Nuclear DNA-Content in Mesenchymal Lesions in Dogs: Its Value as Marker of Malignancy and Extent of Genomic Instability
Abstract
:Abbreviations
| CKC | Canine Kidney Cells |
| CRBC | Chicken Red Blood Cells |
| CV | Coefficient of Variation |
| DI | DNA Index |
| FCM | Flow Cytometry |
| HS | Histiocytic Sarcoma |
| MTB | Malignant Tumor of Bone |
| OS | Osteosarcoma |
| PD | Peridiploid |
| STS | Soft Tissue Sarcoma |
1. Introduction
2. Results
2.1. DNA-Ploidy Status in Primary Lesions
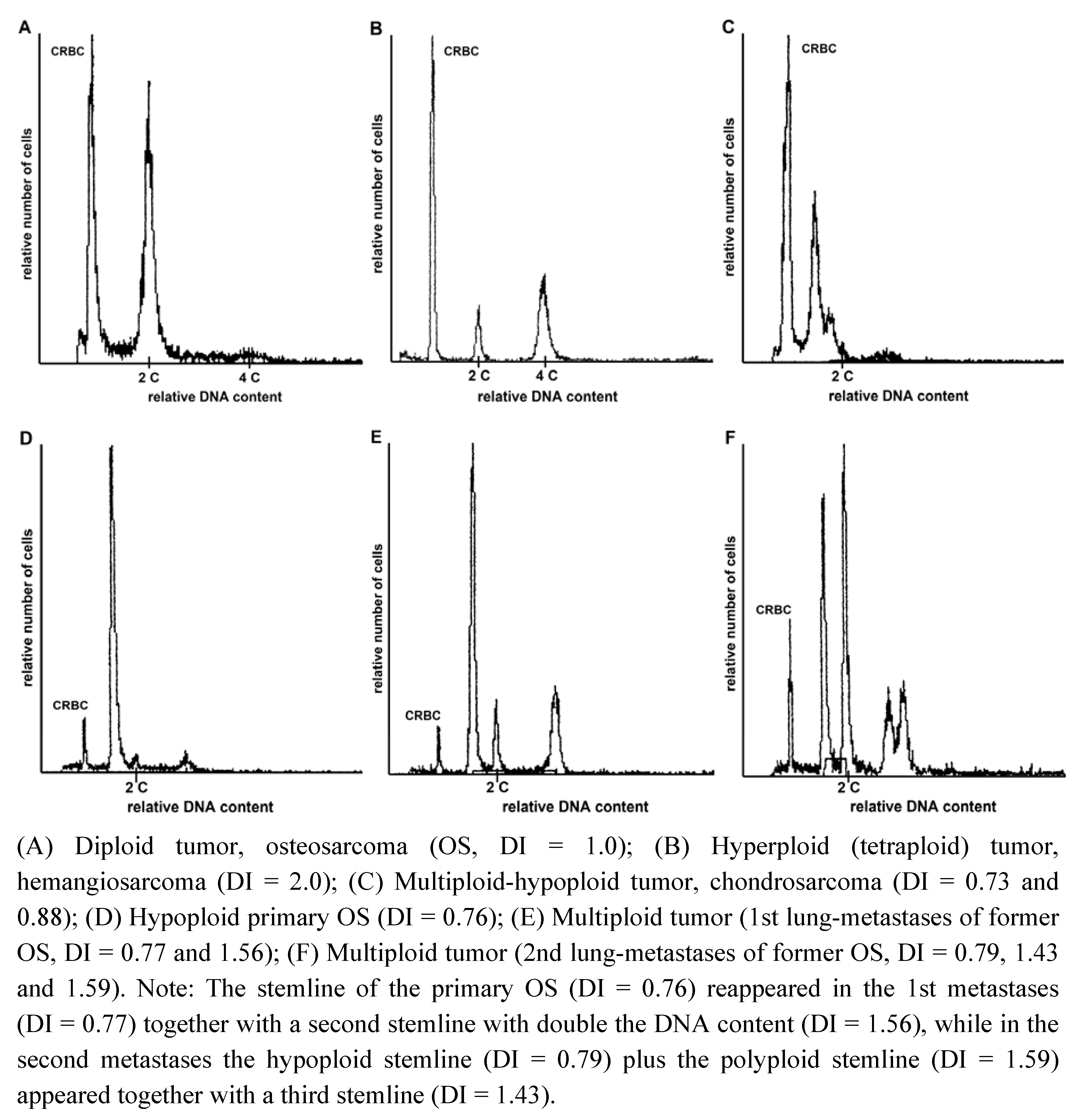
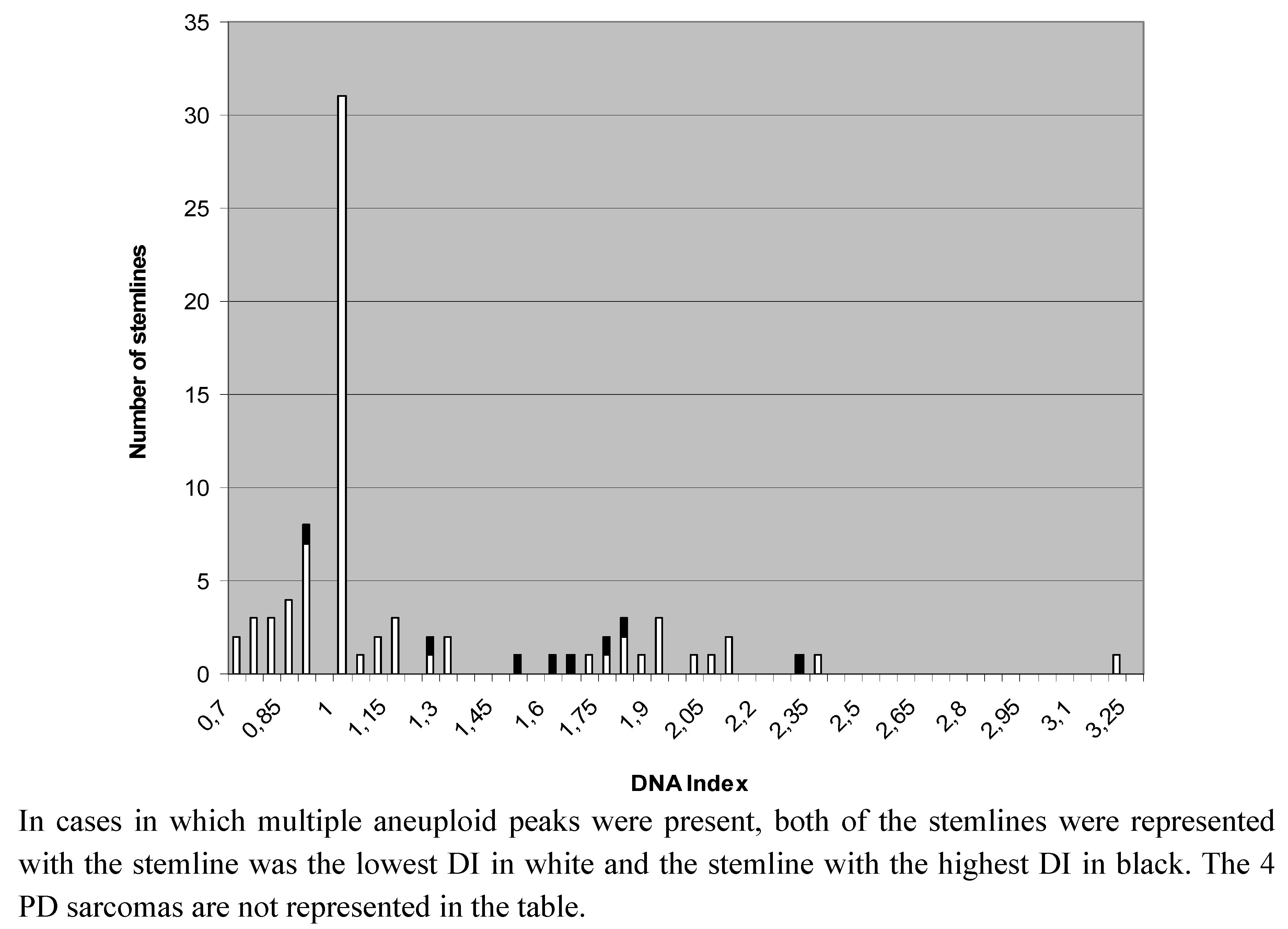
| Subtype | Diploid (n) | Aneuploid (n) | Peridiploid (n) | Total (n) |
|---|---|---|---|---|
| Malignant tumors of bone | ||||
| Osteosarcoma | 9 | 14 | 3 | 26 |
| Chondrosarcoma | 1 | 2 | - | 3 |
| Multilobular tumor of bone | 0 | 1 | - | 1 |
| Total | 10 | 17 | 3 | 30 |
| Soft tissue sarcomas | ||||
| Fibrosarcoma | 2 | 1 | - | 3 |
| Sarcoma-not otherwise specified | 3 | 1 | - | 4 |
| Rhabdomyosarcoma | 2 | 3 | - | 5 |
| Malignant peripheral nerve sheath tumors | 7 | 1 | - | 8 |
| Synovial sarcoma | 0 | 5 | - | 5 |
| Liposarcoma | 0 | 3 | - | 3 |
| Leiomyosarcoma | 6 | 2 | - | 8 |
| Hemangiosarcoma | 1 | 3 | - | 4 |
| Histiocytic sarcoma | 0 | 6 | 1 | 7 |
| Total | 21 | 25 | 1 | 47 |
| Tumor type and grade | Diploid (n) | Peridiploid (n) | Aneuploid (n) |
|---|---|---|---|
| Malignant tumor of bone | |||
| -grade I | 0 | 0 | 0 |
| -grade II | 1 | 1 | 2 |
| -grade III | 8 | 2 | 12 |
| Soft tissue sarcoma | |||
| -grade I | 5 | 0 | 2 |
| -grade II | 5 | 0 | 2 |
| -grade III | 8 | 0 | 11 |
| Diploid | Aneuploid | Peridiploid | |
|---|---|---|---|
| P53-wt | 12 | 14 | 2 |
| P53- alteration | 7 | 9 | 1 |
| Tumor group | Ploidy status | |
|---|---|---|
| Diploid | Aneuploid | |
| Osteosarcomas | ||
| -Metastases | 8 | 12 |
| -No metastases | 0 | 0 |
| Soft tissue sarcomas | ||
| -Metastases | 5 | 17 |
| -No metastases | 8 | 3 |
2.2. Heterogeneity of Ploidy Status or DI
| Case | Primary | Met. 1 | Met. 2 | Met. 3 | Met. 4 |
|---|---|---|---|---|---|
| Osteosarcoma 1 * | 2.10 | 1.10/2.14 | 1.12 | ||
| Osteosarcoma 2 * | 0.76 | 0.77/1.56 | 0.79/1.43/1.59 | 0.79/1.55 | 0.78/1.55 |
| Osteosarcoma 3 * | 1.0 | 1.88 | |||
| Osteosarcoma 4 | 1.0 | 1.0 | 1.0 | ||
| Synovial cell sarcoma * | 0.89/1.80 | 1.77 | |||
| Hemangiosarcoma * | 1.89 | 1.0 | 1.0 | ||
| Sarcoma-NOS * | 1.0 | 1.77 | |||
| Fibrosarcoma | 1.0 | 1.0 | 1.0 | ||
| Malignant Peripheral Nerve Sheet Tumor | 1.0 | 1.0 | |||
| Synovial Cell Sarcoma | 0.72 | 0.72 | |||
| Histiocytic Sarcoma | PD | 0.93 | |||
| Liposarcoma | 2.03 | 1.97 |
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Preparation of Samples
4.3. Histological Examination
4.4. Flow Cytometry Analysis of Nuclear DNA Content
4.5. DNA-Ploidy Assessment
4.6. Mutational Analysis of the p53 Gene
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E. Mortality in North American Dogs from 1984 to 2004: An Investigation into Age-, Size-, and Breed-Related Causes of Death. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef]
