Microfluidic Approaches for Manipulating, Imaging, and Screening C. elegans
Abstract
:1. Introduction
2. Microfluidic Devices to Assist with Routine Procedures
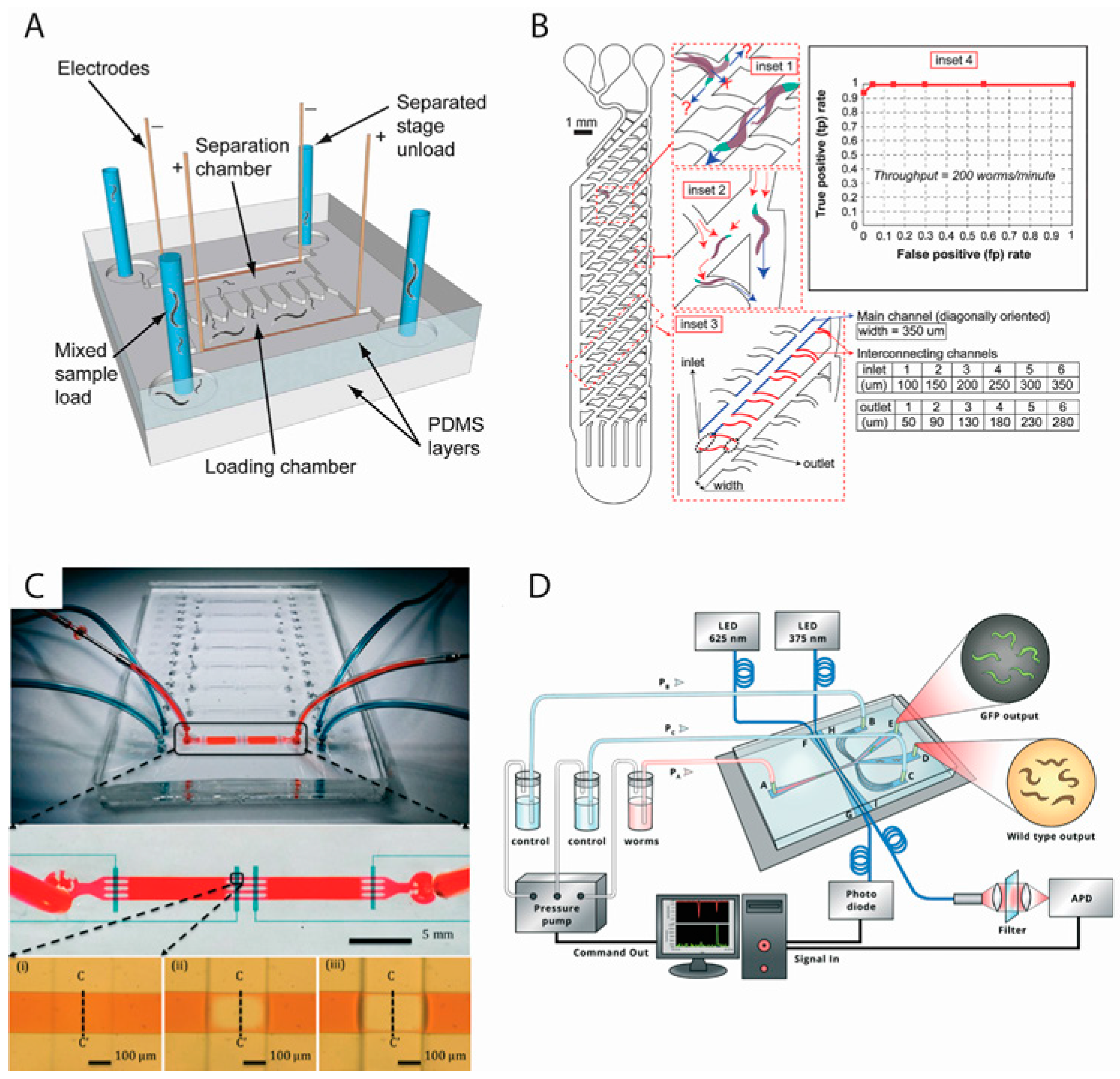
2.1. Worm Sorting
2.2. Worm Culturing
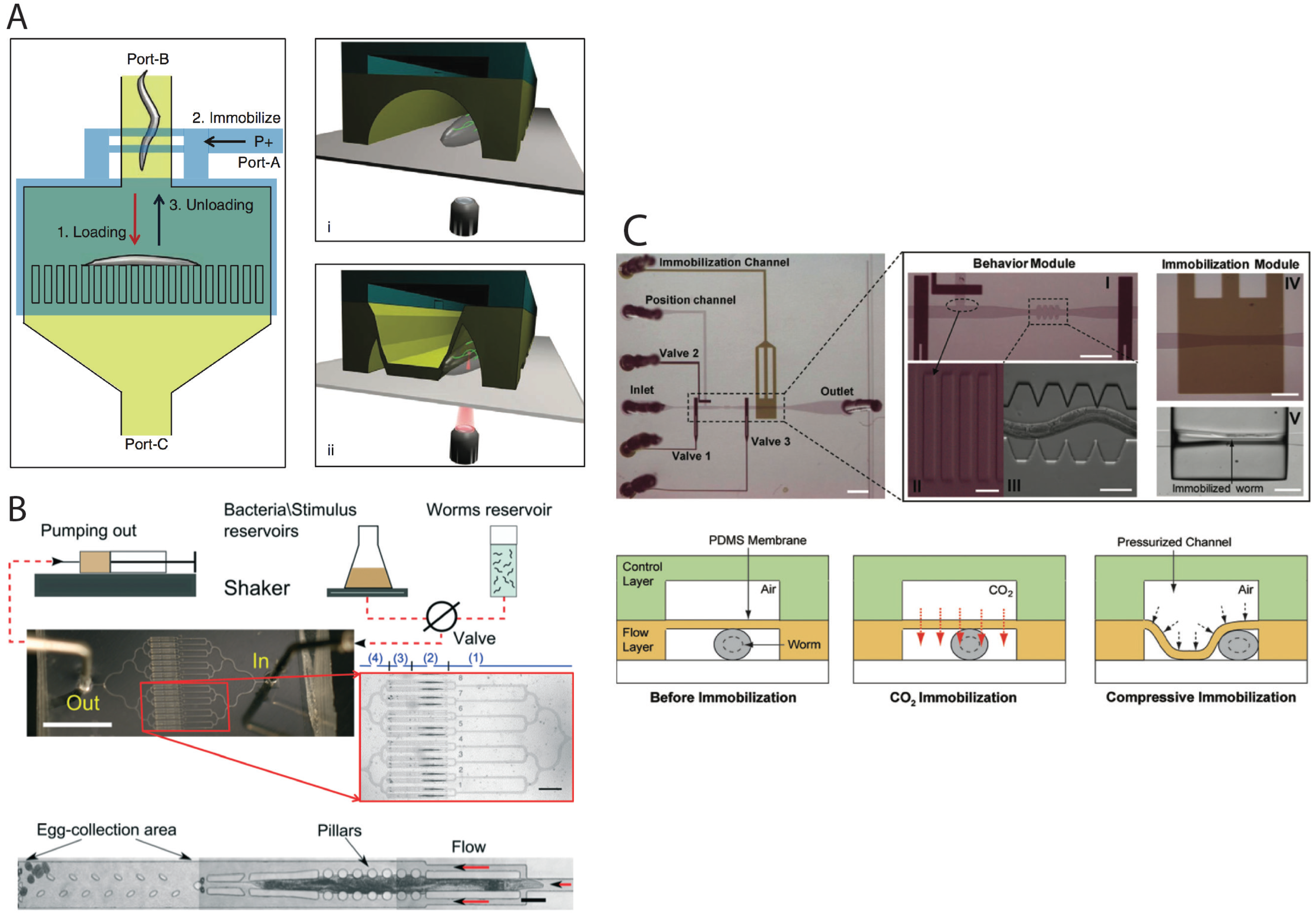
2.3. Worm Immobilization Methods for Developmental, Morphological, and Physiological Studies
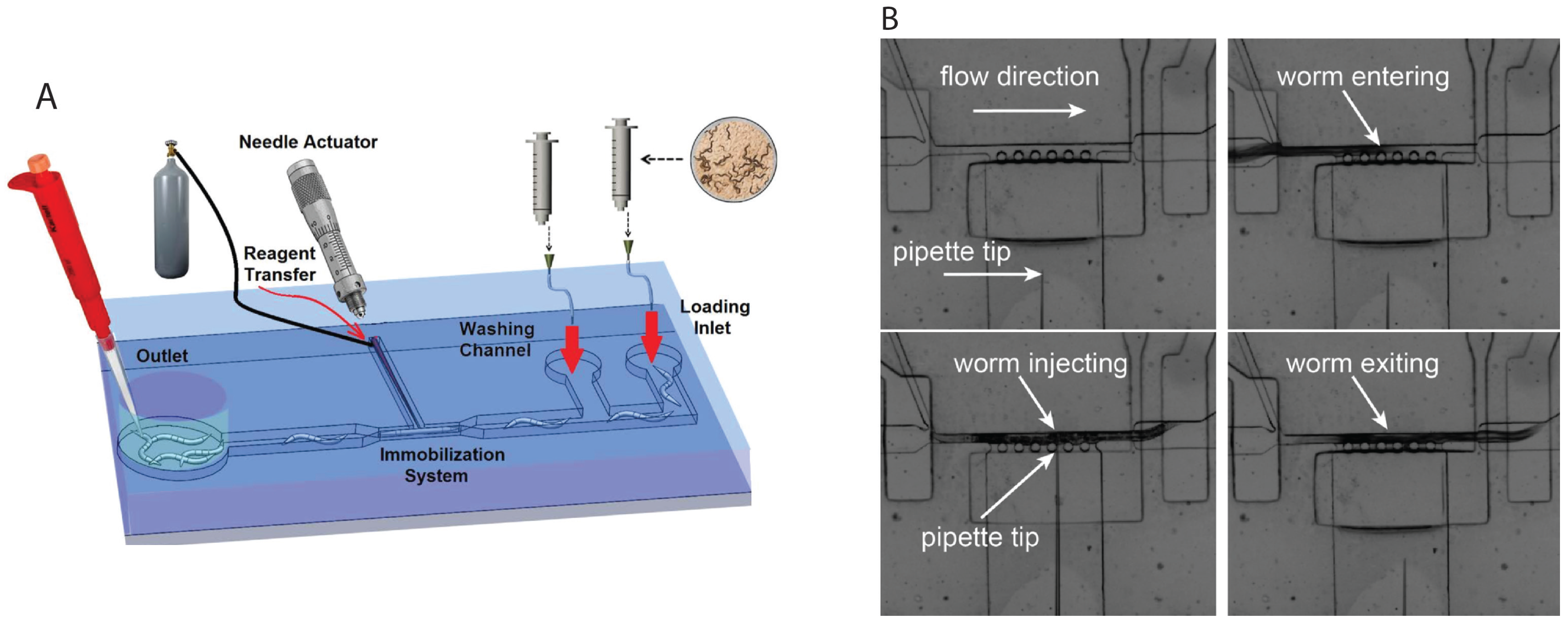
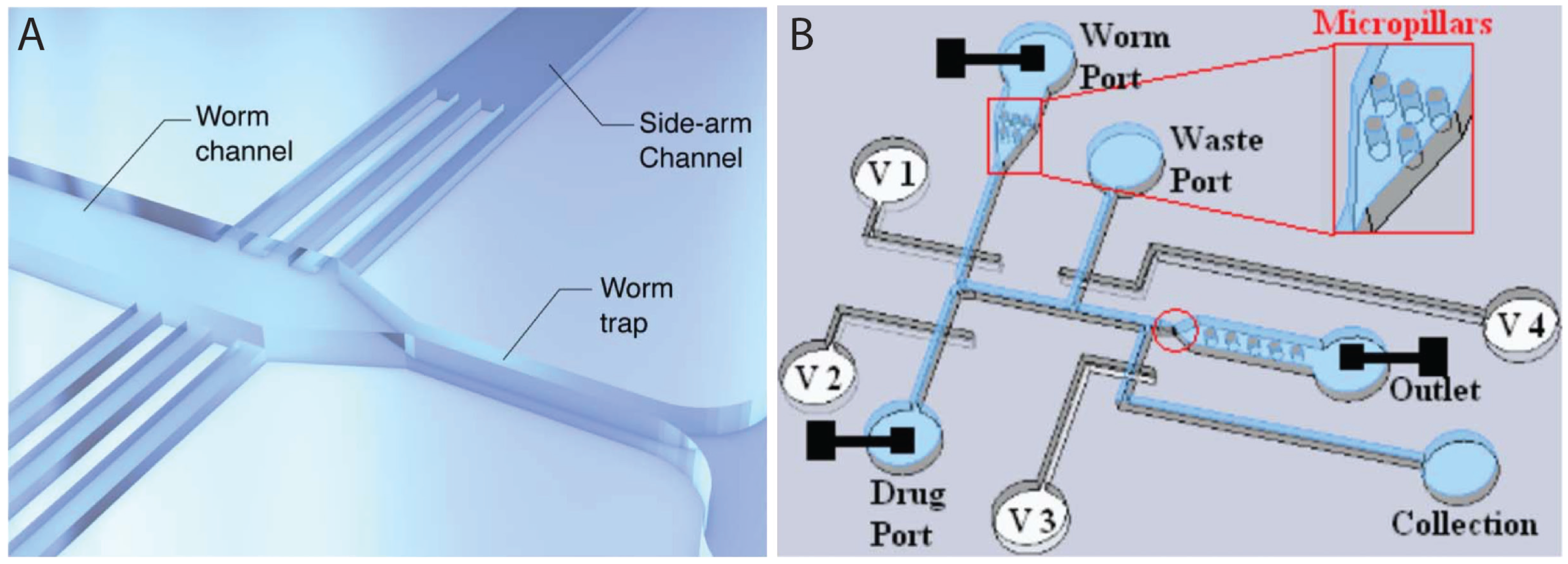
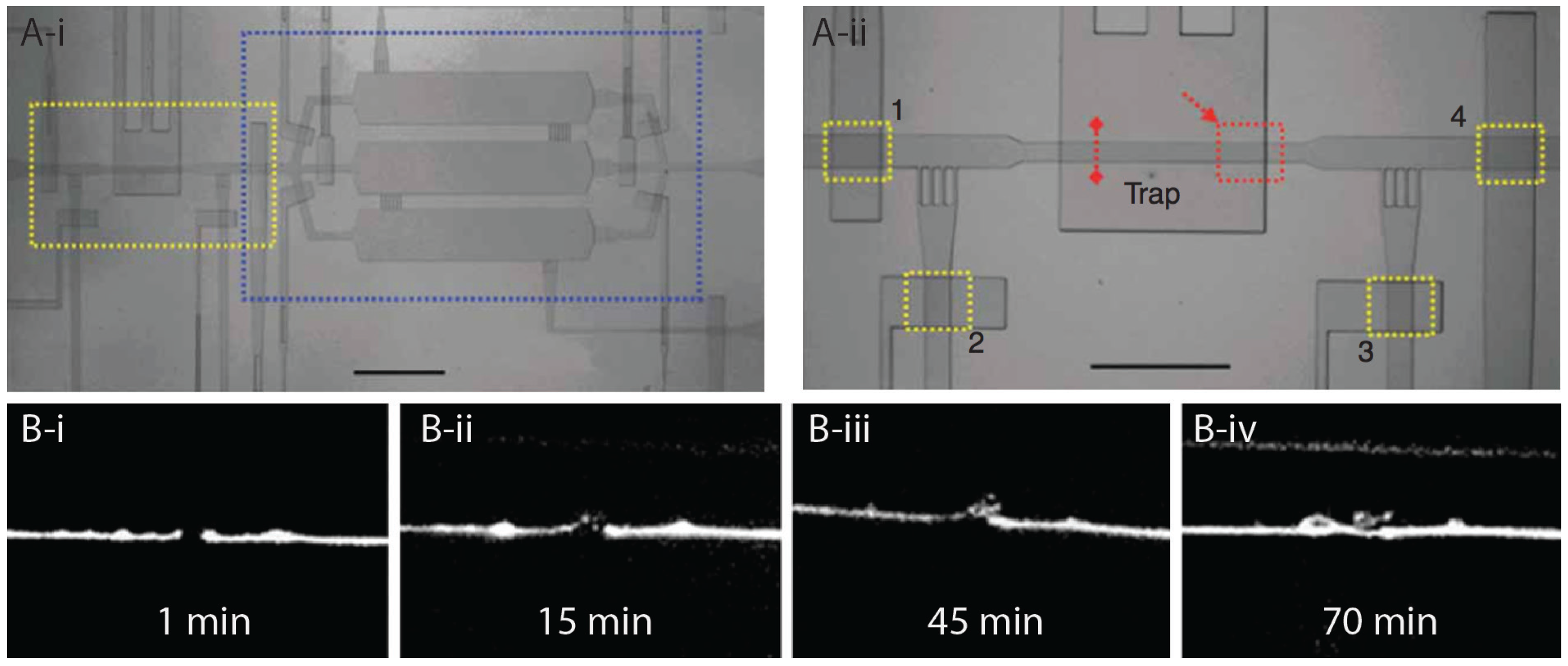
2.4. Microinjection
3. Microfluidic Devices to Assist with Specialized Assays
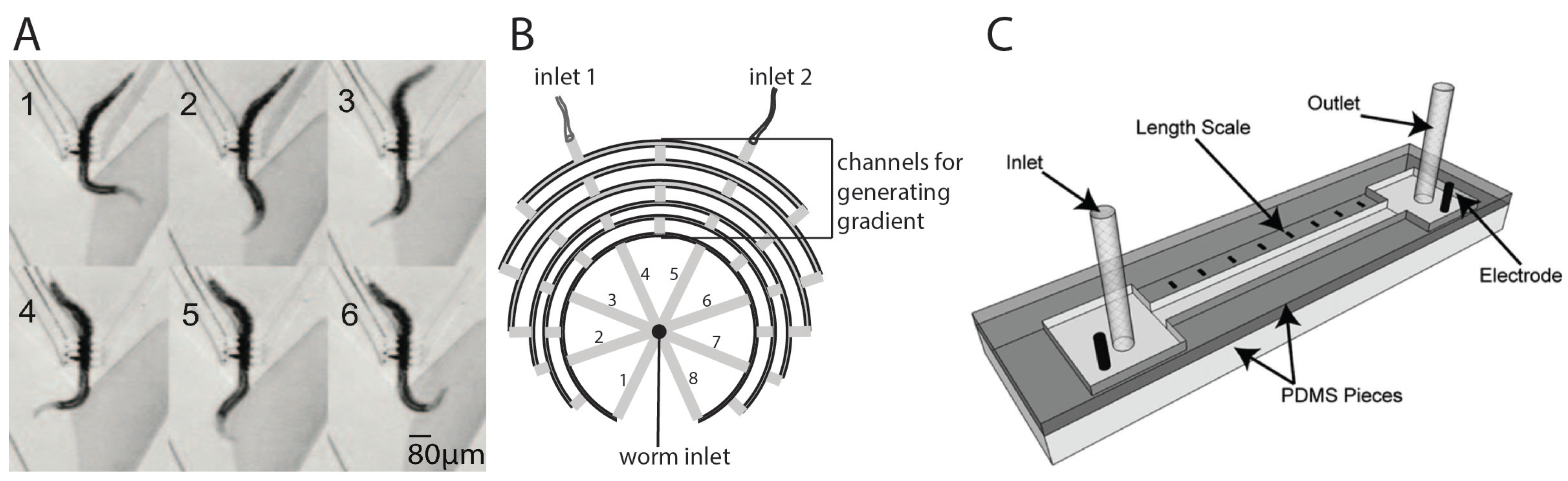
3.1. Study of Movement Responses in Microstructured Environments
3.2. Study of Cellular Processes
