Liquid Crystal Elastomer-Based Microelectrode Array for In Vitro Neuronal Recordings
Abstract
:1. Introduction
2. Materials and Methods
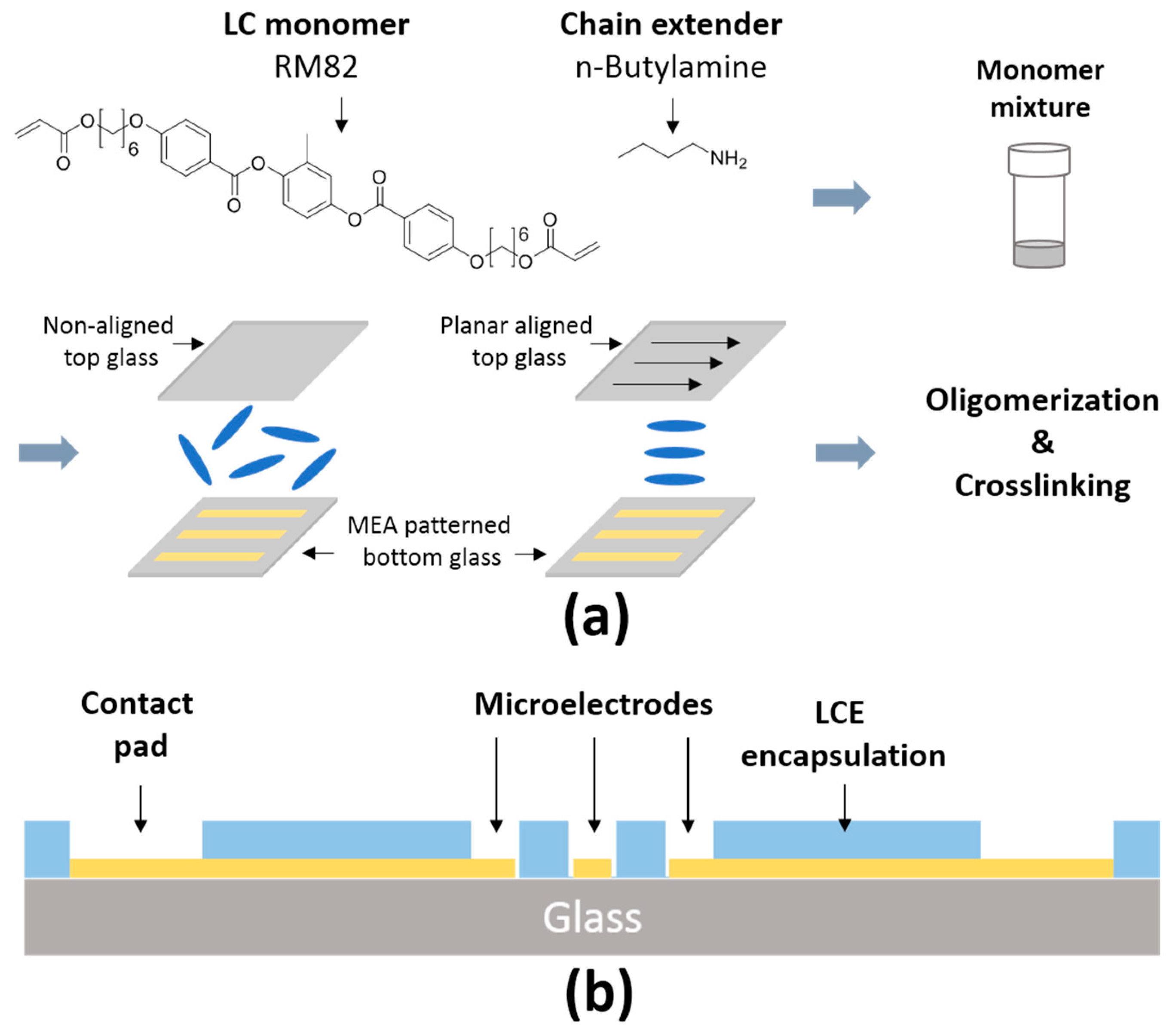
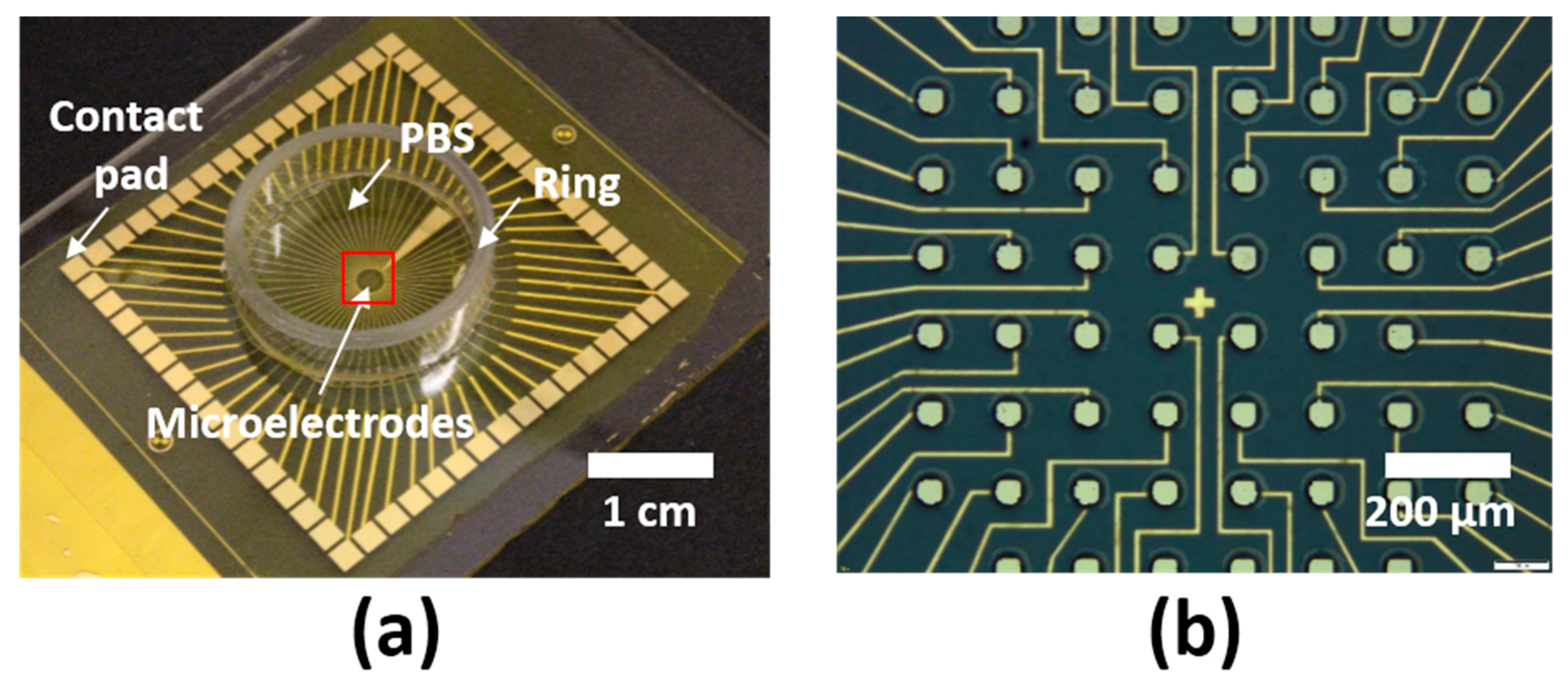
2.1. Fabrication of LCE MEAs
2.2. Electrochemical Characterization of LCE MEAs
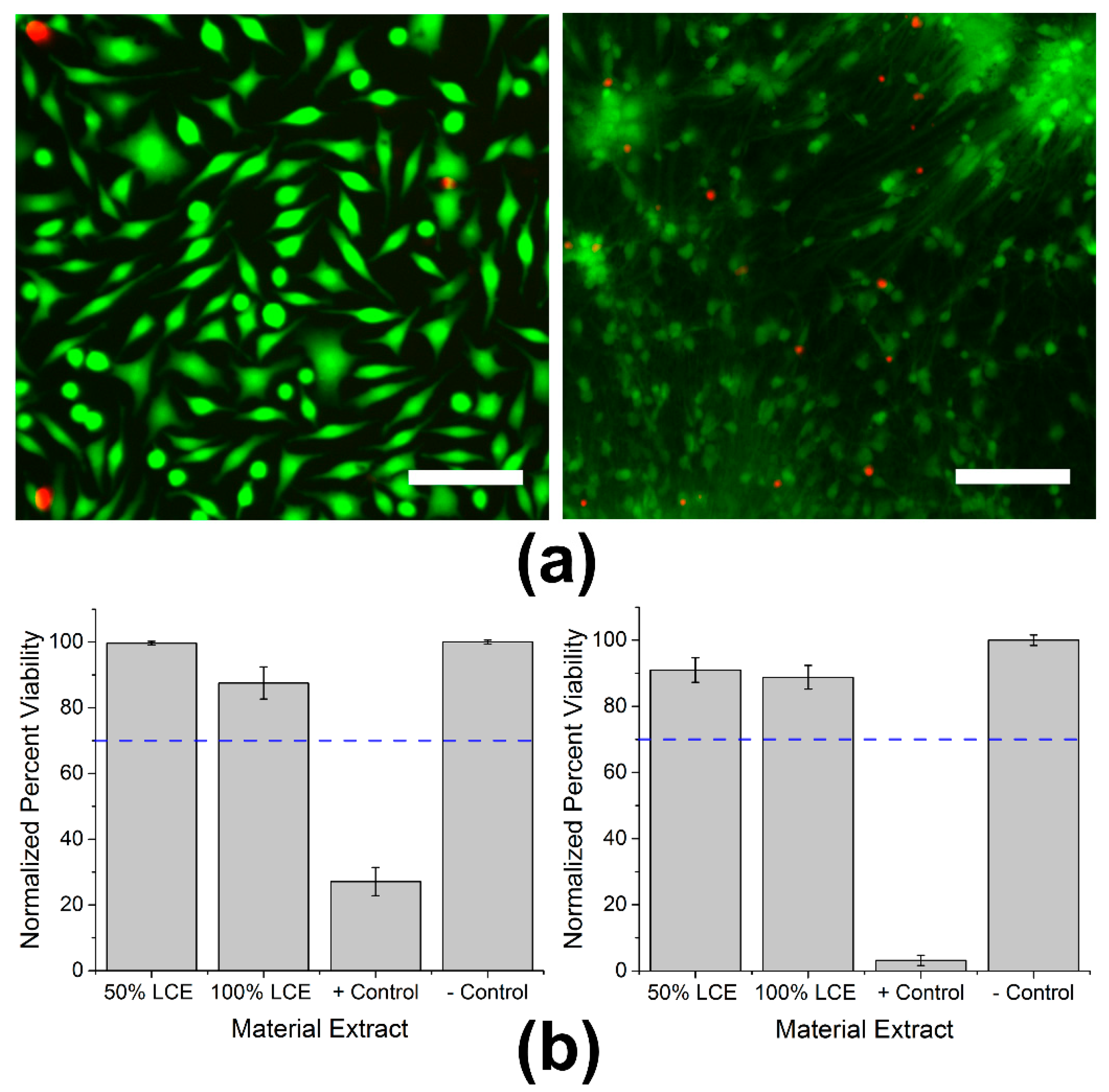
2.3. In Vitro Cytotoxicity Testing
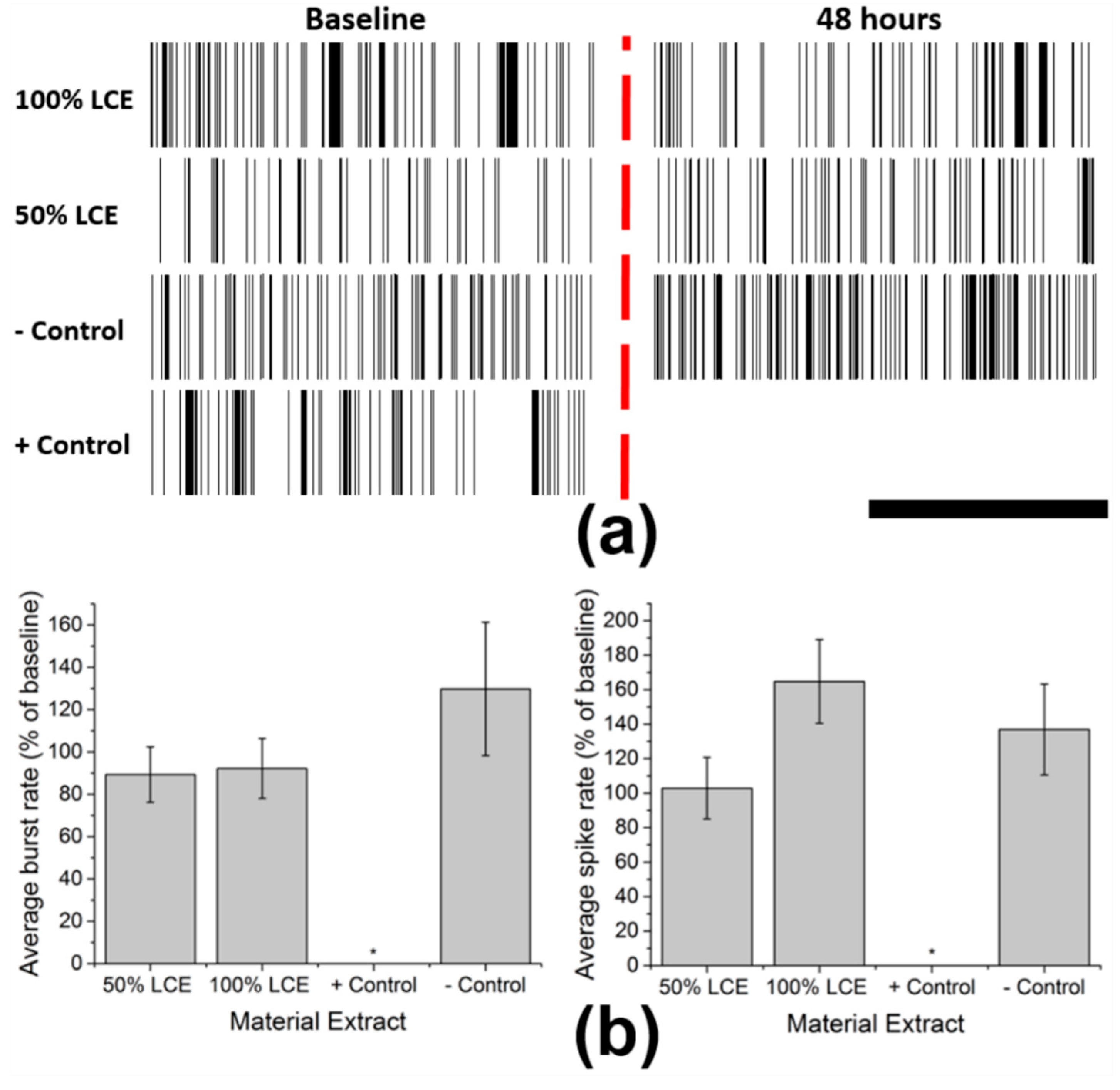
2.4. In Vitro Functional Neurotoxicity Testing
2.5. In Vitro Neuronal Recordings and Pharmacology
2.6. Statistical and Data Analysis
3. Results
3.1. In Vitro Cytotoxicity Testing
3.2. In Vitro Functional Neurotoxicity Testing
3.3. Electrochemical Impedance Stability of MEAs
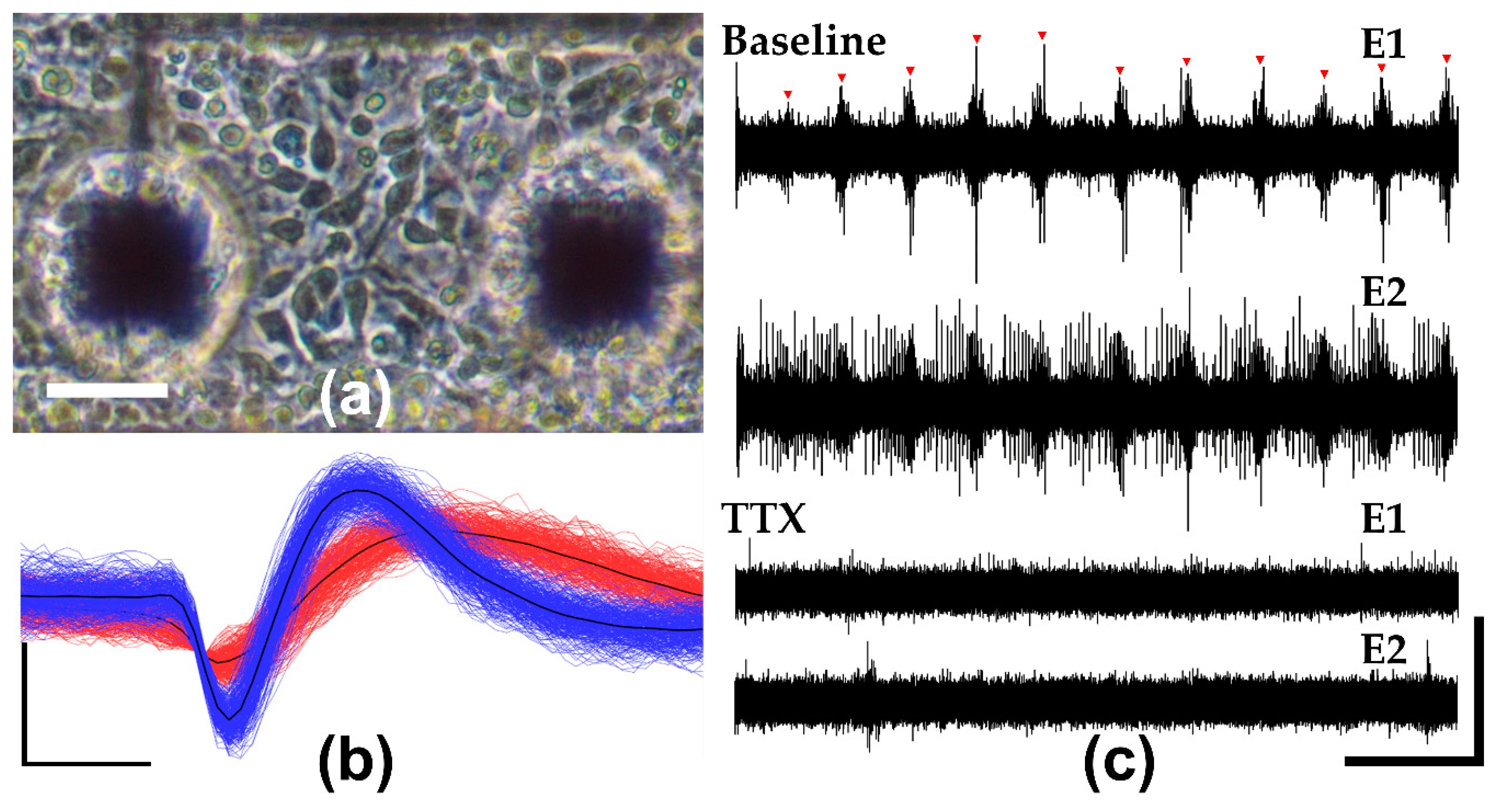
3.4. In Vitro Neuronal Recordings and Pharmacology
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campenot, R.B.; Lund, K.; Mok, S.-A. Production of compartmented cultures of rat sympathetic neurons. Nat. Protoc. 2009. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Pan, L.; Huang, L.; Yu, Z.; Song, X.; Cheng, J.; Xing, W.; Zhou, Y. Microelectrode array-based system for neuropharmacological applications with cortical neurons cultured in vitro. Biosens. Bioelectron. 2007. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Joo, S.; Jung, H.; Hong, N.; Nam, Y. Recent trends in microelectrode array technology for in vitro neural interface platform. Biomed. Eng. Lett. 2014, 4, 129–141. [Google Scholar] [CrossRef]