- Paoloni, M.; Khanna, C. Translation of New Cancer Treatments from Pet Dogs to Humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef]
- Paoloni, M.; Davis, S.; Lana, S.; Withrow, S.; Sangiorgi, L.; Picci, P.; Hewitt, S.; Triche, T.; Meltzer, P.; Khanna, C. Canine Tumor Cross-Species Genomics Uncovers Targets Linked to Osteosarcoma Progression. BMC Genomics 2009, 10, 625. [Google Scholar] [CrossRef]
- Kirpensteijn, J.; Kik, M.; Rutteman, G.R.; Teske, E. Prognostic Significance of a New Histologic Grading System for Canine Osteosarcoma. Vet. Pathol. 2002, 39, 240–246. [Google Scholar] [CrossRef]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine Neoplasia in the UK: Estimates of Incidence Rates from a Population of Insured Dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef]
- Rosenberger, J.A.; Pablo, N.V.; Crawford, P.C. Prevalence of and Intrinsic Risk Factors for AppendicularOsteosarcoma in Dogs: 179 Cases (1996–2005). J. Am. Vet. Med. Assoc. 2007, 231, 1076–1080. [Google Scholar]
- Withrow, S.J.; Vail, D. Small Animal Clinical Oncology, 4th ed; Saunders: St. Louis, MO, USA, 2007. [Google Scholar]
- Kuntz, C.A.; Dernell, W.S.; Powers, B.E.; Devitt, C.; Straw, R.C.; Withrow, S.J. Prognostic Factors for Surgical Treatment of Soft-Tissue Sarcomas in Dogs: 75 Cases (1986–1996). J. Am. Vet. Med. Assoc. 1997, 211, 1147–1151. [Google Scholar]
- Dennis, M.M.; McSporran, K.D.; Bacon, N.J.; Schulman, F.Y.; Foster, R.A.; Powers, B.E. Prognostic Factors for Cutaneous and Subcutaneous Soft Tissue Sarcomas in Dogs. Vet. Pathol. 2011, 48, 73–84. [Google Scholar] [CrossRef]
- Xiang, J.H.; Spanier, S.S.; Benson, N.A.; Braylan, R.C. Flow Cytometric Analysis of DNA in Bone and Soft-Tissue Tumors using Nuclear Suspensions. Cancer 1987, 59, 1951–1958. [Google Scholar]
- Rosenberg, A.E. Pseudosarcomas of Soft Tissue. Arch. Pathol. Lab. Med. 2008, 132, 579–586. [Google Scholar]
- Thway, K. Pathology of Soft Tissue Sarcomas. Clin. Oncol. 2009, 21, 695–705. [Google Scholar]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; de Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-Tissue Sarcomas of Adults; Study of Pathological Prognostic Variables and Definition of a Histopathological Grading System. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef]
- Jones, N.B.; Iwenofu, H.; Scharschmidt, T.; Kraybill, W. Prognostic Factors and Staging for Soft Tissue Sarcomas: An Update. Surg. Oncol. Clin. N. Am. 2012, 21, 187–200. [Google Scholar]
- Barlogie, B.; Raber, M.N.; Schumann, J.; Johnson, T.S.; Drewinko, B.; Swartzendruber, D.E.; Gohde, W.; Andreeff, M.; Freireich, E.J. Flow Cytometry in Clinical Cancer Research. Cancer Res. 1983, 43, 3982–3997. [Google Scholar]
- Kreicbergs, A.; Tribukait, B.; Willems, J.; Bauer, H.C. DNA Flow Analysis of Soft Tissue Tumors. Cancer 1987, 59, 128–133. [Google Scholar]
