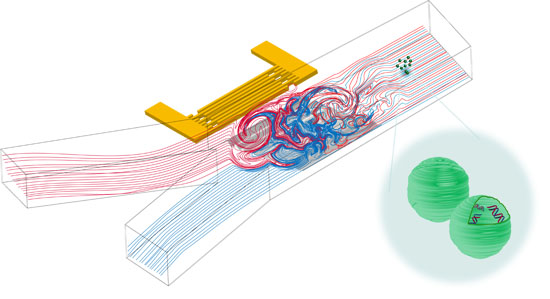
Controllable Acoustic Mixing of Fluids in Microchannels for the Fabrication of Therapeutic Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
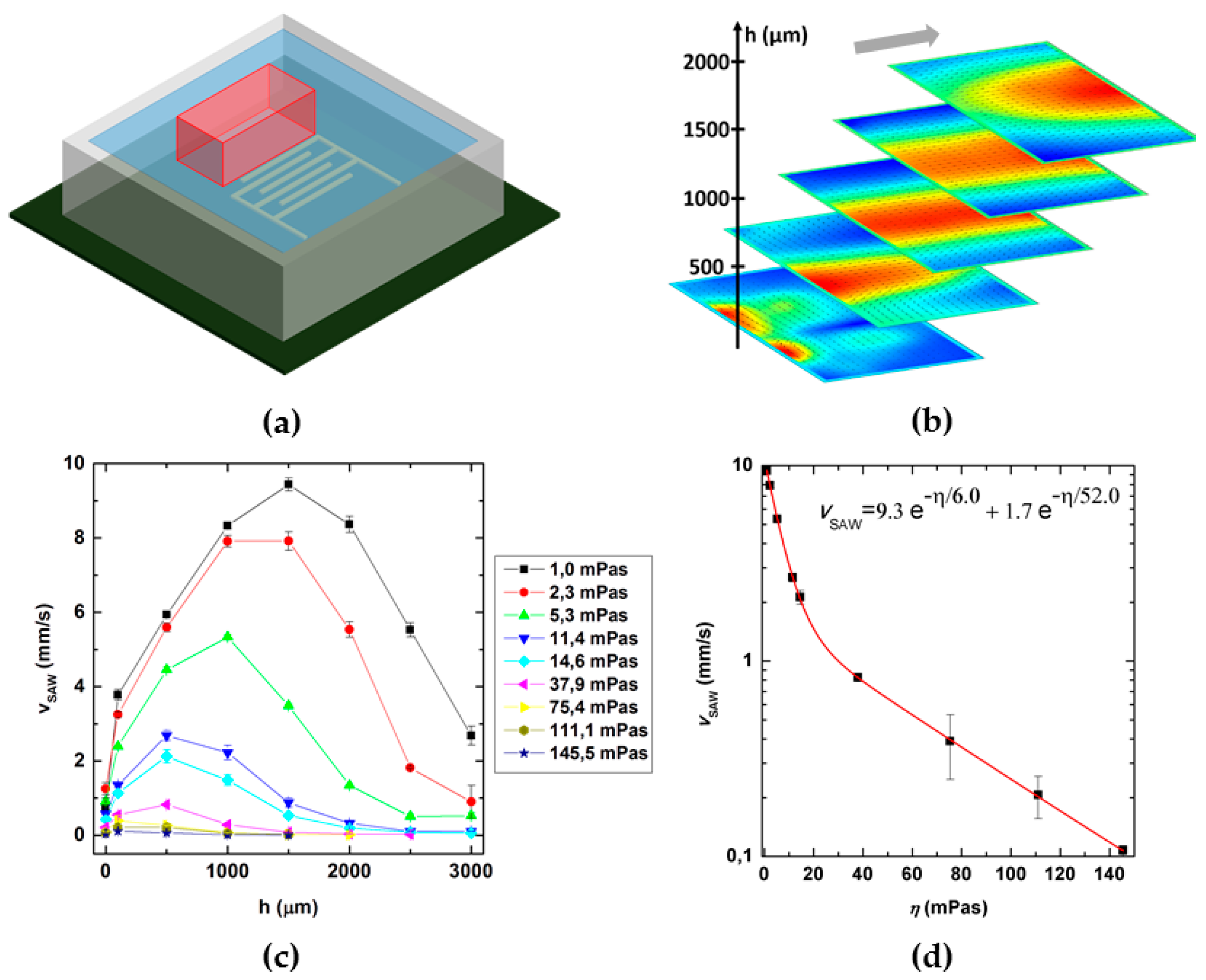
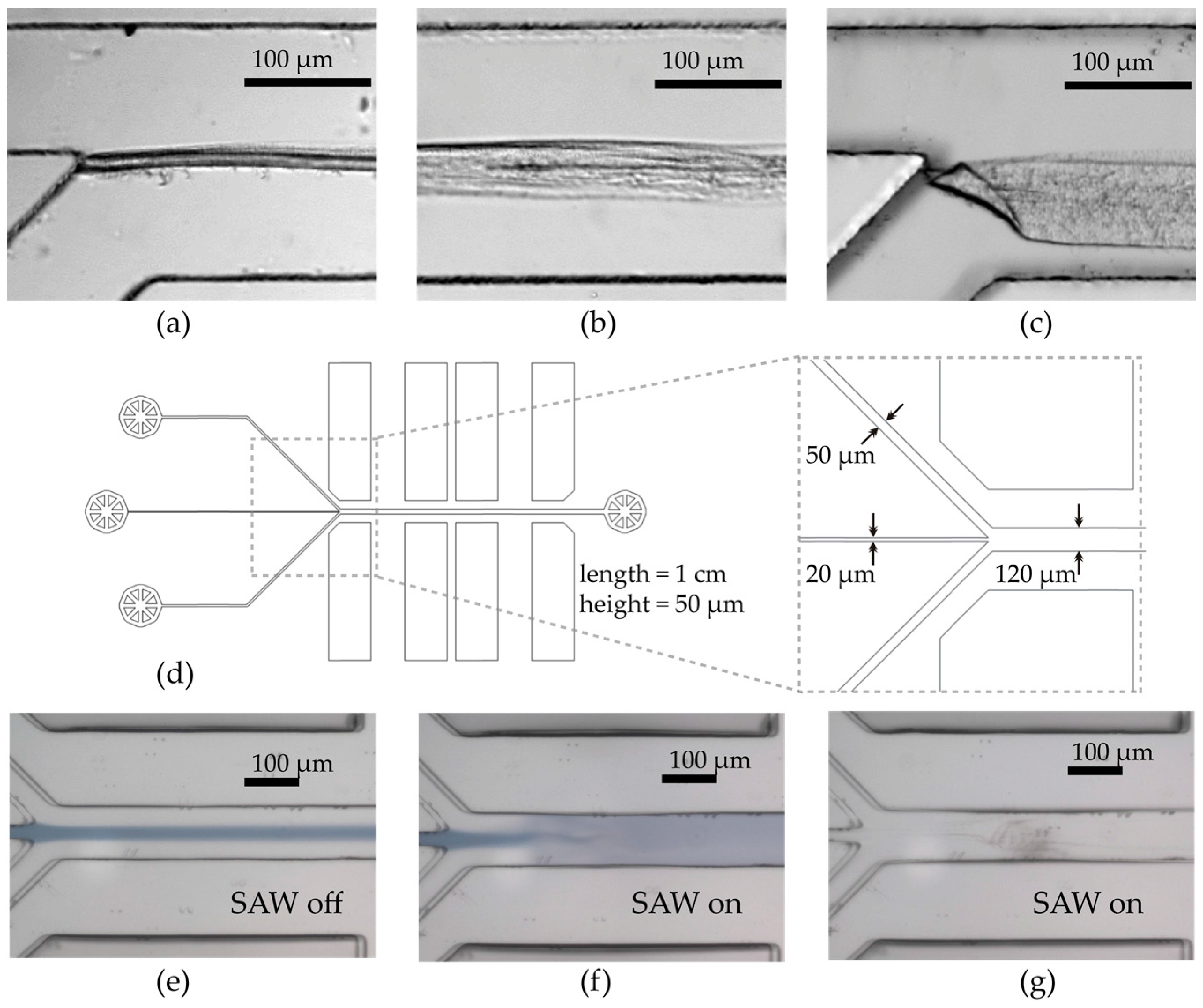
2.1. SAW-Chip and Microchannel Fabrication
2.2. Particle Image Velocimetry Experiments
2.3. Mixing Experiments
2.4. Nanoparticle Synthesis and Sample Evaluation
3. Results and Discussions
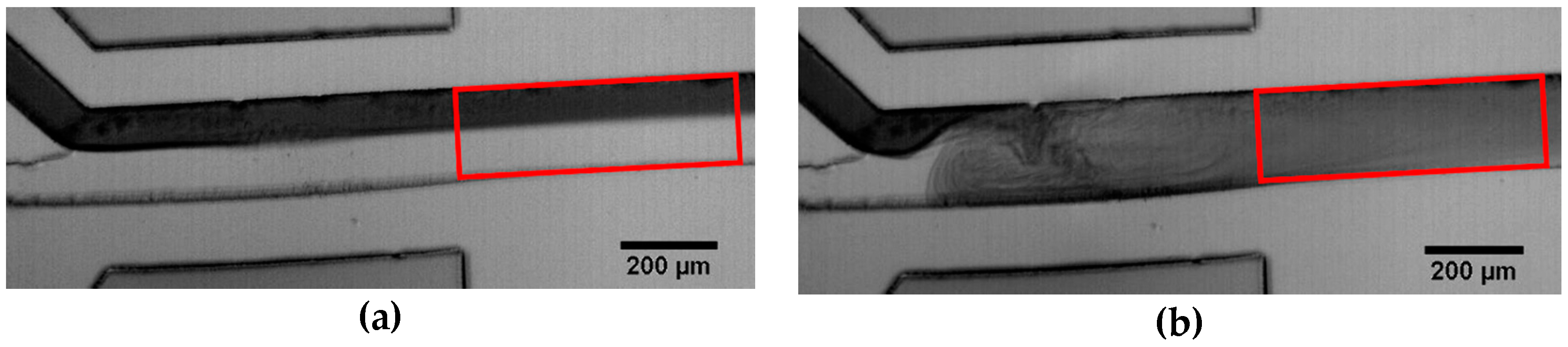
3.1. Mixing Process
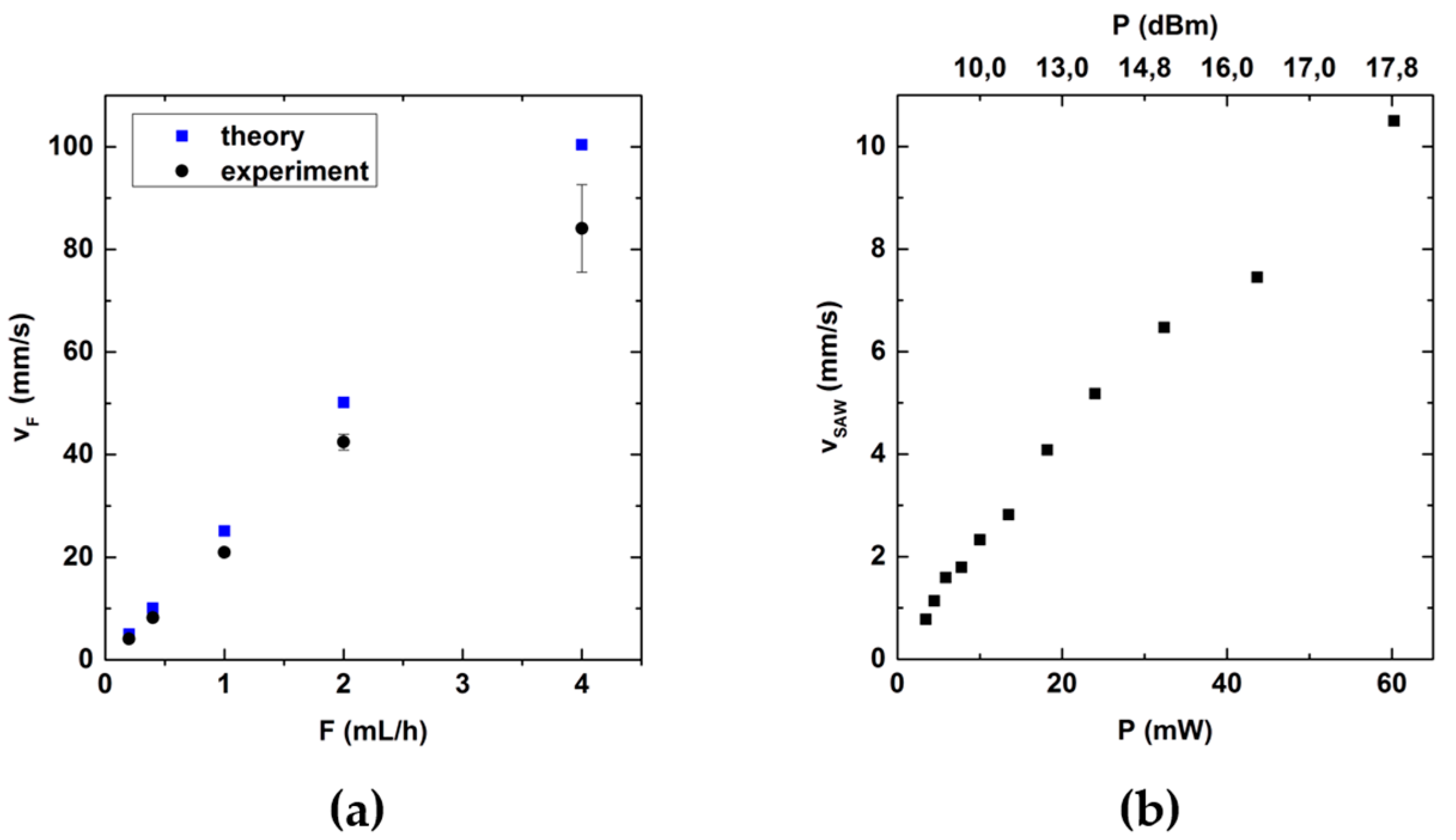
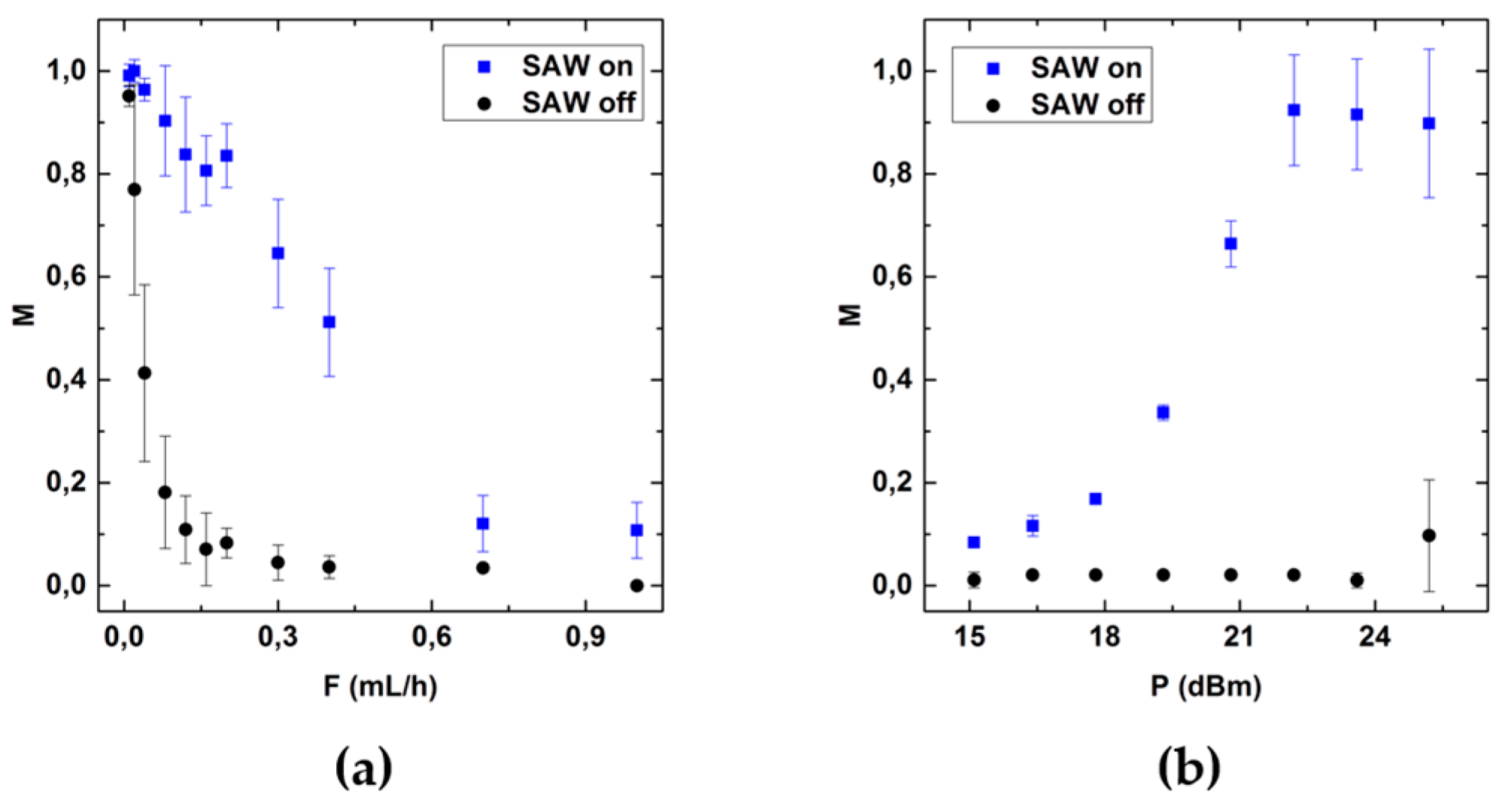
3.2. Role of the Flow Rate and RF-Power
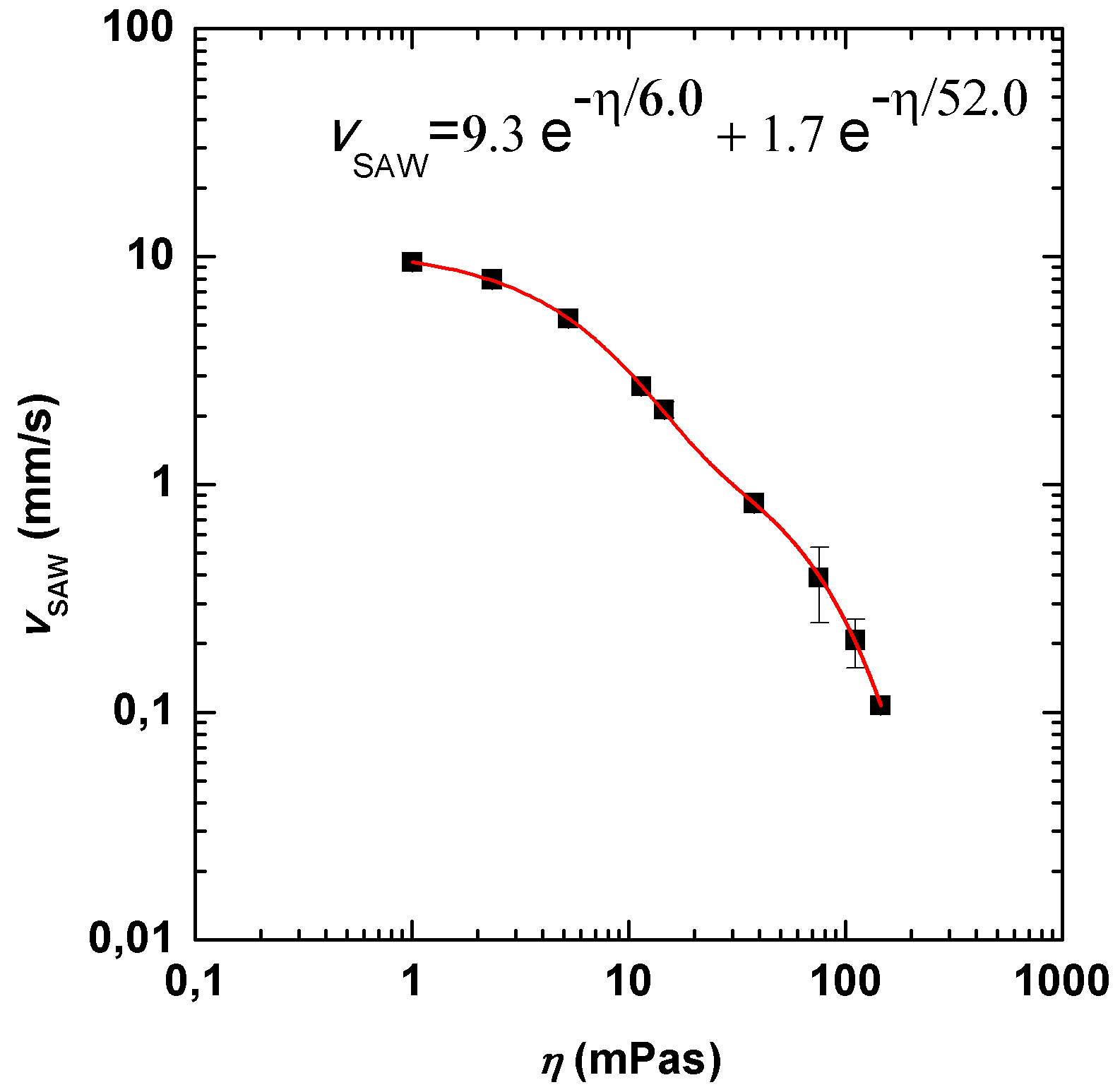
3.3. Role of Viscosity
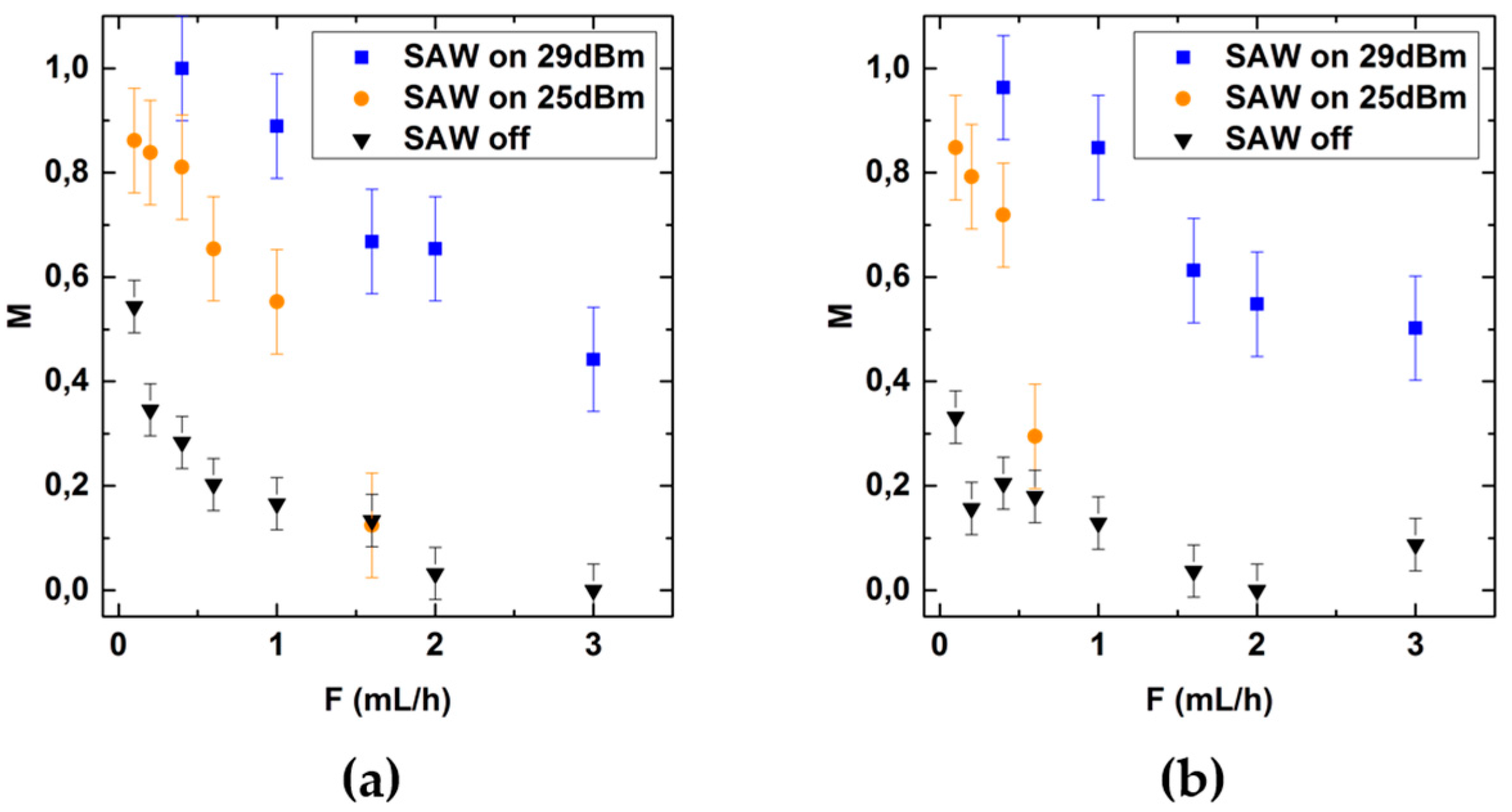
3.4. Mixing Aqueous Solutions with Ethanol and Isopropanol
3.5. Formation and Characterization of Therapeutic Nanoparticles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Nguyen, N.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2004, 15, R1–R16. [Google Scholar] [CrossRef]
- Rudyak, V.; Minakov, A. Modeling and optimization of Y-type micromixers. Micromachines 2014, 5, 886–912. [Google Scholar] [CrossRef]
- Wixforth, A. Acoustically driven planar microfluidics. Superlattices Microstruct. 2003, 33, 389–396. [Google Scholar] [CrossRef]
