Enhanced Axial Resolution of Wide-Field Two-Photon Excitation Microscopy by Line Scanning Using a Digital Micromirror Device
Abstract
:1. Introduction
2. Materials and Methods
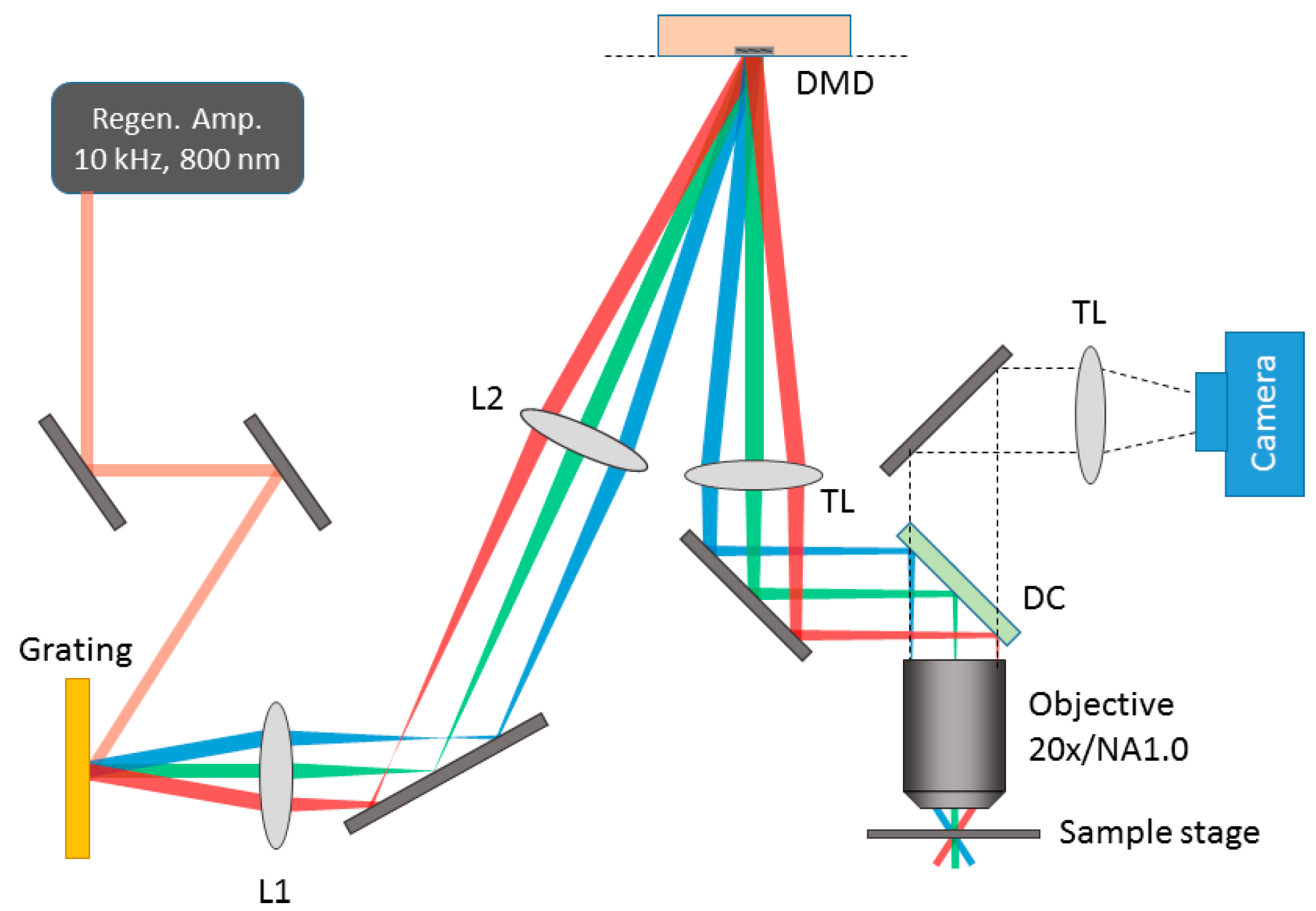
2.1. Digital Micromirror Device (DMD)-Based Line Scanning Temporal Focusing Multi-Photon Microscope
2.2. Preparation of Calibration and Biological Samples
3. Results and Discussion
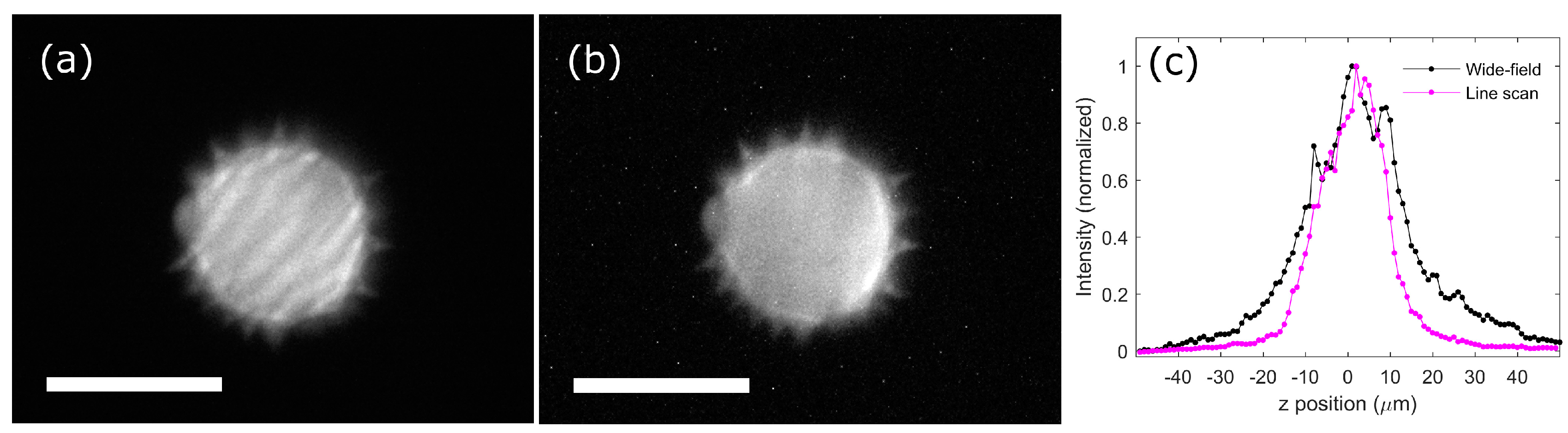
3.1. Axial Resolution Comparison Between Wide-Field and Line-Scanning Temporal Focusing
3.2. Axial Resolution Dependence on the Line Thickness and the Number of Lines
3.3. Imaging of a Pollen Grain and Biological Sample
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Mertz, J. Nonlinear microscopy: New techniques and applications. Curr. Opin. Neurobiol. 2004, 14, 610–616. [Google Scholar] [CrossRef] [PubMed]
- So, P.T.C.; Dong, C.Y.; Masters, B.R.; Berland, K.M. Two-Photon excitation fluorescence microscopy. Annu. Rev. Biomed. Eng. 2000, 2, 399–429. [Google Scholar] [CrossRef] [PubMed]
- Hoover, E.E.; Squier, J.A. Advances in multiphoton microscopy technology. Nat. Photon. 2013, 7, 93–101. [Google Scholar] [CrossRef] [PubMed]
- So, P.T.C.; Yew, E.Y.S.; Rowlands, C. High-throughput nonlinear optical microscopy. Biophys. J. 2013, 105, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Oron, D.; Tal, E.; Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 2005, 13, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; van Howe, J.; Durst, M.; Zipfel, W.; Xu, C. Simultaneous spatial and temporal focusing of femtosecond pulses. Opt. Express 2005, 13, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Therrien, O.D.; Aubé, B.; Pagès, S.; Koninck, P.D.; Côté, D. Wide-field multiphoton imaging of cellular dynamics in thick tissue by temporal focusing and patterned illumination. Biomed. Opt. Express 2011, 2, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Tzeranis, D.S.; Cha, J.W.; Clémenceau, P.; de Jong, S.J.G.; van Geest, L.K.; Moon, J.H.; Yannas, I.V; So, P.T.C. 3D-resolved fluorescence and phosphorescence lifetime imaging using temporal focusing wide-field two-photon excitation. Opt. Express 2012, 20, 26219–26235. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yew, E.Y.S.; Hallacoglu, B.; Fantini, S.; Sheppard, C.J.R.; So, P.T.C. Improvement of axial resolution and contrast in temporally focused widefield two-photon microscopy with structured light illumination. Biomed. Opt. Express 2013, 4, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Andrasfalvy, B.K.; Zemelman, B.V.; Tang, J.; Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl. Acad. Sci. USA 2010, 107, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Papagiakoumou, E.; Anselmi, F.; Begue, A.; de Sars, V.; Gluckstad, J.; Isacoff, E.Y.; Emiliani, V. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 2010, 7, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; So, P.T.C. High-throughput three-dimensional lithographic microfabrication. Opt. Lett. 2010, 35, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Cheng, L.-C.; Chang, C.-Y.; Lien, C.-H.; Campagnola, P.J.; Chen, S.-J. Fast multiphoton microfabrication of freeform polymer microstructures by spatiotemporal focusing and patterned excitation. Opt. Express 2012, 20, 19030–19038. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Yeh, T.-F.; Huang, H.-C.; Chang, H.-Y.; Lin, C.-Y.; Cheng, L.-C.; Chang, C.-Y.; Teng, H.; Chen, S.-J. Graphene oxide-based micropatterns via high-throughput multiphoton-induced reduction and ablation. Opt. Express 2014, 22, 19726–19734. [Google Scholar] [CrossRef] [PubMed]
- Yew, E.Y.S.; Sheppard, C.J.R.; So, P.T.C. Temporally focused wide-field two-photon microscopy: Paraxial to vectorial. Opt. Express 2013, 21, 12951–12963. [Google Scholar] [CrossRef] [PubMed]
- Tal, E.; Oron, D.; Silberberg, Y. Improved depth resolution in video-rate line-scanning multiphoton microscopy using temporal focusing. Opt. Lett. 2005, 30, 1686–1688. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Kruger, N.; Ellman, A.; Shoham, S. Line temporal focusing characteristics in transparent and scattering media. Opt. Express 2013, 21, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Ultrashort optical pulse delivery for nolinear optical microscopy. In Handbook of Biomedical Nonlinear Optical Microscopy; Masters, B.R., So, P.T.C., Eds.; Oxford University Press: New York, NY, USA, 2008; p. 239. [Google Scholar]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef] [PubMed]