- Kroese, M.C.; Rutgers, D.H.; Wils, I.S.; van Unnik, J.A.; Roholl, P.J. The Relevance of the DNA Index and Proliferation Rate in the Grading of Benign and Malignant Soft Tissue Tumors. Cancer 1990, 65, 1782–1788. [Google Scholar] [CrossRef]
- Van den Berg, E.; van Oven, M.W.; de Jong, B.; Dam, A.; Wiersema, J.; Dijkhuizen, T.; Hoekstra, H.J.; Molenaar, W.M. Comparison of Cytogenetic Abnormalities and Deoxyribonucleic Acid Ploidy of Benign, Borderline Malignant, and Different Grades of Malignant Soft Tissue Tumors. Lab. Invest. 1994, 70, 307–313. [Google Scholar]
- Mohamed, A.N.; Zalupski, M.M.; Ryan, J.R.; Koppitch, F.; Balcerzak, S.; Kempf, R.; Wolman, S.R. Cytogenetic Aberrations and DNA Ploidy in Soft Tissue Sarcoma. A Southwest Oncology Group Study. Cancer Genet. Cytogenet. 1997, 99, 45–53. [Google Scholar] [CrossRef]
- Yanong, W.; Daren, S.; Zhenzhou, S.; Renyuan, Z.; Shouye, L. Investigation of Relationships between KI-67 Score, DNA Index, and Histologic Grade in Soft Tissue Sarcomas. Chin. J. Cancer Res. 1988, 8, 55–59. [Google Scholar]
- Plaat, B.E.; Muntinghe, F.L.; Molenaar, W.M.; Hoekstra, H.J.; Bosveld, H.E.; Dam, A.; Dijkhuizen, T.; van den Berg, E. Clinical Outcome of Patients with Previously Untreated Soft Tissue Sarcomas in Relation to Tumor Grade, DNA Ploidy and Karyotype. Int. J. Cancer 1997, 74, 396–402. [Google Scholar]
- Friedlander, M.L.; Hedley, D.W.; Taylor, I.W. Clinical and Biological Significance of Aneuploidy in Human Tumours. J. Clin. Pathol. 1984, 37, 961–974. [Google Scholar] [CrossRef]
- Merkel, D.E.; McGuire, W.L. Ploidy, Proliferative Activity and Prognosis. DNA Flow Cytometry of Solid Tumors. Cancer 1990, 65, 1194–1205. [Google Scholar] [CrossRef]
- Kildal, W.; Abeler, V.M.; Kristensen, G.B.; Jenstad, M.; Thoresen, S.O.; Danielsen, H.E. The Prognostic Value of DNA Ploidy in a Total Population of Uterine Sarcomas. Ann. Oncol. 2009, 20, 1037–1041. [Google Scholar]
- Lopes, J.M.; Hannisdal, E.; Bjerkehagen, B.; Bruland, O.S.; Danielsen, H.E.; Pettersen, E.O.; Sobrinho-Simoes, M.; Nesland, J.M. Synovial Sarcoma. Evaluation of Prognosis with Emphasis on the Study of DNA Ploidy and Proliferation (PCNA and Ki-67) Markers. Anal. Cell. Pathol. 1998, 16, 45–62. [Google Scholar]
- Van De Luijtgaarden, A.C.; van der Graaf, W.T.; Otte-Holler, I.; Schreuder, H.W.; Versleijen-Jonkers, Y.M.; Slootweg, P.J. Targeted Therapy for Ewing’s Sarcoma: Significance of Heterogeneity. Anticancer Res. 2010, 30, 3715–3719. [Google Scholar]
- Chew, I.; Oliva, E. Endometrial Stromal Sarcomas: A Review of Potential Prognostic Factors. Adv. Anat. Pathol. 2010, 17, 113–121. [Google Scholar] [CrossRef]
- Dreinhofer, K.E.; Baldetorp, B.; Akerman, M.; Ferno, M.; Rydholm, A.; Gustafson, P. DNA Ploidy in Soft Tissue Sarcoma: Comparison of Flow and Image Cytometry with Clinical Follow-Up in 93 Patients. Cytometry 2002, 50, 19–24. [Google Scholar]
- Niggli, F.K.; Powell, J.E.; Parkes, S.E.; Ward, K.; Raafat, F.; Mann, J.R.; Stevens, M.C. DNA Ploidy and Proliferative Activity (S-Phase) in Childhood Soft-Tissue Sarcomas: Their Value as Prognostic Indicators. Br. J. Cancer 1994, 69, 1106–1110. [Google Scholar] [CrossRef]
