Azithromycin Synergistically Enhances Anti-Proliferative Activity of Vincristine in Cervical and Gastric Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of AZM and VCR on Viability of Various Cells
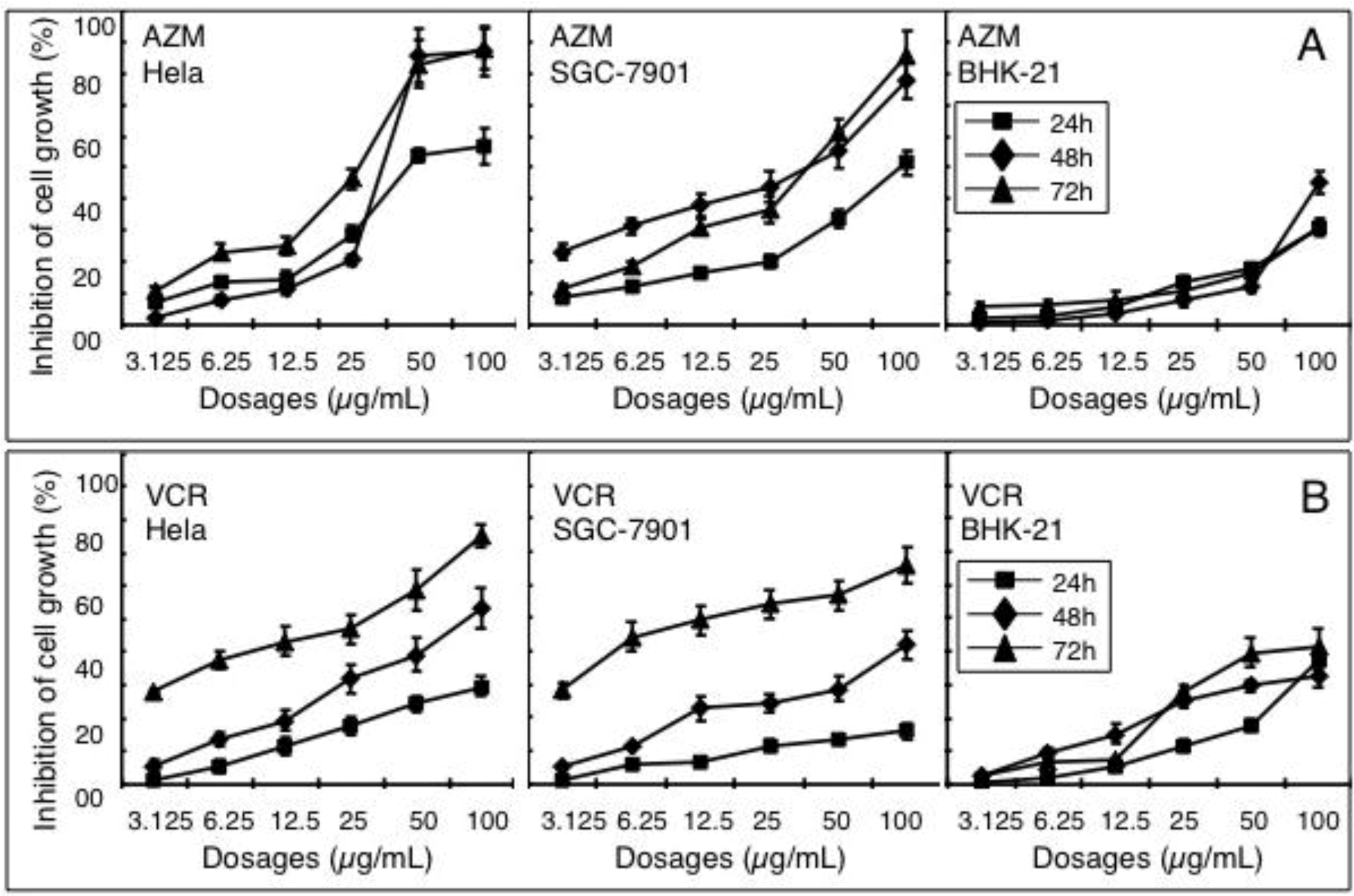
2.2. Synergistic Effect of a Combination of AZM and VCR on the Proliferation of Cells
| Cell lines | VCR/AZM (µg/mL) | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|---|
| Inhibition (%) | CDI | Inhibition (%) | CDI | Inhibition (%) | CDI | ||
| Hela | 12.5/100 | 55.690 ± 0.707 ** | 0.87 | 70.197 ± 0.404 * | 0.66 | 84.444 ± 0.652 ** | 1.22 |
| 12.5/50 | 44.062 ± 0.525 ** | 0.94 | 66.565 ± 0.484 * | 0.65 | 75.021 ± 0.572 ** | 1.55 | |
| 12.5/25 | 41.527 ± 0.777 ** | 0.98 | 61.630 ± 0.623 * | 0.76 | 61.425 ± 0.610 ** | 1.26 | |
| 12.5/12.5 | 35.326 ± 0.841 * | 0.98 | 52.980 ± 0.730 * | 0.88 | 56.577 ± 0.506 * | 0.97 | |
| 12.5/6.25 | 32.461 ± 0.510 ** | 0.88 | 47.519 ± 0.423 ** | 0.91 | 54.743 ± 0.525 ** | 0.89 | |
| 12.5/3.125 | 30.063 ± 0.565 ** | 0.82 | 39.978 ± 0.620 * | 0.83 | 49.810 ± 0.431 | 0.94 | |
| 12.5/0 | 11.753 ± 0.819 | - | 19.322 ± 0.943 | - | 43.143 ± 0.476 | - | |
| SGC-7901 | 12.5/100 | 60.884 ± 1.854 ** | 0.86 | 71.556 ± 0.353 ** | 0.95 | 75.716 ± 1.330 ** | 1.78 |
| 12.5/50 | 56.059 ± 1.798 ** | 0.69 | 69.255 ± 0.718 ** | 0.89 | 71.356 ± 0.574 ** | 1.45 | |
| 12.5/25 | 52.855 ± 1.718 ** | 0.63 | 63.739 ± 0.528 ** | 0.84 | 69.574 ± 1.082 ** | 0.93 | |