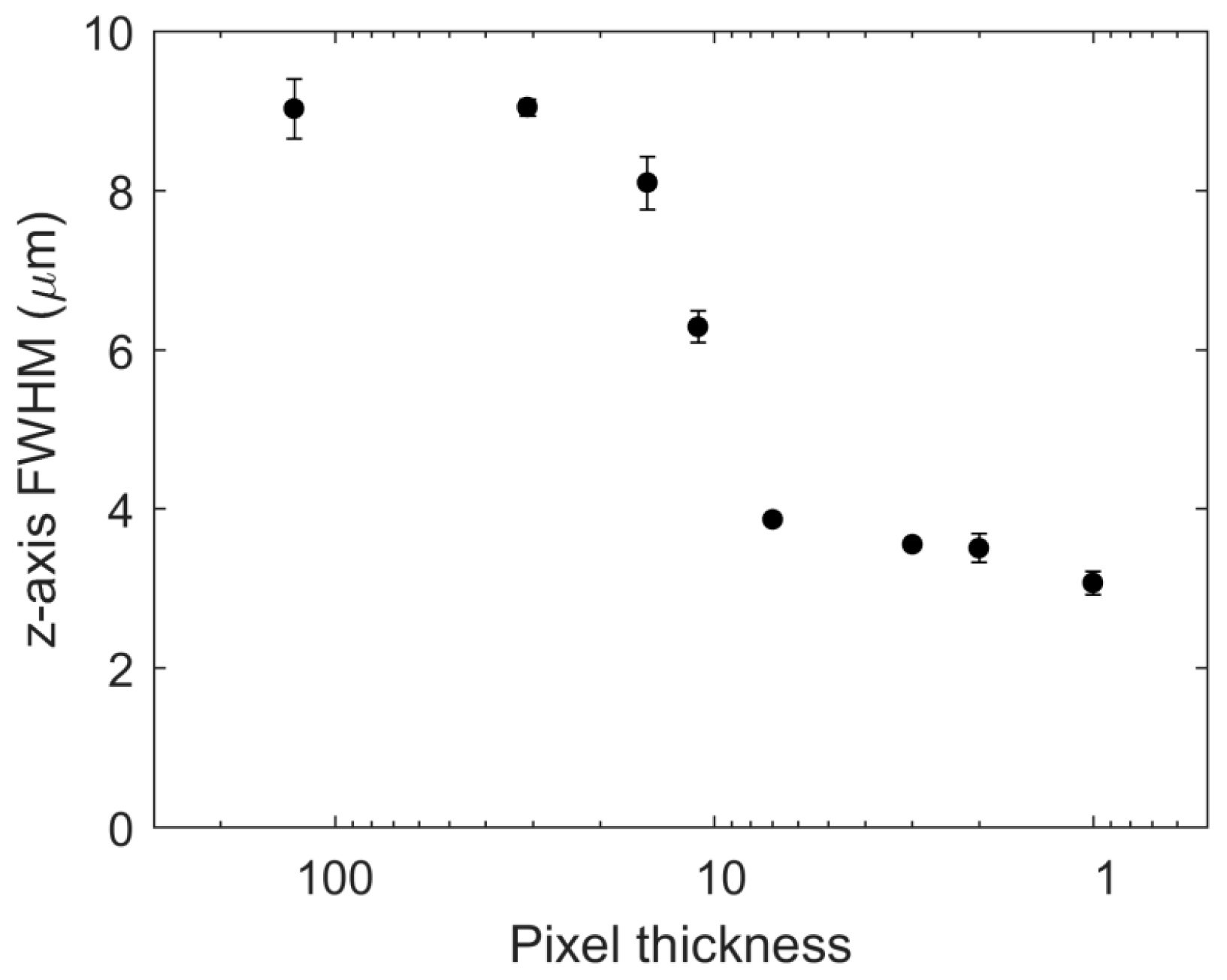

| Pixel Thickness (128 × T) | FWHM (µm) | Standard Error (µm) |
|---|---|---|
| 128 (wide-field) | 9.03 | 0.38 |
| 31 | 9.04 | 0.10 |
| 15 | 8.10 | 0.33 |
| 11 | 6.29 | 0.20 |
| 7 | 3.87 | 0.05 |
| 3 | 3.55 | 0.02 |
| 2 | 3.51 | 0.18 |
| 1 | 3.07 | 0.15 |
| Number of Lines (L × (128 × 3)) | FWHM (µm) | Standard Error (µm) |
|---|---|---|
| 1 | 3.55 | 0.02 |
| 4 | 3.40 | 0.12 |
| 8 | 3.38 | 0.17 |
| 16 | 3.40 | 0.23 |
| 32 | 7.75 | 0.43 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.K.; Rowlands, C.J.; So, P.T.C. Enhanced Axial Resolution of Wide-Field Two-Photon Excitation Microscopy by Line Scanning Using a Digital Micromirror Device. Micromachines 2017, 8, 85. https://doi.org/10.3390/mi8030085
Park JK, Rowlands CJ, So PTC. Enhanced Axial Resolution of Wide-Field Two-Photon Excitation Microscopy by Line Scanning Using a Digital Micromirror Device. Micromachines. 2017; 8(3):85. https://doi.org/10.3390/mi8030085
Chicago/Turabian StylePark, Jong Kang, Christopher J. Rowlands, and Peter T. C. So. 2017. "Enhanced Axial Resolution of Wide-Field Two-Photon Excitation Microscopy by Line Scanning Using a Digital Micromirror Device" Micromachines 8, no. 3: 85. https://doi.org/10.3390/mi8030085