- Samur, M.; Pamir, A.; Akbulut, H.; Erekul, S.; Saglik, Y.; Yildiz, Y.; Dincol, D.; Icli, F. The Clinical Value of Flow Cytometric DNA Content Analysis in Patients with Soft Tissue Sarcomas. Sarcoma 1999, 3, 171–175. [Google Scholar]
- Bauer, H.C.; Kreicbergs, A.; Tribukait, B. DNA Content Prognostic in Soft Tissue Sarcoma. 102 Patients Followed for 1–10 Years. ActaOrthop. Scand. 1991, 62, 187–194. [Google Scholar]
- Mankin, H.J.; Gebhardt, M.C.; Springfield, D.S.; Litwak, G.J.; Kusazaki, K.; Rosenberg, A.E. Flow Cytometric Studies of Human Osteosarcoma. Clin. Orthop. Relat. Res. 1991, 270, 169–180. [Google Scholar]
- Giaretti, W. A Model on the Origin and Evolution of DNA Aneuploidy. Int. J. Oncol. 1993, 2, 165–171. [Google Scholar]
- Shackney, S.E.; Smith, C.A.; Miller, B.W.; Burholt, D.R.; Murtha, K.; Giles, H.R.; Ketterer, D.M.; Pollice, A.A. Model for the Genetic Evolution of Human Solid Tumors. Cancer Res. 1989, 49, 3344–3354. [Google Scholar]
- Dutrillaux, B.; Gerbault-Seureau, M.; Remvikos, Y.; Zafrani, B.; Prieur, M. Breast Cancer Genetic Evolution: I. Data from Cytogenetics and DNA Content. Breast Cancer Res. Treat. 1991, 19, 245–255. [Google Scholar] [CrossRef]
- Gordon, D.J.; Resio, B.; Pellman, D. Causes and Consequences of Aneuploidy in Cancer. Nat. Rev. Genet. 2012, 13, 189–203. [Google Scholar]
- Boveri, T. Zur Frage Der Entstehung Maligner Tumoren; Fischer: Jena, Germany, 1914. [Google Scholar]
- Duesberg, P.; Rasnick, D.; Li, R.; Winters, L.; Rausch, C.; Hehlmann, R. How Aneuploidy may Cause Cancer and Genetic Instability. Anticancer Res. 1999, 19, 4887–4906. [Google Scholar]
- Kolodner, R.D.; Cleveland, D.W.; Putnam, C.D. Cancer. Aneuploidy Drives a Mutator Phenotype in Cancer. Science 2011, 333, 942–943. [Google Scholar] [CrossRef]
- Barrett, M.T.; Pritchard, D.; Palanca-Wessels, C.; Anderson, J.; Reid, B.J.; Rabinovitch, P.S. Molecular Phenotype of Spontaneously Arising 4N (G2-Tetraploid) Intermediates of Neoplastic Progression in Barrett’s Esophagus. Cancer Res. 2003, 63, 4211–4217. [Google Scholar]
- Nowell, P.C.; Croce, C.M. Chromosomes, Genes, and Cancer. Am. J. Pathol. 1986, 125, 7–15. [Google Scholar]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic Instabilities in Human Cancers. Nature 1998, 396, 643–649. [Google Scholar]
- Holland, A.J.; Cleveland, D.W. Losing Balance: The Origin and Impact of Aneuploidy in Cancer. EMBO Rep. 2012, 13, 501–514. [Google Scholar]
- Cornelisse, C.J.; Rutteman, G.R.; Kuipers-Dijkshoorn, N.J.; Hellmen, E. The Difference in DNA Ploidy Pattern between some Canine and Human Neoplasms Appears to be Genuine and a Reflection of Dissimilarities in DNA Aneuploidy Evolution. Anticancer Res. 1994, 14, 1599–1601. [Google Scholar]
- Merlo, L.M.; Wang, L.S.; Pepper, J.W.; Rabinovitch, P.S.; Maley, C.C. Polyploidy, Aneuploidy and the Evolution of Cancer. Adv. Exp. Med. Biol. 2010, 676, 1–13. [Google Scholar] [CrossRef]