- Charkhkar, H.; Arreaga-Salas, D.E.; Tran, T.; Hammack, A.; Voit, W.E.; Pancrazio, J.J.; Gnade, B.E. Novel disposable microelectrode array for cultured neuronal network recording exhibiting equivalent performance to commercially available arrays. Sens. Actuators B Chem. 2016. [Google Scholar] [CrossRef]
- Hammack, A.; Rihani, R.T.; Black, B.J.; Pancrazio, J.J.; Gnade, B.E. A patterned polystyrene-based microelectrode array for in vitro neuronal recordings. Biomed. Microdevices 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, B.C.; Nam, Y. In Vitro Microelectrode Array Technology and Neural Recordings. Crit. Rev. Biomed. Eng. 2011. [Google Scholar] [CrossRef]
- Lacour, S.P.; Benmerah, S.; Tarte, E.; Fitzgerald, J.; Serra, J.; McMahon, S.; Fawcett, J.; Graudejus, O.; Yu, Z.; Morrison, B. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med. Biol. Eng. Comput. 2010, 48, 945–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smela, E.; Jager, E.W.H.; Inganas, O.; Smela, E.; Inganäs, O. Microfabricating Conjugated Polymer Actuators. Science 2000, 290, 1540–1545. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; Van Der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorfi, M.; Skousen, J.L.; Weder, C.; Capadona, J.R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 2015. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.K.; Biran, R.; Underwood, C.J.; Tresco, P.A. Characterization of microglial attachment and cytokine release on biomaterials of differing surface chemistry. Biomaterials 2008. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Capadona, J.R.; Tyler, D.J.; Zorman, C.A.; Rowan, S.J.; Weder, C. Mechanically adaptive nanocomposites for neural interfacing. MRS Bull. 2012. [Google Scholar] [CrossRef]
- Jeong, J.; Bae, S.H.; Seo, J.; Chung, H.; Kim, S.J. Long-term evaluation of a liquid crystal polymer (LCP)-based retinal prosthesis. J. Neural Eng. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.; Park, W.; Yoon, Y. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Kisban, S.; Herwik, S.; Seidl, K.; Rubehn, B.; Jezzini, A.; Umiltà, M.A.; Fogassi, L.; Stieglitz, T.; Paul, O.; Ruther, P. Microprobe Array with Low Impedance Electrodes and Highly Flexible Polyimide Cables for Acute Neural Recording. IEEE Eng. Med. Biol. Soc. 2007, 3, 175–178. [Google Scholar]
- Pang, C.; Cham, J.G.; Nenadic, Z.; Musallam, S.; Tai, Y.; Burdick, J.W.; Andersen, R.A. A New Multi-Site Probe Array with Monolithically Integrated Parylene Flexible Cable for Neural Prostheses. IEEE Eng. Med. Biol. Soc. 2005. [Google Scholar] [CrossRef]