3.3. Study of Chemotaxis, Electrotaxis, and Other Behaviors
3.4. Antimicrobial and Toxicological Studies
3.5. Learning and Memory Studies
3.6. Reproductive Aging and Lifespan Studies
4. Microfluidic Devices to Perform Fast, Automated, and High-Throughput Screening
4.1. Rapid and High-Throughput Screening
4.2. Drug Delivery and Behavioral Screening
4.3. Microsurgery, Regeneration, and Drug Screening
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Markaki, M.; Tavernarakis, N. Modeling human diseases in Caenorhabditis elegans. Biotechnol. J. 2010, 5, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.K. A biochemist’s guide to Caenorhabditis elegans. Anal. Biochem. 2006, 359, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Emmons, S.W. The beginning of connectomics: A commentary on White et al.(1986)‘The structure of the nervous system of the nematode Caenorhabditis elegans’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 2014039. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 314, 1–340. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.A.; Luke, C.J.; Bhatia, S.R.; Long, O.S.; Vetica, A.C.; Perlmutter, D.H.; Pak, S.C. Modeling molecular and cellular aspects of human disease using the nematode Caenorhabditis elegans. Pediatr. Res. 2009, 65, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef] [PubMed]
- Spiller, D.G.; Wood, C.D.; Rand, D.A.; White, M.R. Measurement of single-cell dynamics. Nature 2010, 465, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Rezai, P.; Siddiqui, A.; Selvaganapathy, P.R.; Gupta, B.P. Electrotaxis of Caenorhabditis elegans in a microfluidic environment. Lab Chip 2010, 10, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Rezai, P.; Salam, S.; Selvaganapathy, P.R.; Gupta, B.P. Electrical sorting of Caenorhabditis elegans. Lab Chip 2012, 12, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Maniere, X.; Lebois, F.; Matic, I.; Ladoux, B.; Di Meglio, J.M.; Hersen, P. Running worms: C. elegans self-sorting by electrotaxis. PLoS ONE 2011, 6, e16637. [Google Scholar] [CrossRef] [PubMed]