- Rajagopalan, H.; Lengauer, C. Aneuploidy and Cancer. Nature 2004, 432, 338–341. [Google Scholar]
- Yen, T.J.; Kao, G.D. Mitotic Checkpoint, Aneuploidy and Cancer. Adv. Exp. Med. Biol. 2005, 570, 477–499. [Google Scholar]
- Pihan, G.; Doxsey, S.J. Mutations and Aneuploidy: Co-Conspirators in Cancer? Cancer Cell 2003, 4, 89–94. [Google Scholar] [CrossRef]
- Holland, A.J.; Cleveland, D.W. Boveri Revisited: Chromosomal Instability, Aneuploidy and Tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 478–487. [Google Scholar]
- Frankfurt, O.S.; Slocum, H.K.; Rustum, Y.M.; Arbuck, S.G.; Pavelic, Z.P.; Petrelli, N.; Huben, R.P.; Pontes, E.J.; Greco, W.R. Flow Cytometric Analysis of DNA Aneuploidy in Primary and Metastatic Human Solid Tumors. Cytometry 1984, 5, 71–80. [Google Scholar] [CrossRef]
- Olsson, L.; Paulsson, K.; Bovee, J.V.; Nord, K.H. Clonal Evolution through Loss of Chromosomes and Subsequent Polyploidization in Chondrosarcoma. PLoSOne 2011, 6, e24977. [Google Scholar]
- Thompson, S.L.; Compton, D.A. Proliferation of Aneuploid Human Cells is Limited by a p53-Dependent Mechanism. J. Cell Biol. 2010, 188, 369–381. [Google Scholar] [CrossRef]
- Rutteman, G.R.; Cornelisse, C.J.; Dijkshoorn, N.J.; Poortman, J.; Misdorp, W. Flow Cytometric Analysis of DNA Ploidy in Canine Mammary Tumors. Cancer Res. 1988, 48, 3411–3417. [Google Scholar]
- Verschueren, C.P.; Rutteman, G.R.; Kuipers-Dijkshoorn, N.J.; Sjollema, B.E.; Vos, J.H.; van Dijk, J.E.; Cornelisse, C.J. Flow-Cytometric DNA Ploidy Analysis in Primary and Metastatic Canine Thyroid Carcinomas. Anticancer Res. 1991, 11, 1755–1761. [Google Scholar]
- Hellmen, E.; Lindgren, A.; Linell, F.; Matsson, P.; Nilsson, A. Comparison of Histology and Clinical Variables to DNA Ploidy in Canine Mammary Tumors. Vet. Pathol. 1988, 25, 219–226. [Google Scholar]
- Perez Alenza, M.D.; Rutteman, G.R.; Kuipers-Dijkshoorn, N.J.; Pena, L.; Montoya, A.; Misdorp, W.; Cornelisse, C.J. DNA Flow Cytometry of Canine Mammary Tumours: The Relationship of DNA Ploidy and S-Phase Fraction to Clinical and Histological Features. Res. Vet. Sci. 1995, 58, 238–243. [Google Scholar] [CrossRef]
- Teske, E.; Rutteman, G.R.; Kuipers-Dijkshoorn, N.J.; van Dierendonck, J.H.; van Heerde, P.; Cornelisse, C.J. DNA Ploidy and Cell Kinetic Characteristics in Canine Non-Hodgkin’s Lymphoma. Exp. Hematol. 1993, 21, 579–584. [Google Scholar]
- Collin, F.; Chassevent, A.; Bonichon, F.; Bertrand, G.; Terrier, P.; Coindra, J.M. Flow Cytometric DNA Content Analysis of 185 Soft Tissue Neoplasms Indicates that S-Phase Fraction is a Prognostic Factor for Sarcomas. French Federation of Cancer Centers (FNCLCC) Sarcoma Group. Cancer 1997, 79, 2371–2379. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Hurr, K.; Tu, Z.N.; Teague, K.; Raymond, K.A.; Ayala, A.G.; Murray, J. DNA and RNA Content Analysis by Flow Cytometry in the Pathobiologic Assessment of Bone Tumors. Cytometry 1995, 19, 256–262. [Google Scholar]
