Aberrant Crypt Foci: The Case for Inclusion as a Biomarker for Colon Cancer
Abstract
:1. Historical Background: Discovery of Aberrant Crypt Foci
2. ACF as a Relevant Biomarker for Colon Cancer: Histological Evidence
| Characteristic | Type of ACF | |||
|---|---|---|---|---|
| Non-Dysplastic | Hyperplastic | Dysplastic | Reference | |
| Darker staining | Yes | Yes | Darkest | [29,30] |
| Size | Increased | Increased | Increased | [29,31] |
| Topography | Raised | Raised | Raised | [31] |
| Diameter | Widest | Wide | Wide | [21,30,32] |
| Dilated lumen | Yes | Mixed | Thickened and closing | [30,31] |
| Pericryptal area | Serrated | Mixed | Non-serrated | [6,21,33] |
| Mucin status | Present | Mildly depleted | Depleted | [33,34,35,36,37] |
| Polarity | Ordered | Mixed | Lost | [29] |
| Nuclear morphometry | Round & non-stratified | Mixed | Oval & stratified | [21,29,30] |
| Proliferation pattern | Lower two-thirds of crypt | Progression to upper crypt | Full progression throughout crypt | [8,38] |
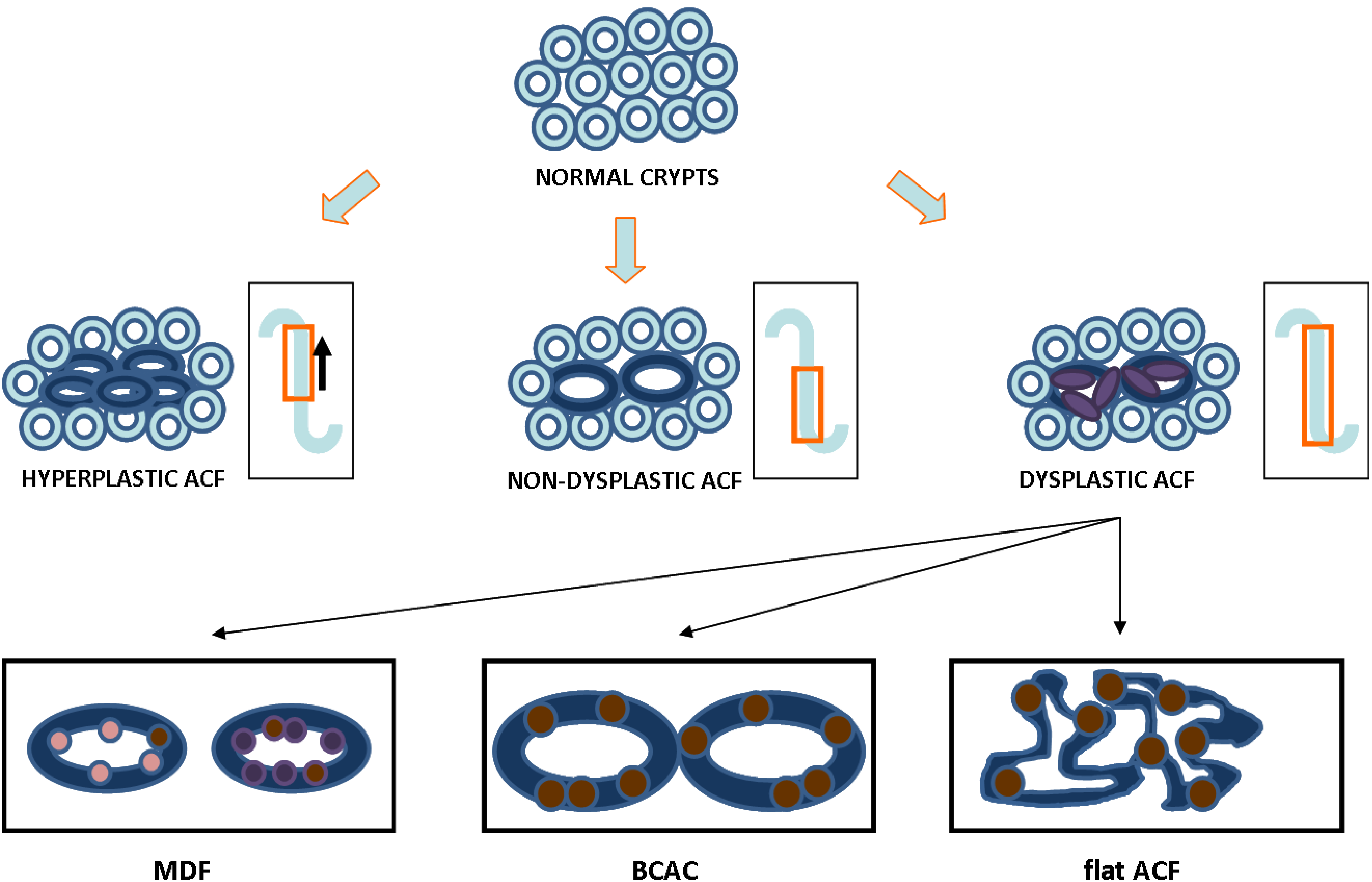
3. ACF as a Relevant Biomarker for Colon Cancer: Molecular and Cellular Evidence
| Molecular Category | Genetic Componentsa | Approximate occurrence in ACF formationb | Likelihood of progression to adenocarcinoma | Reference |
|---|---|---|---|---|
| Epigenetic Silencing | MINT31 | 34% | Majority | [49] |
| SFRP1 | 93% | Possible | [65] | |
| SFRP2 | 87% | Possible | [65] | |
| Genetic Mutation | APC | Less than 10% | Majority | [69] |
| β-catenin | 0 | Possible | [69] | |
| K-ras | 40% | Majority | [46] | |
| p53 | Less than 10% | Possible | [46] | |
| Microsatellite Instabilityc | hMLH 1 | Less than 10% | Probable | [59] |
4. ACF as a Relevant Biomarker for Colon Cancer: Chemoprevention Studies
| Rodent & Species | Method of Induction | Dosea | Route | Timeframe of Exposure of Test Agentb |
|---|---|---|---|---|
| Mouse: CF-1 | Azoxymethane | 2 × 10 | i.p. | Initiation: 1 or 2 weeks before first AOM dose and ending after the second AOM dose |
| Post-Initiation: 8 weeks after final AOM dose | ||||
| Rat: F344 | Azoxymethane | 2 × 15 | i.p. | Initiation: 4 weeks after final AOM dose |
| Post-Initiation: 8 weeks after final AOM dose |
5. ACF as a Relevant Biomarker for Colon Cancer: Clinical Evidence
6. Conclusions
References
- American Cancer Society, Cancer Facts and Figures 2009; American Cancer Society: Atlanta, GA, USA, 2009.
- World Cancer Research Fund/American Institute for Cancer Research, Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund International (WCRF International): London, UK, 2007.
- Roncucci, L.; Pedroni, M.; Vaccina, F.; Benatti, P.; and, L.M.; Pol, A.D. Aberrant crypt foci in colorectal carcinogenesis. Cell and crypt dynamics. Cell Prolif. 2000, 33, 1–18. [Google Scholar] [CrossRef]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987, 37, 147. [Google Scholar] [CrossRef]