| 12.5/12.5 | 51.639 ± 3.291 ** | 0.62 | 61.856 ± 0.736 ** | 0.69 | 66.805 ± 0.415 * | 0.96 | |
| 12.5/6.25 | 49.724 ± 2.047 ** | 0.63 | 57.830 ± 0.880 ** | 0.64 | 60.630 ± 1.010 * | 0.90 | |
| 12.5/3.125 | 44.383 ± 1.842 ** | 0.65 | 56.314 ± 0.753 ** | 0.65 | 54.965 ± 0.921 | 0.92 | |
| 12.5/0 | 6.432 ± 0.498 | - | 22.739 ± 0.697 | - | 49.418 ± 0.547 | - | |
| BHK-21 | 12.5/100 | 50.707 ± 0.652 ** | 0.75 | 74.284 ± 0.546 ** | 0.55 | 65.247 ± 0.649 ** | 0.54 |
| 12.5/50 | 19.801 ± 0.602 ** | 1.03 | 53.194 ± 0.551 ** | 0.63 | 39.077 ± 0.404 ** | 0.79 | |
| 12.5/25 | 13.271 ± 0.549 * | 1.06 | 25.523 ± 0.702 | 1.00 | 29.740 ± 0.579 ** | 0.85 | |
| 12.5/12.5 | 8.426 ± 0.757 | 1.02 | 16.823 ± 0.663 | 1.02 | 24.174 ± 0.842 ** | 0.89 | |
| 12.5/6.25 | 5.146 ± 0.538 | 1.03 | 12.225 ± 0.547 | 1.05 | 21.468 ± 0.345 * | 1.01 | |
| 12.5/3.125 | 2.708 ± 0.644 | 1.05 | 2.561 ± 0.799 | 1.16 | 8.383 ± 0.418 | 1.05 | |
| 12.5/0 | 5.360 ± 0.620 | - | 15.154 ± 0.823 | - | 7.623 ± 0.650 | - | |
2.3. Induction of Cell Apoptosis by AZM and VCR
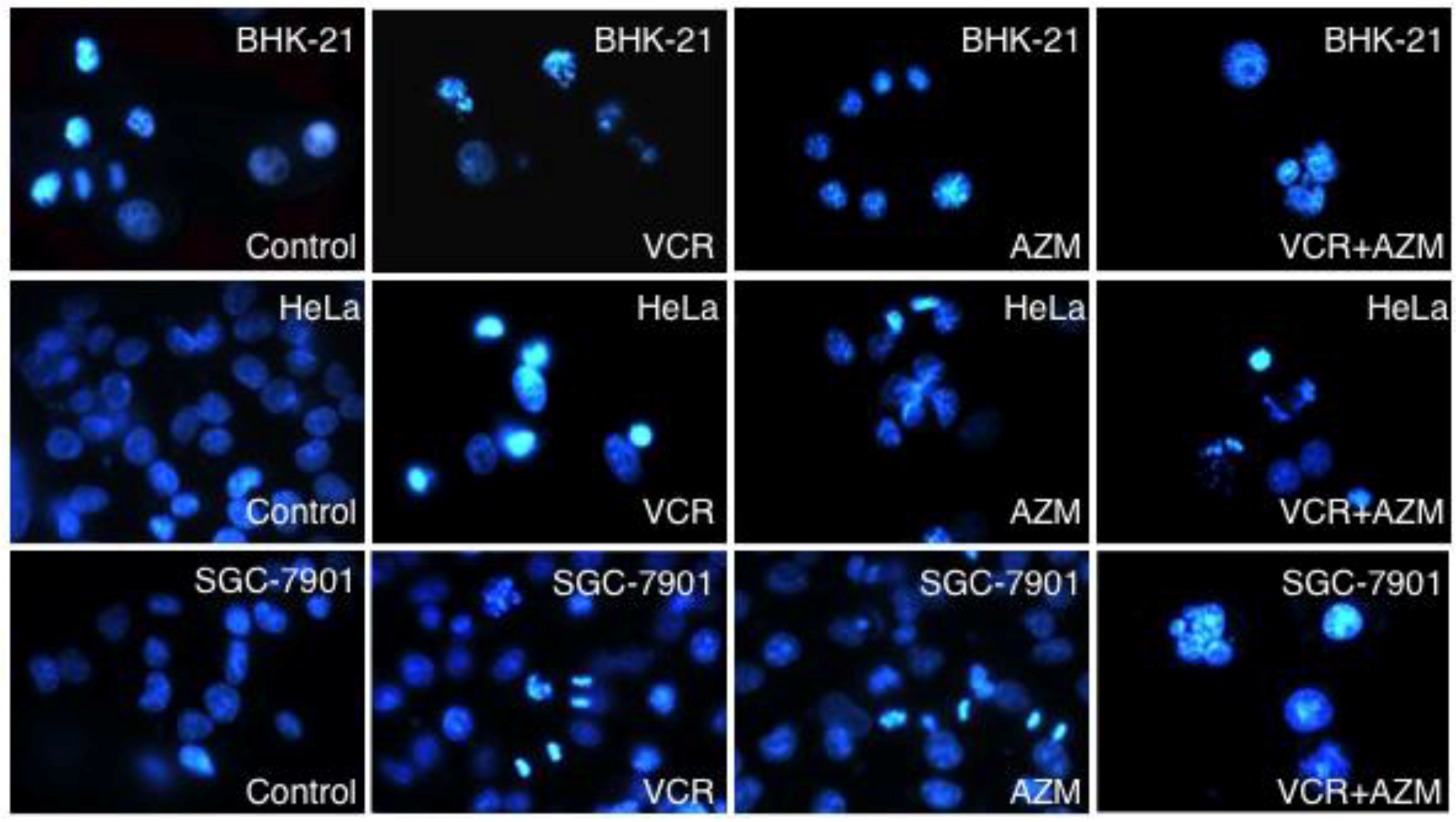
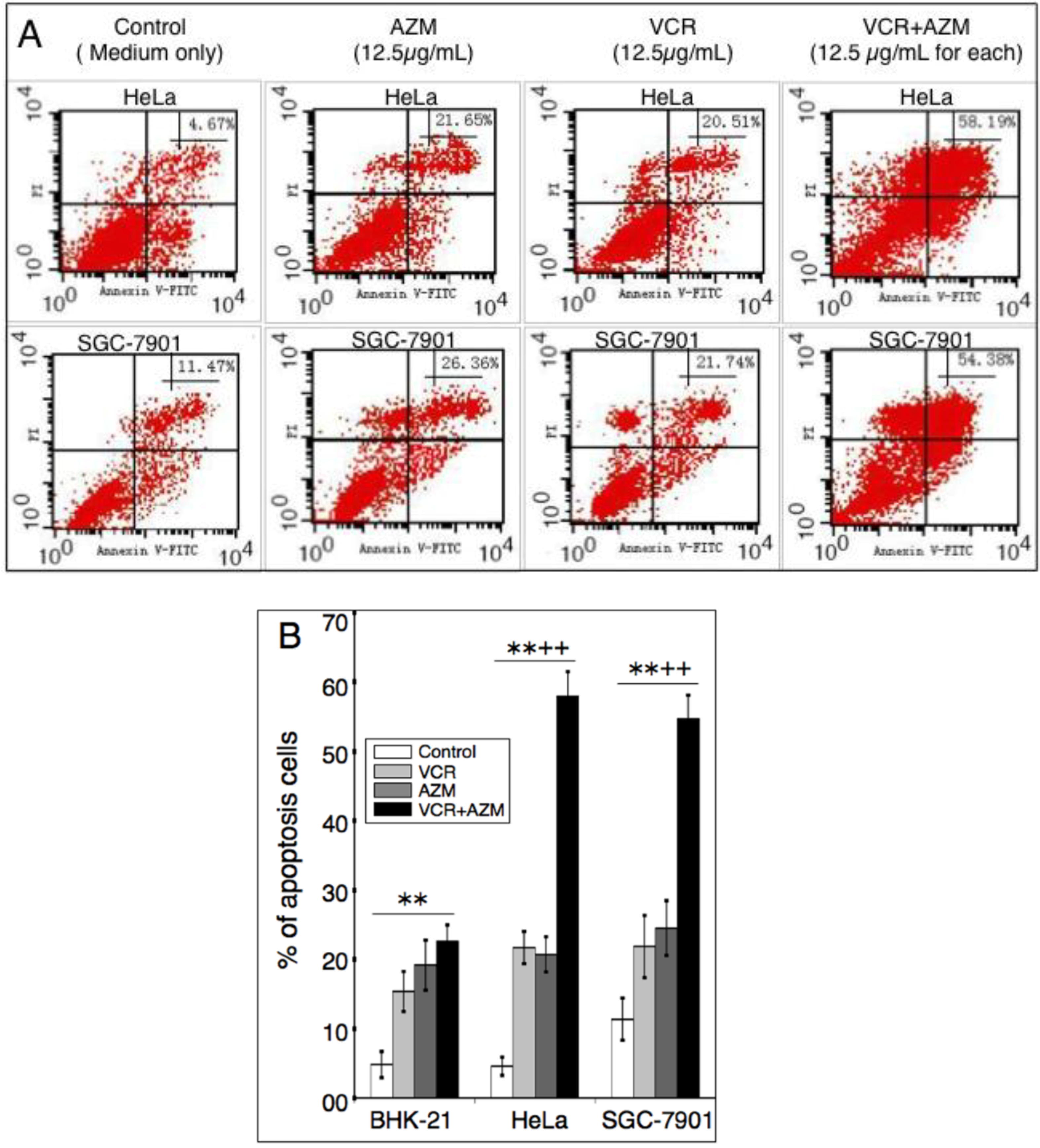
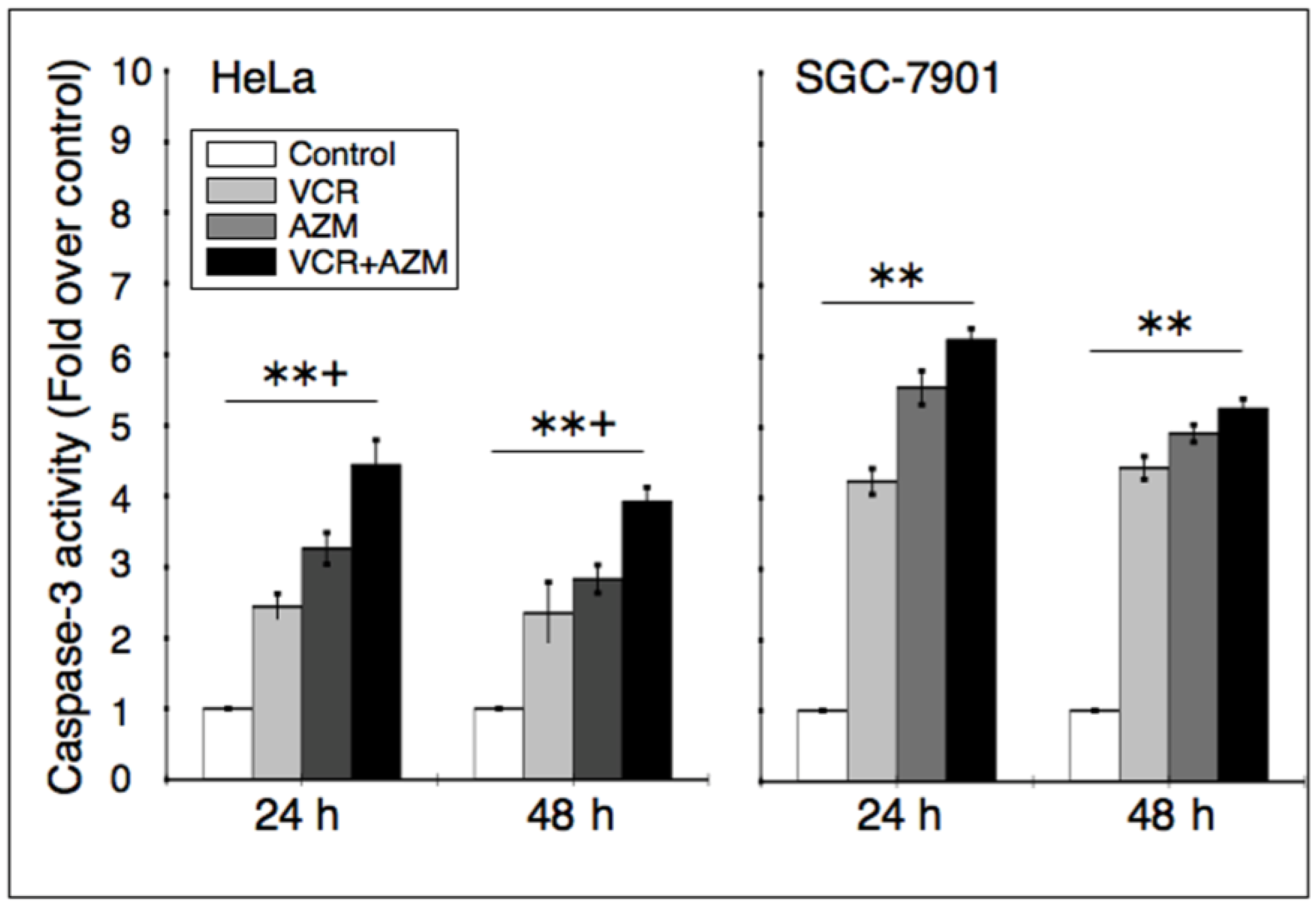
2.4. Effect on Caspase-3 Activity
2.5. Effect on the Expression of Apoptotic Proteins
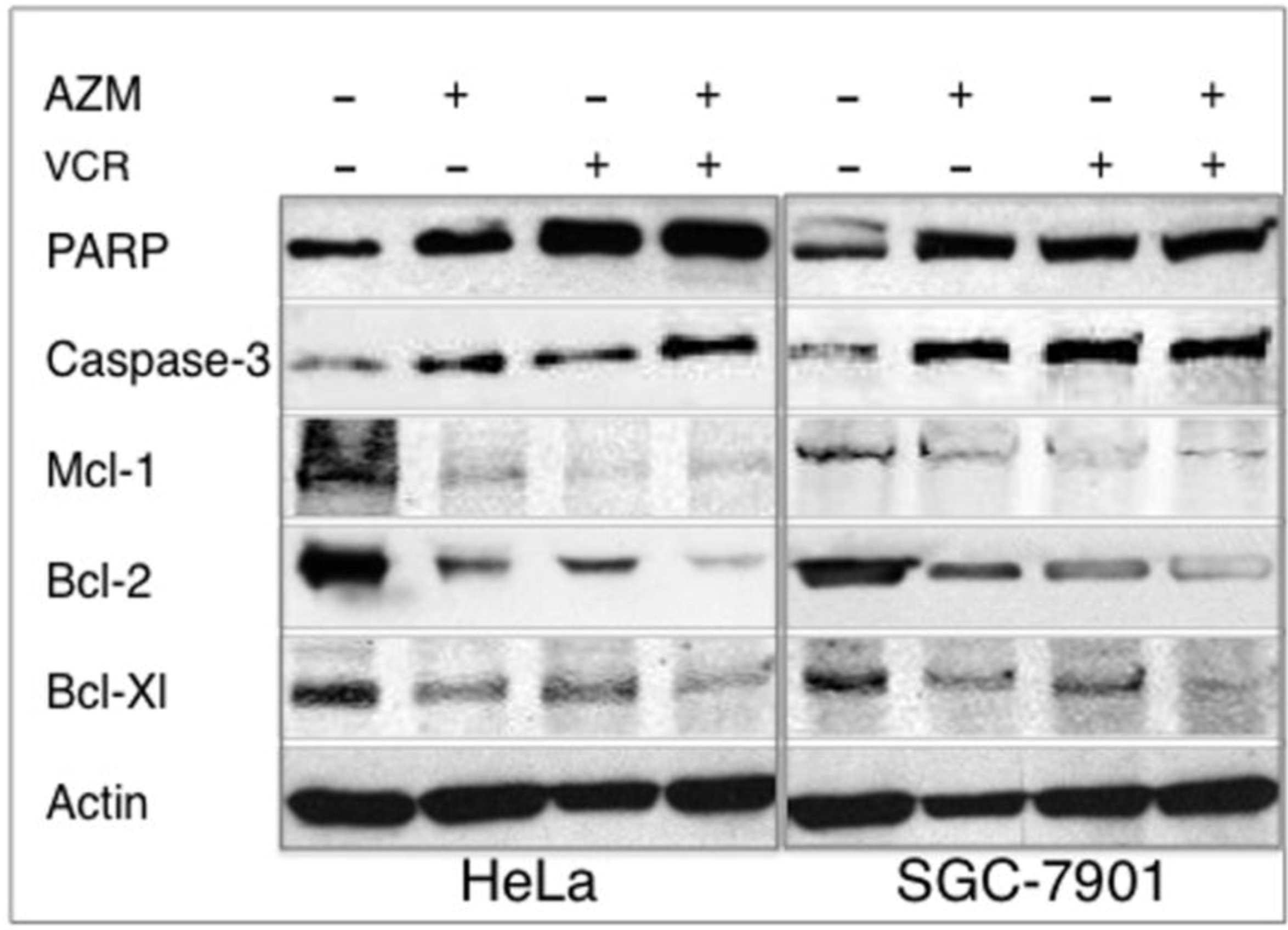
2.6. Discussion
3. Experimental Section
3.1. Reagents and Cell Lines
3.2. Cell Culture and Cell Proliferation Assay
