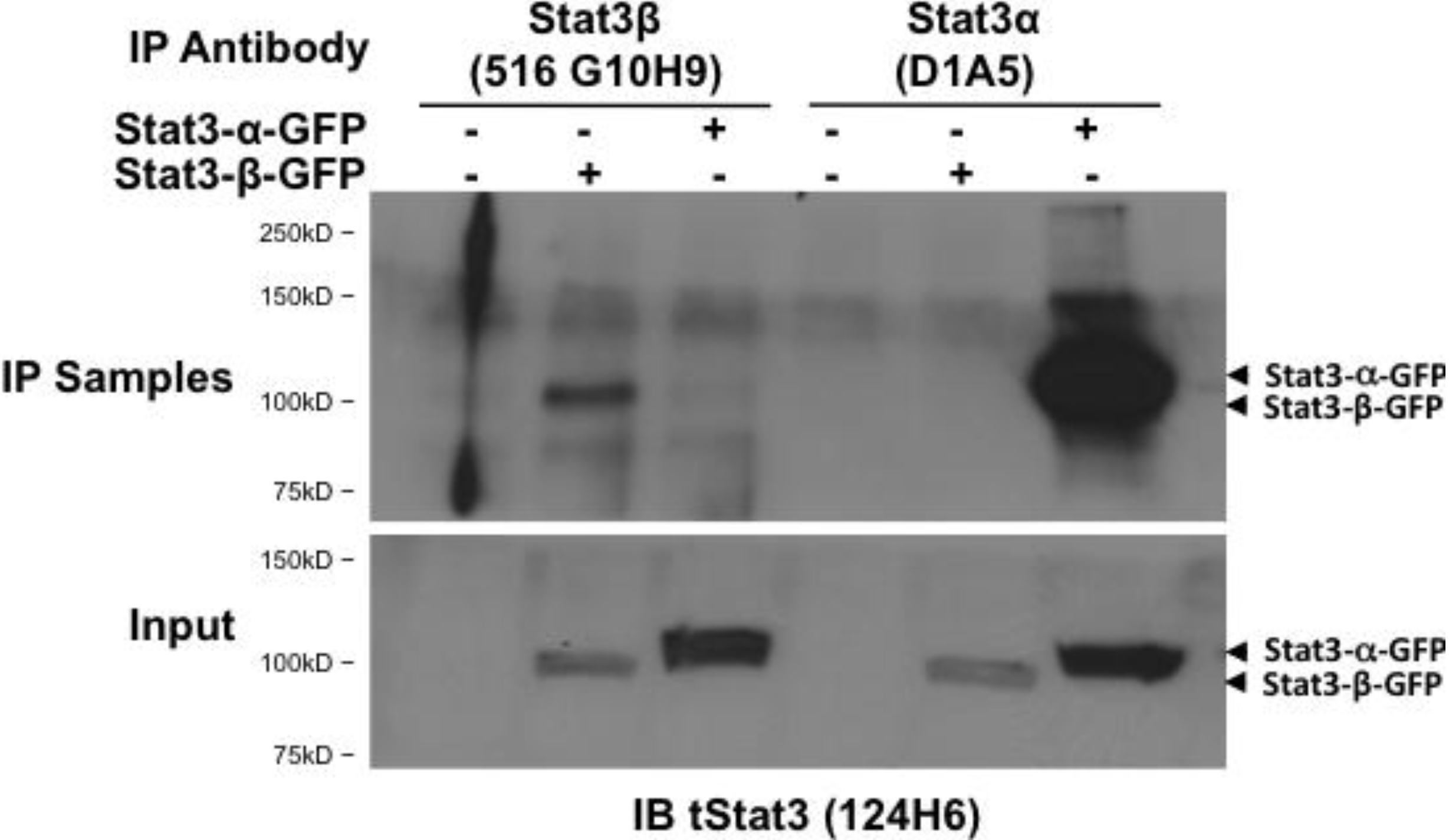
2.6. Stat3β Monoclonal Antibodies Immunoprecipitate Stat3β from Cell Line Lysates
3. Discussion
4. Experimental
4.1. Cell Lines
4.2. Stat3 Plasmids and Purification of Stat3 Protein
4.3. Peptide Design and Mouse Immunization
4.4. Hybridoma Formation
4.5. ELISA for Detecting Anti-Stat3β Activity in Serum and Culture Supernatants
4.6. Purification of IgG from Culture Supernatants
4.7. Immunoblotting
4.8. Immunoprecipitation
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
Author Contributions
Conflicts of Interest
References
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar]
- Bode, J.G.; Albrecht, U.; Haussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins—Regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar]
- He, G.; Karin, M. NF-kappaB and STAT3-key players in liver inflammation and cancer. Cell Res. 2011, 21, 159–168. [Google Scholar]
- Li, N.; Grivennikov, S.I.; Karin, M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell 2011, 19, 429–431. [Google Scholar] [PubMed]
- Bharadwaj, U.; Eckols, T.K.; Kolosov, M.; Kasembeli, M.M.; Adam, A.; Torres, D.; Zhang, X.; Dobrolecki, L.E.; Wei, W.; Lewis, M.T.; et al. Drug-repositioning screening identified piperlongumine as a direct STAT3 inhibitor with potent activity against breast cancer. Oncogene 2014. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Li, M.; Chen, C.; Yao, Q. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol. Cancer Res. 2008, 6, 1755–1765. [Google Scholar] [PubMed]
- Bharadwaj, U.; Li, M.; Zhang, R.; Chen, C.; Yao, Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007, 67, 5479–5488. [Google Scholar] [PubMed]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis 2011, 32, 1013–1024. [Google Scholar] [PubMed]
- Rossa, C., Jr.; Sommer, G.; Spolidorio, L.C.; Rosenzweig, S.A.; Watson, D.K.; Kirkwood, K.L. Loss of expression and function of SOCS3 is an early event in HNSCC: Altered subcellular localization as a possible mechanism involved in proliferation, migration and invasion. PLoS One 2012, 7, e45197. [Google Scholar] [PubMed]
- Ishida, F.; Matsuda, K.; Sekiguchi, N.; Makishima, H.; Taira, C.; Momose, K.; Nishina, S.; Senoo, N.; Sakai, H.; Ito, T.; et al. STAT3 gene mutations and their association with pure red cell aplasia in large granular lymphocyte leukemia. Cancer Sci. 2014, 105, 342–346. [Google Scholar] [PubMed]
- Munoz, J.; Dhillon, N.; Janku, F.; Watowich, S.S.; Hong, D.S. STAT3 Inhibitors: Finding a home in lymphoma and leukemia. Oncologist 2014, 19, 536–544. [Google Scholar] [PubMed]