- Bird, R.P.; McLellan, E.A.; Bruce, W.R. Aberrant crypts, putative precancerous lesions, in the study of the role of diet in the aetiology of colon cancer. Cancer Surv. 1989, 8, 189–200. [Google Scholar]
- McLellan, E.A.; Bird, R.P. Aberrant Crypts: Potential Preneoplastic Lesions in the Murine Colon. Cancer Res. 1988, 48, 6187–6192. [Google Scholar]
- Pretlow, T.P.; Barrow, B.J.; Ashton, W.S.; O'Riordan, M.A.; Pretlow, T.G.; Jurcisek, J.A.; Stellato, T.A. Aberrant Crypts: Putative Preneoplastic Foci in Human Colonic Mucosa. Cancer Res. 1991, 51, 1564–1567. [Google Scholar]
- Renehan, A.G.; O'Dwyer, S.T.; Haboubi, N.J.; Potten, C.S. Early cellular events in colorectal carcinogenesis. Colorectal Disease 2002, 4, 76–89. [Google Scholar]
- Jass, J.R.; Ajioka, Y.; Radojkovic, M.; Allison, L.J.; Lane, M.R. Failure to detect colonic mucosal hyperproliferation in mutation positive members of a family with hereditary non-polyposis colorectal cancer. Histopathology 1997, 30, 201–207. [Google Scholar]
- Stopera, S.A.; Bird, R.P. Immunohistochemical demonstration of mutant p53 tumour suppressor gene product in aberrant crypt foci. Cytobios 1993, 73, 73–88. [Google Scholar]
- Lipkin, M.; Deschner, E. Early proliferative changes in intestinal cells. Cancer Res 1976, 36, 2665–2668. [Google Scholar]
- Mills, S.J.; Mathers, J.C.; Chapman, P.D.; Burn, J.; Gunn, A. Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut 2001, 48, 41–46. [Google Scholar] [CrossRef]
- Green, S.E.; Chapman, P.; Burn, J.; Burt, A.D.; Bennett, M.; Appleton, D.R.; Varma, J.S.; Mathers, J.C. Colonic epithelial cell proliferation in hereditary non-polyposis colorectal cancer. Gut 1998, 43, 85–92. [Google Scholar] [CrossRef]
- Terpstra, O.T.; van Blankenstein, M.; Dees, J.; Eilers, G.A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology 1987, 92, 704–708. [Google Scholar]
- Ponz de Leon, M.; Roncucci, L.; Di Donato, P.; Tassi, L.; Smerieri, O.; Amorico, M.G.; Malagoli, G.; De Maria, D.; Antonioli, A.; Chahin, N.J.; et al. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res. 1988, 48, 4121–4126. [Google Scholar]
- Kubben, F.J.; Peeters-Haesevoets, A.; Engels, L.G.; Baeten, C.G.; Schutte, B.; Arends, J.W.; Stockbrugger, R.W.; Blijham, G.H. Proliferating cell nuclear antigen (PCNA): A new marker to study human colonic cell proliferation. Gut 1994, 35, 530–535. [Google Scholar] [CrossRef]
- Becciolini, A.; Balzi, M.; Faraoni, P.; Tisti, E.; Zappoli Thyrion, G.; Giache, V.; Bandettini, L.; Potten, C.S. Colonic cell proliferation in normal mucosa of patients with colon cancer. Acta Oncol. 1998, 37, 65–71. [Google Scholar] [CrossRef]
- Fujimitsu, Y.; Nakanishi, H.; Inada, K.; Yamachika, T.; Ichinose, M.; Fukami, H.; Tatematsu, M. Development of aberrant crypt foci involves a fission mechanism as revealed by isolation of aberrant cryypts. Jpn. J. Cancer Res. 1996, 87, 1199–1203. [Google Scholar] [CrossRef]