- Helio, H.; Karaharju, E.; Nordling, S. Flow Cytometric Determination of DNA Content in Malignant and Benign Bone Tumours. Cytometry 1985, 6, 165–171. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Radig, K.; Oda, Y.; Mellin, W.; Rys, J.; Niezabitowski, A.; Roessner, A. P53 Gene Mutations in Soft-Tissue Sarcomas—Correlations with p53 Immunohistochemistry and DNA Ploidy. J. Cancer Res. Clin. Oncol. 1997, 123, 211–218. [Google Scholar]
- Balogh, Z.; Szemlaky, Z.; Szendroi, M.; Antal, I.; Papai, Z.; Fonyad, L.; Papp, G.; Changchien, Y.C.; Sapi, Z. Correlation between DNA Ploidy, Metaphase High-Resolution Comparative Genomic Hybridization Results and Clinical Outcome of Synovial Sarcoma. Diagn. Pathol. 2011, 6, 107. [Google Scholar] [CrossRef]
- Kreicbergs, A.; Boquist, L.; Borssen, B.; Larsson, S.E. Prognostic Factors in Chondrosarcoma: A Comparative Study of Cellular DNA Content and Clinicopathologic Features. Cancer 1982, 50, 577–583. [Google Scholar]
- Mertens, F.; Stromberg, U.; Mandahl, N.; Dal Cin, P.; de Wever, I.; Fletcher, C.D.; Mitelman, F.; Rosai, J.; Rydholm, A.; Sciot, R.; et al. Prognostically Important Chromosomal Aberrations in Soft Tissue Sarcomas: A Report of the Chromosomes and Morphology (CHAMP) Study Group. Cancer Res. 2002, 62, 3980–3984. [Google Scholar]
- Hellmen, E.; Svensson, S. Progression of Canine Mammary Tumours as Reflected by DNA Ploidy in Primary Tumours and their Metastases. J. Comp. Pathol. 1995, 113, 327–342. [Google Scholar]
- Johnson, T.S.; Raju, M.R.; Giltinan, R.K.; Gillette, E.L. Ploidy and DNA Distribution Analysis of Spontaneous Dog Tumors by Flow Cytometry. Cancer Res. 1981, 41, 3005–3009. [Google Scholar]
- Fox, M.H.; Armstrong, L.W.; Withrow, S.J.; Powers, B.E.; LaRue, S.M.; Straw, R.C.; Gillette, E.L. Comparison of DNA Aneuploidy of Primary and Metastatic Spontaneous Canine Osteosarcomas. Cancer Res. 1990, 50, 6176–6178. [Google Scholar]
- Mertens, F.; Dal Cin, P.; de Wever, I.; Fletcher, C.D.; Mandahl, N.; Mitelman, F.; Rosai, J.; Rydholm, A.; Sciot, R.; Tallini, G.; et al. Cytogenetic Characterization of Peripheral Nerve Sheath Tumours: A Report of the CHAMP Study Group. J. Pathol. 2000, 190, 31–38. [Google Scholar] [CrossRef]
- Feitz, W.F.; Karthaus, H.F.; Beck, H.L.; Romijn, C.; van der Meyden, A.P.; Debruyne, F.M.; Vooijs, G.P.; Ramaekers, F.C. Tissue-Specific Markers in Flow Cytometry of Urological Cancers. II. Cytokeratin and Vimentin in Renal-Cell Tumors. Int. J. Cancer 1986, 37, 201–207. [Google Scholar] [CrossRef]
- Kilpatrick, S.E.; Teot, L.A.; Geisinger, K.R.; Martin, P.L.; Shumate, D.K.; Zbieranski, N.; Russell, G.B.; Fletcher, C.D. Relationship of DNA Ploidy to Histology and Prognosis in Rhabdomyosarcoma. Comparison of Flow Cytometry and Image Analysis. Cancer 1994, 74, 3227–3233. [Google Scholar] [CrossRef]
- Thompson, S.L.; Bakhoum, S.F.; Compton, D.A. Mechanisms of Chromosomal Instability. Curr. Biol. 2010, 20, R285–R295. [Google Scholar]