- Wixforth, A.; Strobl, C.; Gauer, C.; Toegl, A.; Scriba, J.; Guttenberg, Z.V. Acoustic manipulation of small droplets. Anal. Bioanal. Chem. 2004, 379, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L. On the circulation of air observed in Kundt’s tubes, and on some allied acoustical problems. Phil. Trans. R. Soc. A 1884, 175, 1–21. [Google Scholar] [CrossRef]
- Eckart, C. Vortices and streams caused by sound waves. Phys. Rev. 1948, 73, 68–76. [Google Scholar] [CrossRef]
- Nyborg, W.L. Acoustic streaming due to attenuated plane waves. J. Acoust. Soc. Am. 1953, 25, 68–75. [Google Scholar] [CrossRef]
- Guttenberg, Z.V.; Rathgeber, A.; Keller, S.; Rädler, J.O.; Wixforth, A.; Kostur, M.; Schindler, M.; Talkner, P. Flow profiling of a surface-acoustic-wave nanopump. Phys. Rev. E 2004, 70, 056311. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, K.; Strobl, C.J.; Schneider, M.F.; Wixforth, A.; Guttenberg, Z.V. Acoustic mixing at low Reynold’s numbers. Appl. Phys. Lett. 2006, 88, 054102. [Google Scholar] [CrossRef]
- Jo, M.C.; Guldiken, R. Dual surface acoustic wave-based active mixing in a microfluidic channel. Sens. Actuators A Phys. 2013, 196, 1–7. [Google Scholar] [CrossRef]
- Wixforth, A. Acoustically driven programmable microfluidics for biological and chemical applications. J. Assoc. Lab. Autom. 2006, 11, 399–405. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L.Y. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 2011, 83, 647. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.Y.; Friend, J. Surface Acoustic Wave Microfluidics. Annu. Rev. Fluid Mech. 2014, 46, 379–406. [Google Scholar] [CrossRef]