- McLellan, E.A.; Medline, A.; Bird, R.P. Sequential Analyses of the Growth and Morphological Characteristics of Aberrant Crypt Foci: Putative Preneoplastic Lesions. Cancer Res. 1991, 51, 5270–5274. [Google Scholar]
- Cheng, H.; Bjerknes, M.; Amar, J.; Gardiner, G. Crypt production in nromal and diseased human colonic epithelium. Anat. Rec. 1986, 319, 44. [Google Scholar]
- Bird, R.P. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995, 93, 55–71. [Google Scholar] [CrossRef]
- Orlando, F.A.; Tan, D.; Baltodano, J.D.; Khoury, T.; Gibbs, J.F.; Hassid, V.J.; Ahmed, B.H.; Alrawi, S.J. Aberrant crypt foci as precursors in colorectal cancer progression. J. Surg. Oncol. 2008, 98, 207–213. [Google Scholar] [CrossRef]
- Gupta, A.K.; Schoen, R.E. Aberrant crypt foci: Are they intermediate endpoints of colon carcinogenesis in humans? Curr. Opin. Gastroenterol. 2009, 25, 59–65. [Google Scholar] [CrossRef]
- Gupta, A.K.; Pinsky, P.; Rall, C.; Mutch, M.; Dry, S.; Seligson, D.; Schoen, R.E. Reliability and accuracy of the endoscopic appearance in the identification of aberrant crypt foci. Gastrointest. Endosc. 2009, 70, 322–330. [Google Scholar] [CrossRef]
- Gupta, A.K.; Pretlow, T.P.; Schoen, R.E. Aberrant crypt foci: What we know and what we need to know. Clin. Gastroenterol. Hepatol. 2007, 5, 526–533. [Google Scholar] [CrossRef]
- Khare, S.; Chaudhary, K.; Bissonnette, M.; Carroll, R. Aberrant crypt foci in colon cancer epidemiology. Methods Mol. Biol. 2009, 472, 373–386. [Google Scholar] [CrossRef]
- Lance, P.; Hamilton, S.R. Sporadic aberrant crypt foci are not a surrogate endpoint for colorectal adenoma prevention. Cancer Prev. Res. (Phila Pa) 2008, 1, 4–8. [Google Scholar] [CrossRef]
- Stevens, R.G.; Pretlow, T.P.; Hurlstone, D.P.; Giardina, C.; Rosenberg, D.W. Comment re: "Sporadic aberrant crypt foci are not a surrogate endpoint for colorectal adenoma prevention" and "Aberrant crypt foci in the adenoma prevention with celecoxib trial". Cancer Prev. Res. (Phila Pa) 2008, 1, 215–216, author reply 216. [Google Scholar] [CrossRef]
- Raju, J. Azoxymethane-induced rat aberrant crypt foci: Relevance in studying chemoprevention of colon cancer. World J. Gastroenterol. 2008, 14, 6632–6635. [Google Scholar] [CrossRef]
- Wargovich, M.J.; Chen, C.D.; Jimenez, A.; Steele, V.E.; Velasco, M.; Stephens, L.C.; Price, R.; Gray, K.; Kelloff, G.J. Aberrant crypts as a biomarker for colon cancer: Evaluation of potential chemopreventive agents in the rat. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 355–360. [Google Scholar]
- Paulsen, J.E.; Steffensen, I.-L.; Namork, E.; Alexander, J. Scanning electron microscopy of aberrant crypt foci in rat colon. Carcinogenesis 1994, 15, 2371–2373. [Google Scholar] [CrossRef]
- Fenoglio-Preiser, C.M.; Noffsinger, A. Review Article: Aberrant Crypt Foci: A Review. Toxicol. Pathol. 1999, 27, 632–642. [Google Scholar] [CrossRef]
- Bird, R.P.; Good, C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000, 112–113, 395–402. [Google Scholar] [CrossRef]