- Ware, T.; Simon, D.; Arreaga-salas, D.E.; Reeder, J.; Rennaker, R.; Keefer, E.W.; Voit, W. Fabrication of Responsive, Softening Neural Interfaces. Adv. Funct. Mater. 2012, 22, 3470–3479. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, S.W.; Min, K.S.; Shin, S.; Jun, S.B.; Kim, S.J. Liquid Crystal Polymer ( LCP ), an Attractive Substrate for Retinal Implant. Sens. Mater. 2012, 24, 189–203. [Google Scholar]
- Chen, M.J.; Pham, A.H.; Member, S.; Evers, N.A.; Kapusta, C.; Iannotti, J.; Kornrumpf, W.; Maciel, J.J.; Karabudak, N. Design and Development of a Package Using LCP for RF/Microwave MEMS Switches. IEEE Trans. Microw. Theory Tech. 2006, 54, 4009–4015. [Google Scholar] [CrossRef]
- Lee, S.W.; Min, K.S.; Jeong, J.; Kim, J.; Kim, S.J.; Member, S. Monolithic Encapsulation of Implantable Neuroprosthetic Devices Using Liquid Crystal Polymers. IEEE Trans. Biomed. Eng. 2011, 58, 2255–2263. [Google Scholar]
- Zhu, J.; Dexheimer, M.; Cheng, H. Reconfigurable systems for multifunctional electronics. NPJ Flex. Electron. 2017, 1. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, R.S.; Kim, H.; Boothby, J.M.; Ware, T.H. Liquid crystal elastomer actuators: Synthesis, alignment, and applications. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 395–411. [Google Scholar] [CrossRef] [Green Version]