- Frommelt, T.; Kostur, M.; Wenzel-Schäfer, M.; Talkner, P.; Hänggi, P.; Wixforth, A. Microfluidic mixing via acoustically driven chaotic advection. Phys. Rev. Lett. 2008, 100, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Frommelt, T.; Gogel, D.; Kostur, M.; Talkner, P.; Hanggi, P.; Wixforth, A. Flow patterns and transport in Rayleigh surface acoustic wave streaming: Combined finite element method and raytracing numerics versus experiments. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.; Buhler, O. Streaming by leaky surface acoustic waves. Proc. R. Soc. A Math. Phys. Eng. Sci. 2011, 467, 1779–1800. [Google Scholar] [CrossRef]
- Toegl, A.; Kirchner, R.; Gauer, C.; Wixforth, A. Enhancing results of microarray hybridizations through microagitation. J. Biomol. Tech. 2007, 14, 197–204. [Google Scholar]
- Guttenberg, Z.V.; Müller, H.; Habermüller, H.; Geisbauer, A.; Pipper, J.; Felbel, J.; Kielpinski, M.; Scriba, J.; Wixforth, A. Planar chip device for PCR and hybridization with surface acoustic wave pump. Lab Chip 2005, 5, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.W.; Nuschele, S.; Wixforth, A.; Gorzelanny, C.; Alexander-Katz, A.; Netz, R.R.; Schneider, M.F. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA 2007, 104, 7899–7903. [Google Scholar] [CrossRef] [PubMed]