- Mori, H.; Hata, K.; Yamada, Y.; Kuno, T.; Hara, A. Significance and role of early-lesions in experimental colorectal carcinogenesis. Chem. Biol. Interact. 2005, 155, 1–9. [Google Scholar] [CrossRef]
- Caderni, G.; Femia, A.P.; Giannini, A.; Favuzza, A.; Luceri, C.; Salvadori, M.; Dolara, P. Identification of Mucin-depleted Foci in the Unsectioned Colon of Azoxymethane-treated Rats. Cancer Res. 2003, 63, 2388–2392. [Google Scholar]
- Bara, J.; Forgue-Lafitte, M.E.; Maurin, N.; Flejou, J.F.; Zimber, A. Abnormal expression of gastric mucin in human and rat aberrant crypt foci during colon carcinogenesis. Tumour Biol. 2003, 24, 109–115. [Google Scholar] [CrossRef]
- Femia, A.P.; Paulsen, J.E.; Dolara, P.; Alexander, J.; Caderni, G. Correspondence between flat aberrant crypt foci and mucin-depleted foci in rodent colon carcinogenesis. Anticancer. Res. 2008, 28, 3771–3775. [Google Scholar]
- Jenab, M.; Chen, J.; Thompson, L.U. Sialomucin production in aberrant crypt foci relates to degree of dysplasia and rate of cell proliferation. Cancer Lett. 2001, 165, 19–25. [Google Scholar] [CrossRef]
- Femia, A.P.; Giannini, A.; Fazi, M.; Tarquini, E.; Salvadori, M.; Roncucci, L.; Tonelli, F.; Dolara, P.; Caderni, G. Identification of mucin depleted foci in the human colon. Cancer Prev. Res. (Phila Pa) 2008, 1, 562–567. [Google Scholar] [CrossRef]
- Mori, H.; Yamada, Y.; Kuno, T.; Hirose, Y. Aberrant crypt foci and beta-catenin accumulated crypts; significance and roles for colorectal carcinogenesis. Mutat. Res. 2004, 566, 191–208. [Google Scholar] [CrossRef]
- Paulsen, J.E.; Knutsen, H.; Olstorn, H.B.; Loberg, E.M.; Alexander, J. Identification of flat dysplastic aberrant crypt foci in the colon of azoxymethane-treated A/J mice. Int. J. Cancer 2006, 118, 540–546. [Google Scholar] [CrossRef]
- Cheng, L.; Mao-De, L. Aberrant crypt foci as microscopic precursurs of colorectal cancer. World J. Gastroenterol. 2003, 9, 2642–2649. [Google Scholar]
- Pretlow, T.P.; O'Riordan, M.A.; Pretlow, T.G.; Stellato, T.A. Aberrant crypts in human colonic mucosa: putative preneoplastic lesions. J. Cell. Biochem. Suppl. 1992, 16G, 55–62. [Google Scholar]
- Alrawi, S.J.; Schiff, M.; Carroll, R.E.; Dayton, M.; Gibbs, J.F.; Kulavlat, M.; Tan, D.; Berman, K.; Stoler, D.L.; Anderson, G.R. Aberrant Crypt Foci. Anticancer Res. 2006, 26, 107–119. [Google Scholar]
- Heinen, C.D.; Shivapurkar, N.; Tang, Z.; Groden, J.; Alabaster, O. Microsatellite Instability in Aberrant Crypt Foci from Human Colons. Cancer Res. 1996, 56, 5339–5341. [Google Scholar]
- Shivapurkar, N.; Huang, L.; Ruggeri, B.; Swalsky, P.A.; Bakker, A.; Finkelstein, S.; Frost, A.; Silverberg, S. K-ras and p53 mutations in aberrant crypt foci and colonic tumors from colon cancer patients. Cancer Lett. 1997, 115, 39–46. [Google Scholar] [CrossRef]
- Sena, P.; Saviano, M.; Monni, S.; Losi, L.; Roncucci, L.; Marzona, L.; Pol, A.D. Subcellular localization of [beta]-catenin and APC proteins in colorectal preneoplastic and neoplastic lesions. Cancer Lett. 2006, 241, 203–212. [Google Scholar] [CrossRef]