3.3. Analysis the Interaction of Compounds in Vitro
3.4. Hoechst 33258 Staining
3.5. Annexin V-FITC/PI Staining
3.6. Caspase-3 Activity Assay
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Bhat, U.G.; Halasi, M.; Gartel, A.L. Thiazole antibiotics target foxm1 and induce apoptosis in human cancer cells. PLoS One 2009, 4, e5592. [Google Scholar]
- Chen, D.; Falsetti, S.C.; Frezza, M.; Milacic, V.; Kazi, A.; Cui, Q.C.; Long, T.E.; Turos, E.; Dou, Q.P. Anti-tumor activity of n-thiolated beta-lactam antibiotics. Cancer Lett. 2008, 268, 63–69. [Google Scholar] [CrossRef]
- Dowling, R.J.; Pollak, M.; Sonenberg, N. Current status and challenges associated with targeting mtor for cancer therapy. BioDrugs 2009, 23, 77–91. [Google Scholar] [CrossRef]
- Kadota, J.; Mizunoe, S.; Kishi, K.; Tokimatsu, I.; Nagai, H.; Nasu, M. Antibiotic-induced apoptosis in human activated peripheral lymphocytes. Int. J. Antimicrob. Agents 2005, 25, 216–220. [Google Scholar] [CrossRef]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Rapamycin suppresses ros-dependent apoptosis caused by selenomethionine in a549 lung carcinoma cells. Cancer Chemother. Pharmacol. 2010, 67, 1129–1136. [Google Scholar]
- Chandra, R.; Liu, P.; Breen, J.D.; Fisher, J.; Xie, C.; LaBadie, R.; Benner, R.J.; Benincosa, L.J.; Sharma, A. Clinical pharmacokinetics and gastrointestinal tolerability of a novel extended-release microsphere formulation of azithromycin. Clin. Pharmacokinet. 2007, 46, 247–259. [Google Scholar] [CrossRef]