- Shao, H.; Quintero, A.J.; Tweardy, D.J. Identification and characterization of cis elements in the STAT3 gene regulating STAT3 alpha and STAT3 beta messenger RNA splicing. Blood 2001, 98, 3853–3856. [Google Scholar] [PubMed]
- Kato, T.; Sakamoto, E.; Kutsuna, H.; Kimura-Eto, A.; Hato, F.; Kitagawa, S. Proteolytic conversion of STAT3alpha to STAT3gamma in human neutrophils: Role of granule-derived serine proteases. J. Biol. Chem. 2004, 279, 31076–31080. [Google Scholar] [PubMed]
- Biethahn, S.; Alves, F.; Wilde, S.; Hiddemann, W.; Spiekermann, K. Expression of granulocyte colony-stimulating factor- and granulocyte-macrophage colony-stimulating factor-associated signal transduction proteins of the JAK/STAT pathway in normal granulopoiesis and in blast cells of acute myelogenous leukemia. Exp. Hematol. 1999, 27, 885–894. [Google Scholar] [PubMed]
- Chakraborty, A.; White, S.M.; Schaefer, T.S.; Ball, E.D.; Dyer, K.F.; Tweardy, D.J. Granulocyte colony-stimulating factor activation of Stat3 alpha and Stat3 beta in immature normal and leukemic human myeloid cells. Blood 1996, 88, 2442–2449. [Google Scholar] [PubMed]
- Caldenhoven, E.; van Dijk, T.B.; Solari, R.; Armstrong, J.; Raaijmakers, J.A.; Lammers, J.W.; Koenderman, L.; de Groot, R.P. STAT3beta, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J. Biol. Chem. 1996, 271, 13221–13227. [Google Scholar] [PubMed]
- Kunz, D.; Zimmermann, R.; Heisig, M.; Heinrich, P.C. Identification of the promoter sequences involved in the interleukin-6 dependent expression of the rat alpha 2-macroglobulin gene. Nucleic Acids Res. 1989, 17, 1121–1138. [Google Scholar] [PubMed]
- Kushner, I. The phenomenon of the acute phase response. Ann. N. Y. Acad. Sci. 1982, 389, 39–48. [Google Scholar] [PubMed]
- Schaefer, T.S.; Sanders, L.K.; Nathans, D. Cooperative transcriptional activity of Jun and Stat3beta, a short form of Stat3. Proc. Natl. Acad. Sci. USA 1995, 92, 9097–9101. [Google Scholar] [PubMed]
- Schaefer, T.S.; Sanders, L.K.; Park, O.K.; Nathans, D. Functional differences between Stat3alpha and Stat3beta. Mol. Cell. Biol. 1997, 17, 5307–5316. [Google Scholar] [PubMed]
- Ivanov, V.N.; Bhoumik, A.; Krasilnikov, M.; Raz, R.; Owen-Schaub, L.B.; Levy, D.; Horvath, C.M.; Ronai, Z. Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol. Cell 2001, 7, 517–528. [Google Scholar] [PubMed]