- Nascimbeni, R.; Villanacci, V.; Mariani, P.P.; Di Betta, E.; Ghirardi, M.; Donato, F.; Salerni, B. Aberrant Crypt Foci in the Human Colon: Frequency and Histologic Patterns in Patients With Colorectal Cancer or Diverticular Disease. Am. J. Surg. Path. 1999, 23, 1256. [Google Scholar] [CrossRef]
- Chan, A.O.-O.; Broaddus, R.R.; Houlihan, P.S.; Issa, J.-P.J.; Hamilton, S.R.; Rashid, A. CpG Island Methylation in Aberrant Crypt Foci of the Colorectum. Am. J. Pathol. 2002, 160, 1823–1830. [Google Scholar] [CrossRef]
- Coral, V.A.W.; Takeshi, K.; Michael, D.W.; Barbara, A.L.; Joanne, Y.; Jeremy, R.J. DNA methylation patterns in adenomas from FAP, multiple adenoma and sporadic colorectal carcinoma patients. Int. J. Cancer 2006, 118, 907–915. [Google Scholar] [CrossRef]
- Takahashi, M.; Mutoh, M.; Kawamori, T.; Sugimura, T.; Wakabayashi, K. Altered expression of {beta}-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis 2000, 21, 1319–1327. [Google Scholar] [CrossRef]
- Takahashi, M.; Fukuda, K.; Sugimura, T.; Wakabayashi, K. {beta}-Catenin Is Frequently Mutated and Demonstrates Altered Cellular Location in Azoxymethane-induced Rat Colon Tumors. Cancer Res. 1998, 58, 42–46. [Google Scholar]
- Tsujii, M.; DuBois, R.N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995, 83, 493–501. [Google Scholar] [CrossRef]
- Kazanov, D.; Shapira, I.; Pick, M.; Kolker, O.; Liberman, E.; Deutsch, V.; Strier, L.; Dvory-Sobol, H.; Kunik, T.; Arber, N. Oncogenic Transformation of Normal Enterocytes by Overexpression of Cyclin D1. Dig. Dis. Sci. 2003, 48, 1251–1261. [Google Scholar] [CrossRef]
- Bala, S.; Peltomaki, P. CYCLIN D1 as a Genetic Modifier in Hereditary Nonpolyposis Colorectal Cancer. Cancer Res. 2001, 61, 6042–6045. [Google Scholar]
- Peltomäki, P. DNA mismatch repair and cancer. Mutat. Res./Rev. Mutat. Res. 2001, 488, 77–85. [Google Scholar] [CrossRef]
- Duval, A.; Rolland, S.; Tubacher, E.; Bui, H.; Thomas, G.; Hamelin, R. The Human T-Cell Transcription Factor-4 Gene: Structure, Extensive Characterization of Alternative Splicings, and Mutational Analysis in Colorectal Cancer Cell Lines. Cancer Res. 2000, 60, 3872–3879. [Google Scholar]
- Toyota, M.; Itoh, F.; Imai, K. DNA methylation and gastrointestinal malignancies: Functional consequences and clinical implications. J. Gastroenterol. 2000, 35, 727–734. [Google Scholar] [CrossRef]
- Pedroni, M.; Sala, E.; Scarselli, A.; Borghi, F.; Menigatti, M.; Benatti, P.; Percesepe, A.; Rossi, G.; Foroni, M.; Losi, L.; Di Gregorio, C.; Anto De, P.; Nascimbeni, R.; Di Betta, E.; Salerni, B.; Ponz de Leon, M.; Roncucci, L. Microsatellite Instability and Mismatch-Repair Protein Expression in Hereditary and Sporadic Colorectal Carcinogenesis. Cancer Res. 2001, 61, 896–899. [Google Scholar]