- Zuckerman, J.M. The newer macrolides: Azithromycin and clarithromycin. Infect. Dis. Clin. N. Am. 2000, 14, 449–462. [Google Scholar] [CrossRef]
- Stamatiou, R.; Boukas, K.; Paraskeva, E.; Molyvdas, P.A.; Hatziefthimiou, A. Azithromycin reduces the viability of human bronchial smooth muscle cells. J. Antibiot. (Tokyo) 2010, 63, 71–75. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Hiwatashi, Y.; Maeda, M.; Fukushima, H.; Onda, K.; Tanaka, S.; Utsumi, H.; Hirano, T. Azithromycin suppresses proliferation, interleukin production and mitogen-activated protein kinases in human peripheral-blood mononuclear cells stimulated with bacterial superantigen. J. Pharm. Pharmacol. 2011, 63, 1320–1326. [Google Scholar]
- Tsai, W.C.; Hershenson, M.B.; Zhou, Y.; Sajjan, U. Azithromycin increases survival and reduces lung inflammation in cystic fibrosis mice. Inflamm. Res. 2009, 58, 491–501. [Google Scholar] [CrossRef]
- Koch, C.C.; Esteban, D.J.; Chin, A.C.; Olson, M.E.; Read, R.R.; Ceri, H.; Morck, D.W.; Buret, A.G. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: Effects of streptococcus pneumoniae. J. Antimicrob. Chemother. 2000, 46, 19–26. [Google Scholar] [CrossRef]
- Mizunoe, S.; Kadota, J.; Tokimatsu, I.; Kishi, K.; Nagai, H.; Nasu, M. Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of bcl-xl. Int. Immunopharmacol. 2004, 4, 1201–1207. [Google Scholar] [CrossRef]