- Steppich, D.; Griesbauer, J.; Frommelt, T.; Appelt, W.; Wixforth, A.; Schneider, M. Thermomechanic-electrical coupling in phospholipid monolayers near the critical point. Phys. Rev. E 2010, 81, 061123. [Google Scholar] [CrossRef] [PubMed]
- Fallah, M.A.; Myles, V.M.; Krüger, T.; Sritharan, K.; Wixforth, A.; Varnik, F.; Schneider, S.W.; Schneider, M.F. Acoustic driven flow and lattice Boltzmann simulations to study cell adhesion in biofunctionalized μ-fluidic channels with complex geometry. Biomicrofluidics 2010, 4, 024106. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Stamp, M.; Kmeth, R.; Buchegger, S.; Stritzker, B.; Saldamli, B.; Burgkart, R.; Schneider, M.F.; Wixforth, A. A novel tool for dynamic cell adhesion studies—The De-Adhesion Number Investigator DANI. Lab Chip 2014, 14, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Stamp, M.E.M.; Brugger, M.S.; Wixforth, A.; Westerhausen, C. Acoustotaxis–in vitro stimulation in a wound healing assay employing surface acoustic waves. Biomater. Sci. 2016, 4, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Friend, J.R.; Yeo, L.Y.; Arifin, D.R.; Mechler, A. Evaporative self-assembly assisted synthesis of polymeric nanoparticles by surface acoustic wave atomization. Nanotechnology 2008, 19, 145301. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.D.; Phan, V.N.; Nguyen, N.T. High-throughput micromixers based on acoustic streaming induced by surface acoustic wave. Microfluid. Nanofluid. 2010, 10, 619–625. [Google Scholar] [CrossRef]