- Liu, T.; Yan, H.; Kuismanen, S.; Percesepe, A.; Bisgaard, M.-L.; Pedroni, M.; Benatti, P.; Kinzler, K.W.; Vogelstein, B.; Ponz de Leon, M.; Peltomaki, P.; Lindblom, A. The Role of hPMS1 and hPMS2 in Predisposing to Colorectal Cancer. Cancer Res. 2001, 61, 7798–7802. [Google Scholar]
- Lucci-Cordisco, E.; Rovella, V.; Carrara, S.; Percesepe, A.; Pedroni, M.; Bellacosa, A.; Caluseriu, O.; Forasarig, M.; Anti, M.; Neri, G.; de Leon, M.P.; Viel, A.; Genuardi, M. Mutations of the 'minor' mismatch repair gene MSH6 in typical and atypical hereditary nonpolyposis colorectal cancer. Fam. Cancer 2001, 1, 95–101. [Google Scholar] [CrossRef]
- Luo, L.; Shen, G.-Q.; Stiffler, K.A.; Wang, Q.K.; Pretlow, T.G.; Pretlow, T.P. Loss of heterozygosity in human aberrant crypt foci (ACF), a putative precursor of colon cancer. Carcinogenesis 2006, 27, 1153–1159. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The Epigenomics of Cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef]
- Rashid, A.; Shen, L.; Morris, J.S.; Issa, J.-P.J.; Hamilton, S.R. CpG Island Methylation in Colorectal Adenomas. Am. J. Pathol. 2001, 159, 1129–1135. [Google Scholar] [CrossRef]
- Suzuki, H.; Watkins, D.N.; Jair, K.-W.; Schuebel, K.E.; Markowitz, S.D.; Dong Chen, W.; Pretlow, T.P.; Yang, B.; Akiyama, Y.; van Engeland, M.; Toyota, M.; Tokino, T.; Hinoda, Y.; Imai, K.; Herman, J.G.; Baylin, S.B. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 2004, 36, 417–422. [Google Scholar]
- Toyota, M.; Ohe-Toyota, M.; Ahuja, N.; Issa, J.-P.J. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc. Natl. Acad. Sci. 2000, 97, 710–715. [Google Scholar] [CrossRef]
- Wang, L.; Bani-Hani, A.; Montoya, D.P.; Roche, P.C.; Thibodeau, S.N.; Bugart, L.J.; Roberts, L.R. hMLH1 and hMSH2 expression in human hepatocellular carcinoma. Int. J. Oncol. 2001, 19, 567–570. [Google Scholar]
- Greenspan, E.J.; Cyr, J.L.; Pleau, D.C.; Levine, J.; Rajan, T.V.; Rosenberg, D.W.; Heinen, C.D. Microsatellite instability in aberrant crypt foci from patients without concurrent colon cancer. Carcinogenesis 2007, 28, 769–776. [Google Scholar]
- Takayama, T.; Ohi, M.; Hayashi, T.; Miyanishi, K.; Nobuoka, A.; Nakajima, T.; Satoh, T.; Takimoto, R.; Kato, J.; Sakamaki, S.; Niitsu, Y. Analysis of K-ras, APC, and [beta]-Catenin in Aberrant Crypt Foci in Sporadic Adenoma, Cancer, and Familial Adenomatous Polyposis. Gastroenterol. 2001, 121, 599–611. [Google Scholar]
- Corpet, D.E.; Tache, S. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr. Cancer 2002, 43, 1–21. [Google Scholar] [CrossRef]
- Wargovich, M.J.; Jimenez, A.; McKee, K.; Steele, V.E.; Velasco, M.; Woods, J.; Price, R.; Gray, K.; Kelloff, G.J. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis 2000, 21, 1149–1155. [Google Scholar] [CrossRef]
- Steele, V.E.; Moon, R.C.; Lubet, R.A.; Grubbs, C.J.; Reddy, B.S.; Wargovich, M.; McCormick, D.L.; Pereira, M.A.; Crowell, J.A.; Bagheri, D.; et al. Preclinical efficacy evaluation of potential chemopreventive agents in animal carcinogenesis models: methods and results from the NCI Chemoprevention Drug Development Program. J. Cell. Biochem. Suppl. 1994, 20, 32–54. [Google Scholar]