- Yoo, J.Y.; Huso, D.L.; Nathans, D.; Desiderio, S. Specific ablation of Stat3beta distorts the pattern of Stat3-responsive gene expression and impairs recovery from endotoxic shock. Cell 2002, 108, 331–344. [Google Scholar] [PubMed]
- Maritano, D.; Sugrue, M.L.; Tininini, S.; Dewilde, S.; Strobl, B.; Fu, X.; Murray-Tait, V.; Chiarle, R.; Poli, V. The STAT3 isoforms alpha and beta have unique and specific functions. Nat. Immunol. 2004, 5, 401–409. [Google Scholar] [PubMed]
- Dewilde, S.; Vercelli, A.; Chiarle, R.; Poli, V. Of alphas and betas: Distinct and overlapping functions of STAT3 isoforms. Front. Biosci. 2008, 13, 6501–6514. [Google Scholar] [PubMed]
- Hevehan, D.L.; Miller, W.M.; Papoutsakis, E.T. Differential expression and phosphorylation of distinct STAT3 proteins during granulocytic differentiation. Blood 2002, 99, 1627–1637. [Google Scholar] [PubMed]
- Minami, M.; Inoue, M.; Wei, S.; Takeda, K.; Matsumoto, M.; Kishimoto, T.; Akira, S. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc. Natl. Acad. Sci. USA 1996, 93, 3963–3966. [Google Scholar] [PubMed]
- Huang, Y.; Qiu, J.; Dong, S.; Redell, M.S.; Poli, V.; Mancini, M.A.; Tweardy, D.J. Stat3 isoforms, alpha and beta, demonstrate distinct intracellular dynamics with prolonged nuclear retention of Stat3beta mapping to its unique C-terminal end. J. Biol. Chem. 2007, 282, 34958–34967. [Google Scholar] [PubMed]
- Ng, I.H.; Ng, D.C.; Jans, D.A.; Bogoyevitch, M.A. Selective STAT3-alpha or -beta expression reveals spliceform-specific phosphorylation kinetics, nuclear retention and distinct gene expression outcomes. Biochem. J. 2012, 447, 125–136. [Google Scholar] [PubMed]
- Sasse, J.; Hemmann, U.; Schwartz, C.; Schniertshauer, U.; Heesel, B.; Landgraf, C.; Schneider-Mergener, J.; Heinrich, P.C.; Horn, F. Mutational analysis of acute-phase response factor/Stat3 activation and dimerization. Mol. Cell. Biol. 1997, 17, 4677–4686. [Google Scholar] [PubMed]
- Foley, H.A.; Ofori-Acquah, S.F.; Yoshimura, A.; Critz, S.; Baliga, B.S.; Pace, B.S. Stat3 beta inhibits gamma-globin gene expression in erythroid cells. J. Biol. Chem. 2002, 277, 16211–16219. [Google Scholar] [PubMed]
- Zammarchi, F.; de Stanchina, E.; Bournazou, E.; Supakorndej, T.; Martires, K.; Riedel, E.; Corben, A.D.; Bromberg, J.F.; Cartegni, L. Antitumorigenic potential of STAT3 alternative splicing modulation. Proc. Natl. Acad. Sci. USA 2011, 108, 17779–17784. [Google Scholar] [PubMed]
- Christensen, K.; Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030, USA. Unpublished data. 2013.