- Asakura, E.; Nakayama, H.; Sugie, M.; Zhao, Y.L.; Nadai, M.; Kitaichi, K.; Shimizu, A.; Miyoshi, M.; Takagi, K.; Hasegawa, T. Azithromycin reverses anticancer drug resistance and modifies hepatobiliary excretion of doxorubicin in rats. Eur. J. Pharmacol. 2004, 484, 333–339. [Google Scholar] [CrossRef]
- Gidding, C.E.; Kellie, S.J.; Kamps, W.A.; de Graaf, S.S. Vincristine revisited. Crit. Rev. Oncol. Hematol. 1999, 29, 267–287. [Google Scholar] [CrossRef]
- Moore, A.; Pinkerton, R. Vincristine: Can its therapeutic index be enhanced? Pediatr. Blood Cancer 2009, 53, 1180–1187. [Google Scholar] [CrossRef]
- Kivisto, K.T.; Kroemer, H.K.; Eichelbaum, M. The role of human cytochrome p450 enzymes in the metabolism of anticancer agents: Implications for drug interactions. Br. J. Clin. Pharmacol. 1995, 40, 523–530. [Google Scholar] [CrossRef]
- Mondesire, W.H.; Jian, W.; Zhang, H.; Ensor, J.; Hung, M.C.; Mills, G.B.; Meric-Bernstam, F. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin. Cancer Res. 2004, 10, 7031–7042. [Google Scholar]
- Romano, M.F.; Avellino, R.; Petrella, A.; Bisogni, R.; Romano, S.; Venuta, S. Rapamycin inhibits doxorubicin-induced nf-kappab/rel nuclear activity and enhances the apoptosis of melanoma cells. Eur. J. Cancer 2004, 40, 2829–2836. [Google Scholar] [CrossRef]