- Tseng, W.; Lin, J.; Sung, W.; Chen, S.; Lee, G. Active micro-mixers using surface acoustic waves on Y-cut 128° LiNbO3. J. Micromech. Microeng. 2006, 16, 539–548. [Google Scholar] [CrossRef]
- Zeng, Q.; Guo, F.; Yao, L.; Zhu, H.W.; Zheng, L.; Guo, Z.X.; Liu, W.; Chen, Y.; Guo, S.S.; Zhao, X.Z. Milliseconds mixing in microfluidic channel using focused surface acoustic wave. Sens. Actuators B. Chem. 2011, 160, 1552–1556. [Google Scholar] [CrossRef]
- Nguyen, J.; Walsh, C.L.; Motion, J.P.M.; Perttu, E.K.; Szoka, F. Controlled Nucleation of Lipid Nanoparticles. Pharm. Res. 2012, 29, 2236–2248. [Google Scholar] [CrossRef] [PubMed]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Lächelt, U.; Wagner, E. Nucleic acid therapeutics using polyplexes: A journey of 50 years (and beyond). Chem. Rev. 2015, 115, 11043–11078. [Google Scholar] [CrossRef] [PubMed]
- Chee, G.K.; Kang, X.; Xie, Y.; Fei, Z.; Guan, J.; Yu, B.; Zhang, X.; Lee, L.J. Delivery of polyethylenimine/DNA complexes assembled in a microfluidics device. Mol. Pharm. 2009, 6, 1333–1342. [Google Scholar]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, 18440–18450. [Google Scholar] [CrossRef] [PubMed]