- Milne, B.S.; Hoather, T.; O'Brien, P.C.; Yang, F.; Ferguson-Smith, M.A.; Dobson, J.; Sargan, D. Karyotype of Canine Soft Tissue Sarcomas: A Multi-Colour, Multi-Species Approach to Canine Chromosome Painting. Chromosome Res. 2004, 12, 825–835. [Google Scholar] [CrossRef]
- Aguirre-Hernandez, J.; Milne, B.S.; Queen, C.; O'Brien, P.C.; Hoather, T.; Haugland, S.; Ferguson-Smith, M.A.; Dobson, J.M.; Sargan, D.R. Disruption of Chromosome 11 in Canine Fibrosarcomas Highlights an Unusual Variability of CDKN2B in Dogs. BMC Vet. Res. 2009, 5, 27. [Google Scholar]
- Tap, O.T.; Rutteman, G.R.; Zijlstra, C.; de Haan, N.A.; Bosma, A.A. Analysis of Chromosome Aberrations in a Mammary Carcinoma Cell Line from a Dog by using Canine Painting Probes. Cytogenet. Cell Genet. 1998, 82, 75–79. [Google Scholar] [CrossRef]
- Mayr, B.; Kramberger-Kaplan, E.; Loupal, G.; Schleger, W. Analysis of Complex Cytogenetic Alterations in Three Canine Mammary Sarcomas. Res. Vet. Sci. 1992, 53, 205–211. [Google Scholar]
- Maeda, J.; Yurkon, C.R.; Fujisawa, H.; Kaneko, M.; Genet, S.C.; Roybal, E.J.; Rota, G.W.; Saffer, E.R.; Rose, B.J.; Hanneman, W.H.; et al. Genomic Instability and Telomere Fusion of Canine Osteosarcoma Cells. PLoSOne 2012, 7, e43355. [Google Scholar]
- Mellink, C.H.; Bosma, A.A.; Rutteman, G.R. Cytogenetic Analysis of Cell Lines Derived from Metastases of a Mammary Carcinoma in a Dog. Anticancer Res. 1989, 9, 1241–1244. [Google Scholar]
- Reimann, N.; Nolte, I.; Bartnitzke, S.; Bullerdiek, J. Re: Sit, DNA, Sit: Cancer Genetics Going to the Dog. J. Natl. Cancer Inst. 1999, 91, 1688–1689. [Google Scholar] [CrossRef]
- Breen, M. Canine Cytogenetics—From Band to Basepair. Cytogenet. Genome Res. 2008, 120, 50–60. [Google Scholar]
- Breen, M.; Modiano, J.F. Evolutionarily Conserved Cytogenetic Changes in Hematological Malignancies of Dogs and Humans—Man and His Best Friend Share More than Companionship. Chromosome Res. 2008, 16, 145–154. [Google Scholar] [CrossRef]
- Hedan, B.; Thomas, R.; Motsinger-Reif, A.; Abadie, J.; Andre, C.; Cullen, J.; Breen, M. Molecular Cytogenetic Characterization of Canine Histiocytic Sarcoma: A Spontaneous Model for Human Histiocytic Cancer Identifies Deletion of Tumor Suppressor Genes and Highlights Influence of Genetic Background on Tumor Behavior. BMC Cancer 2011, 11, 201. [Google Scholar]
- Breen, M.; Thomas, A. Canine Cytogenetics and Chromosome Maps. In Genetics of the Dog, 2nd; Ostrander, E.A., Ruvinsky, A., Eds.; CAB International: London, UK, 2012; pp. 245–254. [Google Scholar]
- Daniel, J.; Coulter, J.; Woo, J.H.; Wilsbach, K.; Gabrielson, E. High Levels of the Mps1 Checkpoint Protein are Protective of Aneuploidy in Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5384–5389. [Google Scholar]
- Solomon, D.A.; Kim, T.; Diaz-Martinez, L.A.; Fair, J.; Elkahloun, A.G.; Harris, B.T.; Toretsky, J.A.; Rosenberg, S.A.; Shukla, N.; Ladanyi, M.; et al. Mutational Inactivation of STAG2 Causes Aneuploidy in Human Cancer. Science 2011, 333, 1039–1043. [Google Scholar]