- Alvarado, Y.; Mita, M.M.; Vemulapalli, S.; Mahalingam, D.; Mita, A.C. Clinical activity of mammalian target of rapamycin inhibitors in solid tumors. Target Oncol. 2011, 6, 69–94. [Google Scholar] [CrossRef]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Valentino, J.D.; Gulhati, P.; Mark Evers, B. Mtor inhibitors in cancer therapy. Cancer Lett. 2012, 319, 1–7. [Google Scholar] [CrossRef]
- Smith, D.M.; Kazi, A.; Smith, L.; Long, T.E.; Heldreth, B.; Turos, E.; Dou, Q.P. A novel beta-lactam antibiotic activates tumor cell apoptotic program by inducing DNA damage. Mol. Pharmacol. 2002, 61, 1348–1358. [Google Scholar] [CrossRef]
- Kazi, A.; Hill, R.; Long, T.E.; Kuhn, D.J.; Turos, E.; Dou, Q.P. Novel n-thiolated beta-lactam antibiotics selectively induce apoptosis in human tumor and transformed, but not normal or nontransformed, cell. Biochem. Pharmacol. 2004, 67, 365–374. [Google Scholar]
- Frezza, M.; Garay, J.; Chen, D.; Cui, C.; Turos, E.; Dou, Q.P. Induction of tumor cell apoptosis by a novel class of n-thiolated beta-lactam antibiotics with structural modifications at n1 and c3 of the lactam ring. Int. J. Mol. Med. 2008, 21, 689–695. [Google Scholar]
- Yatsunami, J.; Fukuno, Y.; Nagata, M.; Tominaga, M.; Aoki, S.; Tsuruta, N.; Kawashima, M.; Taniguchi, S.; Hayashi, S. Antiangiogenic and antitumor effects of 14-membered ring macrolides on mouse b16 melanoma cells. Clin. Exp. Metastasis 1999, 17, 361–367. [Google Scholar]