- Ochiai, M.; Ushigome, M.; Fujiwara, K.; Ubagai, T.; Kawamori, T.; Sugimura, T.; Nagao, M.; Nakagama, H. Characterization of dysplastic aberrant crypt foci in the rat colon induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Am. J. Pathol. 2003, 163, 1607–1614. [Google Scholar] [CrossRef]
- Qin, X.; Zarkovic, M.; Nakatsuru, Y.; Arai, M.; Oda, H.; Ishikawa, T. DNA adduct formation and assessment of aberrant crypt foci in vivo in the rat colon mucosa after treatment with N-methyl-N-nitrosourea. Carcinogenesis 1994, 15, 851–855. [Google Scholar] [CrossRef]
- Xu, M.; Chen, R.; Dashwood, R.H. Effect of carcinogen dose fractionation, diet and source of F344 rat on the induction of colonic aberrant crypts by 2-amino-3-methylimidazo[4,5-f]quinoline. Carcinogenesis 1999, 20, 2293–2298. [Google Scholar] [CrossRef]
- Corpet, D. Chemoprevention Database Colorectal Cancer Prevention. INRA: Paris, France, 2010. [Google Scholar]
- Hurlstone, D.P.; Karajeh, M.; Sanders, D.S.; Drew, S.K.; Cross, S.S. Rectal Aberrant Crypt Foci Identified Using High-Magnification-Chromoscopic Colonoscopy: Biomarkers for Flat and Depressed Neoplasia. Am. J. Gastroenterol. 2005, 100, 1283–1289. [Google Scholar] [CrossRef]
- Takayama, T.; Katsuki, S.; Takahashi, Y.; Ohi, M.; Nojiri, S.; Sakamaki, S.; Kato, J.; Kogawa, K.; Miyake, H.; Niitsu, Y. Aberrant Crypt Foci of the Colon as Precursors of Adenoma and Cancer. N. Engl. J. Med. 1998, 339, 1277–1284. [Google Scholar] [CrossRef]
- Cho, N.; Redston, M.; Zauber, A.; Carothers, A.; Hornick, J.; Wilton, A.; Sontag, S.; Nishioka, N.; Giardiello, F.; Saltzman, J.; Gostout, C.; Eagle, C.; Hawk, E.; Bertagnolli, M. Aberrant Crypt Foci in the Adenoma Prevention with Celecoxib Trial. Cancer Prev. Res. 2008, 1, 21–31. [Google Scholar] [CrossRef]
- Suehiro, Y.; Hinoda, Y. Genetic and epigenetic changes in aberrant crypt foci and serrated polyps. Cancer Sci. 2008, 99, 1071–1076. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wargovich, M.J.; Brown, V.R.; Morris, J. Aberrant Crypt Foci: The Case for Inclusion as a Biomarker for Colon Cancer. Cancers 2010, 2, 1705-1716. https://doi.org/10.3390/cancers2031705
Wargovich MJ, Brown VR, Morris J. Aberrant Crypt Foci: The Case for Inclusion as a Biomarker for Colon Cancer. Cancers. 2010; 2(3):1705-1716. https://doi.org/10.3390/cancers2031705
Chicago/Turabian StyleWargovich, Michael J., Vondina R. Brown, and Jay Morris. 2010. "Aberrant Crypt Foci: The Case for Inclusion as a Biomarker for Colon Cancer" Cancers 2, no. 3: 1705-1716. https://doi.org/10.3390/cancers2031705
APA StyleWargovich, M. J., Brown, V. R., & Morris, J. (2010). Aberrant Crypt Foci: The Case for Inclusion as a Biomarker for Colon Cancer. Cancers, 2(3), 1705-1716. https://doi.org/10.3390/cancers2031705