- Kasembeli, M.M.; Section of Infectious Disease, Department of Medicine, Baylor College of Medicine, Houston, TX 77030, USA. Unpublished data. 2014.
- Xu, X.; Kasembeli, M.M.; Jiang, X.; Tweardy, B.J.; Tweardy, D.J. Chemical probes that competitively and selectively inhibit Stat3 activation. PLoS One 2009, 4, e4783. [Google Scholar] [PubMed]
- Alten, J.A.; Moran, A.; Tsimelzon, A.I.; Mastrangelo, M.A.; Hilsenbeck, S.G.; Poli, V.; Tweardy, D.J. Prevention of hypovolemic circulatory collapse by IL-6 activated Stat3. PLoS One 2008, 3, e1605. [Google Scholar] [PubMed]
- Moran, A.; Arikan, A.A.; Mastrangelo, M.A.; Wu, Y.; Yu, B.; Poli, V.; Tweardy, D.J. Prevention of trauma and hemorrhagic shock-mediated liver apoptosis by activation of stat3alpha. Int. J. Clin. Exp. Med. 2008, 1, 213–247. [Google Scholar] [PubMed]
- Moran, A.; Thacker, S.A.; Arikan, A.A.; Mastrangelo, M.A.; Wu, Y.; Yu, B.; Tweardy, D.J. IL-6-mediated activation of Stat3alpha prevents trauma/hemorrhagic shock-induced liver inflammation. PLoS One 2011, 6, e21449. [Google Scholar] [PubMed]
- Moran, A.; Tsimelzon, A.I.; Mastrangelo, M.A.; Wu, Y.; Yu, B.; Hilsenbeck, S.G.; Poli, V.; Tweardy, D.J. Prevention of trauma/hemorrhagic shock-induced lung apoptosis by IL-6-mediated activation of Stat3. Clin. Transl. Sci. 2009, 2, 41–49. [Google Scholar]
- Scholz, A.; Heinze, S.; Detjen, K.M.; Peters, M.; Welzel, M.; Hauff, P.; Schirner, M.; Wiedenmann, B.; Rosewicz, S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology 2003, 125, 891–905. [Google Scholar] [PubMed]
- Turkson, J.; Bowman, T.; Garcia, R.; Caldenhoven, E.; de Groot, R.P.; Jove, R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 1998, 18, 2545–2552. [Google Scholar] [PubMed]
- Discovery Services. Available online: http://dtp.nci.nih.gov/docs/misc/common_files/mda-mb-435-update.html (accessed on 22 August 2014).
- Burke, W.M.; Jin, X.; Lin, H.J.; Huang, M.; Liu, R.; Reynolds, R.K.; Lin, J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene 2001, 20, 7925–7934. [Google Scholar] [PubMed]
- Ni, Z.; Lou, W.; Leman, E.S.; Gao, A.C. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res. 2000, 60, 1225–1228. [Google Scholar] [PubMed]
- Niu, G.; Heller, R.; Catlett-Falcone, R.; Coppola, D.; Jaroszeski, M.; Dalton, W.; Jove, R.; Yu, H. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999, 59, 5059–5063. [Google Scholar] [PubMed]
- Benekli, M.; Xia, Z.; Donohue, K.A.; Ford, L.A.; Pixley, L.A.; Baer, M.R.; Baumann, H.; Wetzler, M. Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease-free survival. Blood 2002, 99, 252–257. [Google Scholar] [PubMed]
- Xia, Z.; Sait, S.N.; Baer, M.R.; Barcos, M.; Donohue, K.A.; Lawrence, D.; Ford, L.A.; Block, A.M.; Baumann, H.; Wetzler, M.; et al. Truncated STAT proteins are prevalent at relapse of acute myeloid leukemia. Leuk. Res. 2001, 25, 473–482. [Google Scholar] [PubMed]
- Ramadoss, P.; Unger-Smith, N.E.; Lam, F.S.; Hollenberg, A.N. STAT3 targets the regulatory regions of gluconeogenic genes in vivo. Mol. Endocrinol. 2009, 23, 827–837. [Google Scholar] [PubMed]