- Kawashima, M.; yatsunami, J.; Fukuno, Y.; Nagata, M.; Tominaga, M.; Hayashi, S. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung 2002, 180, 73–89. [Google Scholar]
- Vaishnav, P.; Demain, A.L. Unexpected applications of secondary metabolites. Biotechnol. Adv. 2010, 29, 223–229. [Google Scholar] [CrossRef]
- O'Reilly, K.E.; Rojo, F.; She, Q.B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. Mtor inhibition induces upstream receptor tyrosine kinase signaling and activates akt. Cancer Res. 2006, 66, 1500–1508. [Google Scholar]
- Beuvink, I.; Boulay, A.; Fumagalli, S.; Zilbermann, F.; Ruetz, S.; O’Reilly, T.; Natt, F.; Hall, J.; Lane, H.A.; Thomas, G. The mtor inhibitor rad001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 2005, 120, 747–759. [Google Scholar] [CrossRef]
- Mikasa, K.; Sawaki, M.; Kita, E.; Hamada, K.; Teramoto, S.; Sakamoto, M.; Maeda, K.; Konishi, M.; Narita, N. Significant survival benefit to patients with advanced non-small-cell lung cancer from treatment with clarithromycin. Chemotherapy 1997, 43, 288–296. [Google Scholar] [CrossRef]
- Sassa, K.; Mizushima, Y.; Fujishita, T.; Oosaki, R.; Kobayashi, M. Therapeutic effect of clarithromycin on a transplanted tumor in rats. Antimicrob. Agents Chemother. 1999, 43, 67–72. [Google Scholar]
- Hamada, K.; Kita, E.; Sawaki, M.; Mikasa, K.; Narita, N. Antitumor effect of erythromycin in mice. Chemotherapy 1995, 41, 59–69. [Google Scholar] [CrossRef]