- Zhigaltsev, I.V.; Tam, Y.K.; Leung, A.K.K.; Cullis, P.R. Production of limit size nanoliposomal systems with potential utility as ultra-small drug delivery agents. J. Liposome Res. 2015, 2104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhigaltsev, I.V.; Belliveau, N.; Hafez, I.; Leung, A.K.K.; Huft, J.; Hansen, C.; Cullis, P.R. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir 2012, 28, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.; Foradada, M.; Estelrich, J. Liposomes obtained by the ethanol injection method. Int. J. Pharm. 1993, 95, 51–56. [Google Scholar] [CrossRef]
- Rudorf, S.; Rädler, J.O. Self-assembly of stable monomolecular nucleic acid lipid particles with a size of 30 nm. J. Am. Chem. Soc. 2012, 134, 11652–11658. [Google Scholar] [CrossRef] [PubMed]
- Streibl, M.; Wixforth, A.; Kotthaus, J.P.; Gorov, A.O.; Kadow, C.; Gossard, A.C. Imaging of carrier dynamics in semiconductor heterostructures by surface acoustic waves. Appl. Phys. Lett. 1999, 75, 4139–4141. [Google Scholar] [CrossRef]
- Shilton, R.J.; Travagliati, M.; Beltram, F.; Cecchini, M. Nanoliter-droplet acoustic streaming via ultra high frequency surface acoustic waves. Adv. Mater. 2014, 26, 4941–4946. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Thielicke, W.; Stamhuis, E.J. PIVlab—Towards User-Friendly, Affordable and Accurate Digital Particle Image Velocimetry in MATLAB. 2014. Available online: http://openresearchsoftware.metajnl.com/articles/10.5334/jors.bl/ (accessed on 18 August 2016).