- Küpfer, J.; Finkelmann, H. Nematic liquid single crystal elastomers. Die Makromol. Chem. Rapid Commun. 1991, 12, 717–726. [Google Scholar] [CrossRef]
- Ware, T.H.; McConney, M.E.; Wie, J.J.; Tondiglia, V.P.; White, T.J. Voxelated liquid crystal elastomers. Science 2015, 347, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Wermter, H.; Finkelmann, H. Liquid crystalline elastomers as artificial muscles. E-Polymers 2001, 1, 1–13. [Google Scholar] [CrossRef]
- Gelebart, A.H.; Mulder, D.J.; Varga, M.; Konya, A.; Vantomme, G.; Meijer, E.W.; Selinger, L.B.R.; Broer, D.J. Making waves in a photoactive polymer film. Nature 2017, 546, 632–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Ikeda, T. Photocontrollable liquid-crystalline actuators. Adv. Mater. 2011. [Google Scholar] [CrossRef] [PubMed]
- Boothby, J.M.; Kim, H.; Ware, T.H. Shape changes in chemoresponsive liquid crystal elastomers. Sens. Actuators B Chem. 2017, 240, 511–518. [Google Scholar] [CrossRef]
- De Haan, L.T.; Verjans, J.M.N.; Broer, D.J.; Bastiaansen, C.W.M.; Schenning, A.P.H.J. Humidity-responsive liquid crystalline polymer actuators with an asymmetry in the molecular trigger that bend, fold, and curl. J. Am. Chem. Soc. 2014. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004, 7, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Gugel, Z.; Li, X.; Gilgunn, P.J.; Khilwani, R.; Ozdoganlar, O.B.; Fedder, G.K.; Weber, D.J.; Cui, X.T. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials 2014. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Na, K.; Zhang, H.; Kozai, T.D.Y.; Kotov, N.A.; Yoon, E.; Chestek, C.A. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 2015. [Google Scholar] [CrossRef] [PubMed]
- Guitchounts, G.; Markowitz, J.E.; Liberti, W.A.; Gardner, T.J. A carbon-fiber electrode array for long-term neural recording. J. Neural Eng. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yakacki, C.M.; Saed, M.; Nair, D.P.; Gong, T.; Reed, S.M.; Bowman, C.N. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol-acrylate reaction. RSC Adv. 2015. [Google Scholar] [CrossRef]
- Sharma, A.; Neshat, A.; Mahnen, C.J.; Nielsen, A.D.; Snyder, J.; Stankovich, T.L.; Daum, B.G.; Laspina, E.M.; Beltrano, G.; Gao, Y.; et al. Biocompatible, biodegradable and porous liquid crystal elastomer scaffolds for spatial cell cultures. Macromol. Biosci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; Paoli, P.; Pioner, J.M.; Sacconi, L.; Coppini, R.; Santini, L.; Lulli, M.; Cerbai, E.; Wiersma, D.S.; Poggesi, C.; et al. Liquid Crystalline Networks toward Regenerative Medicine and Tissue Repair. Small 2017. [Google Scholar] [CrossRef] [PubMed]