- Loske, C.; Neumann, A.; Cunningham, A.M.; Nichol, K.; Schinzel, R.; Riederer, P.; Munch, G. Cytotoxicity of advanced glycation endproducts is mediated by oxidative stress. J. Neural. Transm. 1998, 105, 1005–1015. [Google Scholar] [CrossRef]
- Cao, S.S.; Zhen, Y.S. Potentiation of antimetabolite antitumor activity in vivo by dipyridamole and amphotericin b. Cancer Chemother. Pharmacol. 1989, 24, 181–186. [Google Scholar] [CrossRef]
- Xu, S.P.; Sun, G.P.; Shen, Y.X.; Wei, W.; Peng, W.R.; Wang, H. Antiproliferation and apoptosis induction of paeonol in hepg2 cells. World J. Gastroenterol. 2007, 13, 250–256. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, X.; Zhang, Y.; Li, Y.; Hao, X.; Liu, X.; Wang, Y. Azithromycin Synergistically Enhances Anti-Proliferative Activity of Vincristine in Cervical and Gastric Cancer Cells. Cancers 2012, 4, 1318-1332. https://doi.org/10.3390/cancers4041318
Zhou X, Zhang Y, Li Y, Hao X, Liu X, Wang Y. Azithromycin Synergistically Enhances Anti-Proliferative Activity of Vincristine in Cervical and Gastric Cancer Cells. Cancers. 2012; 4(4):1318-1332. https://doi.org/10.3390/cancers4041318
Chicago/Turabian StyleZhou, Xuezhang, Yuyan Zhang, Yong Li, Xiujing Hao, Xiaoming Liu, and Yujiong Wang. 2012. "Azithromycin Synergistically Enhances Anti-Proliferative Activity of Vincristine in Cervical and Gastric Cancer Cells" Cancers 4, no. 4: 1318-1332. https://doi.org/10.3390/cancers4041318