- Marotta, L.L.; Almendro, V.; Marusyk, A.; Shipitsin, M.; Schemme, J.; Walker, S.R.; Bloushtain-Qimron, N.; Kim, J.J.; Choudhury, S.A.; Maruyama, R.; et al. The JAK2/STAT3 signaling pathway is required for growth of CD44CD24 stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011, 121, 2723–2735. [Google Scholar] [PubMed]
- Bonnal, S.; Vigevani, L.; Valcarcel, J. The spliceosome as a target of novel antitumour drugs. Nat. Rev. Drug Discov. 2012, 11, 847–859. [Google Scholar] [PubMed]
- Elsarraj, H.S.; Hong, Y.; Valdez, K.; Carletti, M.; Salah, S.M.; Raimo, M.; Taverna, D.; Prochasson, P.; Bharadwaj, U.; Tweardy, D.J.; et al. A novel role of microRNA146b in promoting mammary alveolar progenitor cell maintenance. J. Cell Sci. 2013, 126, 2446–2458. [Google Scholar] [PubMed]
- Xia, H.; Qi, Y.; Ng, S.S.; Chen, X.; Li, D.; Chen, S.; Ge, R.; Jiang, S.; Li, G.; Chen, Y.; et al. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009, 1269, 158–165. [Google Scholar] [PubMed]
- Walker, S.R.; Xiang, M.; Frank, D.A. STAT3 Activity and Function in Cancer: Modulation by STAT5 and miR-146b. Cancers (Basel) 2014, 6, 958–968. [Google Scholar]
- Xiang, M.; Birkbak, N.J.; Vafaizadeh, V.; Walker, S.R.; Yeh, J.E.; Liu, S.; Kroll, Y.; Boldin, M.; Taganov, K.; Groner, B.; et al. STAT3 induction of miR-146b forms a feedback loop to inhibit the NF-kappaB to IL-6 signaling axis and STAT3-driven cancer phenotypes. Sci. Signal. 2014. [Google Scholar] [CrossRef]
- Costa-Pereira, A.P.; Tininini, S.; Strobl, B.; Alonzi, T.; Schlaak, J.F.; Is’harc, H.; Gesualdo, I.; Newman, S.J.; Kerr, I.M.; Poli, V.; et al. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc. Natl. Acad. Sci. USA 2002, 99, 8043–8047. [Google Scholar] [PubMed]
- Shao, H.; Cheng, H.Y.; Cook, R.G.; Tweardy, D.J. Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res. 2003, 63, 3923–3930. [Google Scholar] [PubMed]
- ImageJ: Image Processing and Analysis in Java. Available online: http://imagej.nih.gov/ij/ (accessed on 22 August 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharadwaj, U.; Kasembeli, M.M.; Eckols, T.K.; Kolosov, M.; Lang, P.; Christensen, K.; Edwards, D.P.; Tweardy, D.J. Monoclonal Antibodies Specific for STAT3β Reveal Its Contribution to Constitutive STAT3 Phosphorylation in Breast Cancer. Cancers 2014, 6, 2012-2034. https://doi.org/10.3390/cancers6042012
Bharadwaj U, Kasembeli MM, Eckols TK, Kolosov M, Lang P, Christensen K, Edwards DP, Tweardy DJ. Monoclonal Antibodies Specific for STAT3β Reveal Its Contribution to Constitutive STAT3 Phosphorylation in Breast Cancer. Cancers. 2014; 6(4):2012-2034. https://doi.org/10.3390/cancers6042012
Chicago/Turabian StyleBharadwaj, Uddalak, Moses M. Kasembeli, T. Kris Eckols, Mikhail Kolosov, Paul Lang, Kurt Christensen, Dean P. Edwards, and David J. Tweardy. 2014. "Monoclonal Antibodies Specific for STAT3β Reveal Its Contribution to Constitutive STAT3 Phosphorylation in Breast Cancer" Cancers 6, no. 4: 2012-2034. https://doi.org/10.3390/cancers6042012
APA StyleBharadwaj, U., Kasembeli, M. M., Eckols, T. K., Kolosov, M., Lang, P., Christensen, K., Edwards, D. P., & Tweardy, D. J. (2014). Monoclonal Antibodies Specific for STAT3β Reveal Its Contribution to Constitutive STAT3 Phosphorylation in Breast Cancer. Cancers, 6(4), 2012-2034. https://doi.org/10.3390/cancers6042012