- Boothby, J.M.; Ware, T.H. Dual-responsive, shape-switching bilayers enabled by liquid crystal elastomers. Soft Matter 2017, 13, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Charkhkar, H.; Frewin, C.; Nezafati, M.; Knaack, G.L.; Peixoto, N.; Saddow, S.E.; Pancrazio, J.J. Use of cortical neuronal networks for in vitro material biocompatibility testing. Biosens. Bioelectron. 2014, 53, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Black, B.J.; Atmaramani, R.; Pancrazio, J.J. Spontaneous and Evoked Activity from Murine Ventral Horn Cultures on Microelectrode Arrays. Front. Cell. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rihani, R.T.; Kim, H.; Black, B.J.; Atmaramani, R.; Saed, M.O.; Pancrazio, J.J.; Ware, T.H. Liquid Crystal Elastomer-Based Microelectrode Array for In Vitro Neuronal Recordings. Micromachines 2018, 9, 416. https://doi.org/10.3390/mi9080416
Rihani RT, Kim H, Black BJ, Atmaramani R, Saed MO, Pancrazio JJ, Ware TH. Liquid Crystal Elastomer-Based Microelectrode Array for In Vitro Neuronal Recordings. Micromachines. 2018; 9(8):416. https://doi.org/10.3390/mi9080416
Chicago/Turabian StyleRihani, Rashed T., Hyun Kim, Bryan J. Black, Rahul Atmaramani, Mohand O. Saed, Joseph J. Pancrazio, and Taylor H. Ware. 2018. "Liquid Crystal Elastomer-Based Microelectrode Array for In Vitro Neuronal Recordings" Micromachines 9, no. 8: 416. https://doi.org/10.3390/mi9080416