- Thielicke, W.; Stamhuis, E. PIVlab—Time-Resolved Digital Particle Image Velocimetry Tool for MATLAB. 2014. Available online: https://figshare.com/articles/PIVlab_version_1_35/1092508 (accessed on 18 August 2016).
- Thielicke, W. The Flapping Flight of Birds—Analysis And Application. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2014. [Google Scholar]
- Cheng, N. Formula for the viscosity of a glycerol—Water mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Tabeling, P. Introduction to Microfluidics; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Frommelt, T. Mischen und Sortieren Mit SAW-Fluidik in Simulation und Experiment. Ph.D. Thesis, University of Augsburg, Augsburg, Germany, January 2007. [Google Scholar]
- Riaud, A.; Baudoin, M.; Matar, O.B.; Thomas, J.-L.; Brunet, P. Influence of viscosity on acoustic streaming in sessile droplets: An experimental and a numerical study with a streaming source spatial filtering (SSSF) method. Fluid Dyn. 2016. [Google Scholar]
- Senn, S.; Richardson, W. The first t-test. Stat. Med. 1994, 13, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Aryal, S.; Hu, C.-M.J.; Zhang, L. Quick synthesis of lipid−polymer hybrid nanoparticles with low polydispersity using a single-step sonication method. Langmuir 2010, 26, 16958–16962. [Google Scholar] [CrossRef] [PubMed]