- Kramer, A.; Maier, B.; Bartek, J. Centrosome Clustering and Chromosomal (in)Stability: A Matter of Life and Death. Mol. Oncol. 2011, 5, 324–335. [Google Scholar] [CrossRef]
- Bunz, F.; Fauth, C.; Speicher, M.R.; Dutriaux, A.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B.; Lengauer, C. Targeted Inactivation of p53 in Human Cells does Not Result in Aneuploidy. Cancer Res. 2002, 62, 1129–1133. [Google Scholar]
- Li, M.; Fang, X.; Baker, D.J.; Guo, L.; Gao, X.; Wei, Z.; Han, S.; van Deursen, J.M.; Zhang, P. The ATM-p53 Pathway Suppresses Aneuploidy-Induced Tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 14188–14193. [Google Scholar]
- Aylon, Y.; Oren, M. P53: Guardian of Ploidy. Mol. Oncol. 2011, 5, 315–323. [Google Scholar]
- Nasir, L.; Rutteman, G.R.; Reid, S.W.; Schulze, C.; Argyle, D.J. Analysis of p53 Mutational Events and MDM2 Amplification in Canine Soft-Tissue Sarcomas. Cancer Lett. 2001, 174, 83–89. [Google Scholar] [CrossRef]
- Kirpensteijn, J.; Kik, M.; Teske, E.; Rutteman, G.R. TP53 Gene Mutations in Canine Osteosarcoma. Vet. Surg. 2008, 37, 454–460. [Google Scholar] [CrossRef]
- Fabarius, A.; Li, R.; Yerganian, G.; Hehlmann, R.; Duesberg, P. Specific Clones of Spontaneously Evolving Karyotypes Generate Individuality of Cancers. Cancer Genet. Cytogenet 2008, 180, 89–99. [Google Scholar] [CrossRef]
- Affolter, V.K.; Moore, P.F. Localized and Disseminated Histiocytic Sarcoma of Dendritic Cell Origin in Dogs. Vet. Pathol. 2002, 39, 74–83. [Google Scholar] [CrossRef]
- Maas, C.P.; ter Haar, G.; van der Gaag, I.; Kirpensteijn, J. Reclassification of Small Intestinal and Cecal Smooth Muscle Tumors in 72 Dogs: Clinical, Histologic, and Immunohistochemical Evaluation. Vet. Surg. 2007, 36, 302–313. [Google Scholar] [CrossRef]
- Vindelov, L.L.; Christensen, I.J.; Nissen, N.I. A Detergent-Trypsin Method for the Preparation of Nuclei for Flow Cytometric DNA Analysis. Cytometry 1983, 3, 323–327. [Google Scholar]
- Canis Lupus familiaris tumor protein. p. 53. Available online: http://www.ncbi.nlm.nih.gov/nuccore/NM_001003210.1/ (accessed on 16 November 2012).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boerkamp, K.M.; Rutteman, G.R.; Kik, M.J.L.; Kirpensteijn, J.; Schulze, C.; Grinwis, G.C.M. Nuclear DNA-Content in Mesenchymal Lesions in Dogs: Its Value as Marker of Malignancy and Extent of Genomic Instability. Cancers 2012, 4, 1300-1317. https://doi.org/10.3390/cancers4041300
Boerkamp KM, Rutteman GR, Kik MJL, Kirpensteijn J, Schulze C, Grinwis GCM. Nuclear DNA-Content in Mesenchymal Lesions in Dogs: Its Value as Marker of Malignancy and Extent of Genomic Instability. Cancers. 2012; 4(4):1300-1317. https://doi.org/10.3390/cancers4041300
Chicago/Turabian StyleBoerkamp, Kim M., Gerard R. Rutteman, Marja J. L. Kik, Jolle Kirpensteijn, Christoph Schulze, and Guy C. M. Grinwis. 2012. "Nuclear DNA-Content in Mesenchymal Lesions in Dogs: Its Value as Marker of Malignancy and Extent of Genomic Instability" Cancers 4, no. 4: 1300-1317. https://doi.org/10.3390/cancers4041300