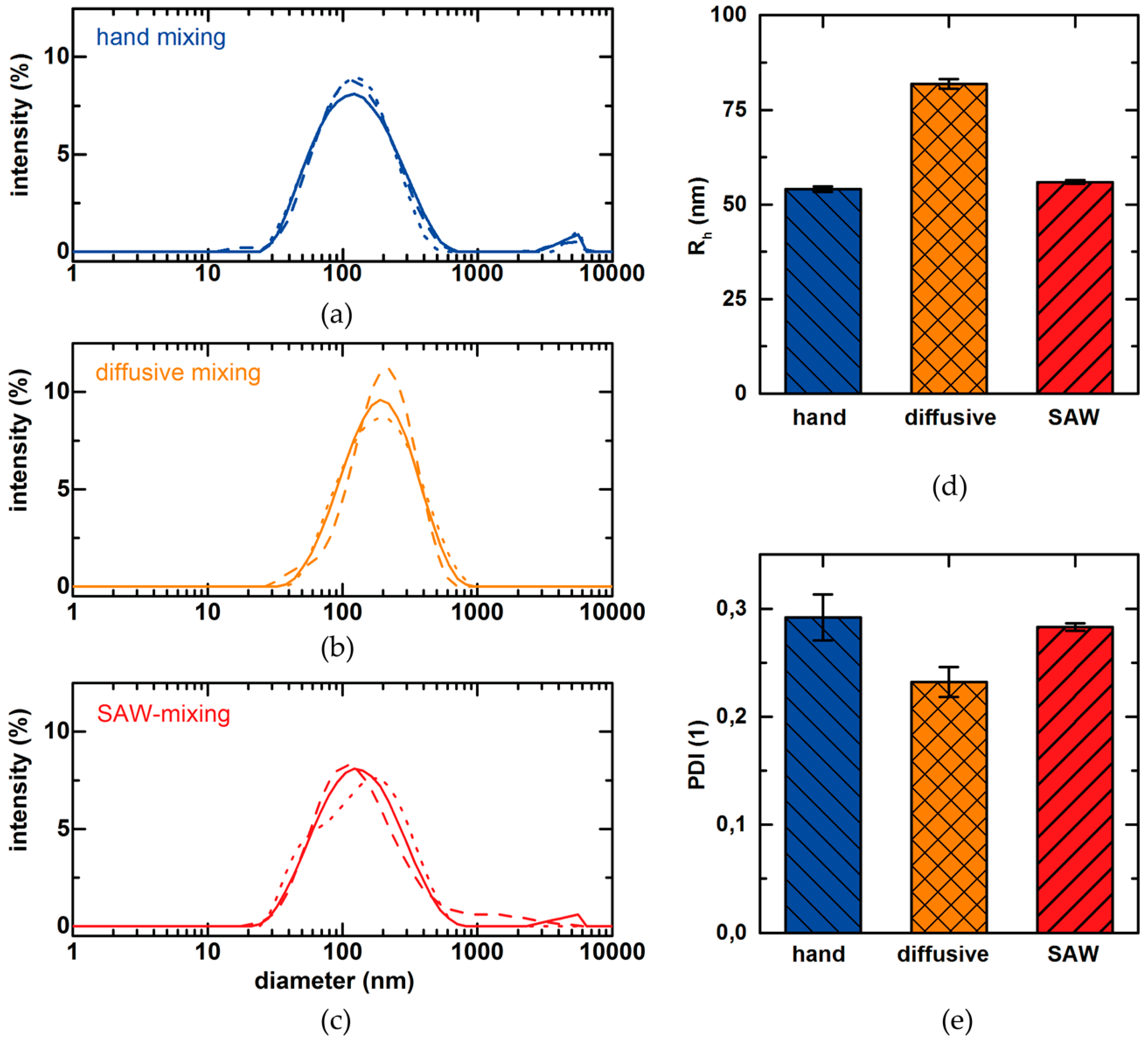
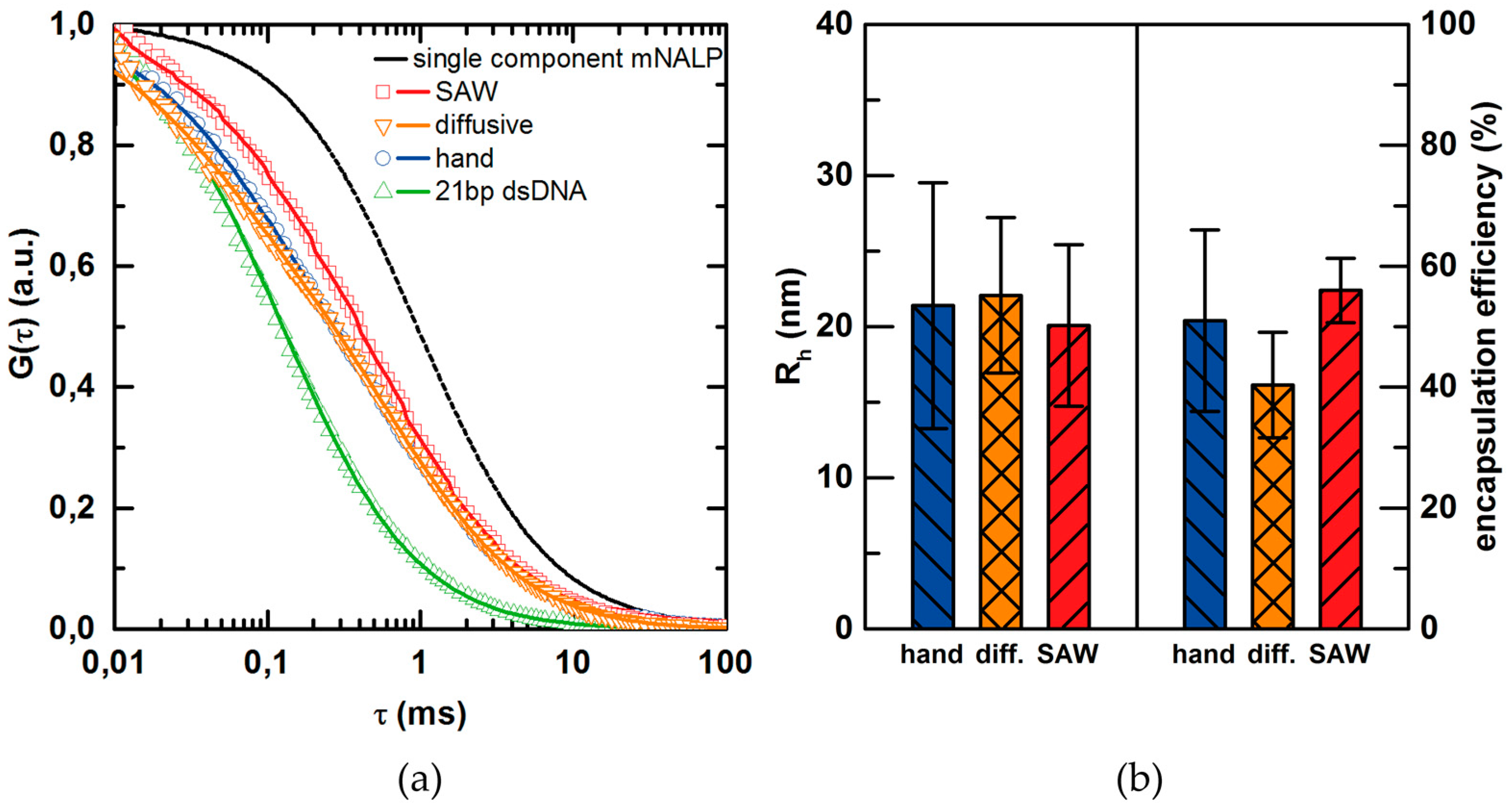
| Student’s t-Test Results | Rh | Encapsulation | Rh | Encapsulation |
|---|---|---|---|---|
| Comparison | SAW vs. hand mixing | SAW vs. hand mixing | SAW vs. diffusive mixing | SAW vs. diffusive mixing |
| t-value | 1.263 | 4.483 | 1.922 | 14.101 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westerhausen, C.; Schnitzler, L.G.; Wendel, D.; Krzysztoń, R.; Lächelt, U.; Wagner, E.; Rädler, J.O.; Wixforth, A. Controllable Acoustic Mixing of Fluids in Microchannels for the Fabrication of Therapeutic Nanoparticles. Micromachines 2016, 7, 150. https://doi.org/10.3390/mi7090150
Westerhausen C, Schnitzler LG, Wendel D, Krzysztoń R, Lächelt U, Wagner E, Rädler JO, Wixforth A. Controllable Acoustic Mixing of Fluids in Microchannels for the Fabrication of Therapeutic Nanoparticles. Micromachines. 2016; 7(9):150. https://doi.org/10.3390/mi7090150
Chicago/Turabian StyleWesterhausen, Christoph, Lukas G. Schnitzler, Dominik Wendel, Rafał Krzysztoń, Ulrich Lächelt, Ernst Wagner, Joachim O. Rädler, and Achim Wixforth. 2016. "Controllable Acoustic Mixing of Fluids in Microchannels for the Fabrication of Therapeutic Nanoparticles" Micromachines 7, no. 9: 150. https://doi.org/10.3390/mi7090150