- Solvas, X.C.i.; Geier, F.M.; Leroi, A.M.; Bundy, J.G.; Edel, J.B.; DeMello, A.J. High-throughput age synchronisation of Caenorhabditis elegans. Chem. Commun. 2011, 47, 9801–9803. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Kim, D.; Ko, U.H.; Shin, J.H. A sorting strategy for C. elegans based on size-dependent motility and electrotaxis in a micro-structured channel. Lab Chip 2012, 12, 4128–4134. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Zhuo, W.; Liang, Q.; McGrath, P.T.; Lu, H. A high-throughput device for size based separation of C. elegans developmental stages. Lab Chip 2014, 14, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ng, L.F.; Ng, L.T.; Choi, K.B.; Gruber, J.; Bettiol, A.A.; Thakor, N.V. A continuous-flow C. elegans sorting system with integrated optical fiber detection and laminar flow switching. Lab Chip 2014, 14, 4000–4006. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, R.; Ge, A.; Hu, L.; Wang, S.; Feng, X.; Du, W.; Liu, B.F. Highly efficient microfluidic sorting device for synchronizing developmental stages of C. elegans based on deflecting electrotaxis. Lab Chip 2015, 15, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Cornaglia, M.; Lehnert, T.; Gijs, M.A. Versatile size-dependent sorting of C. elegans nematodes and embryos using a tunable microfluidic filter structure. Lab Chip 2016, 16, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Gabel, C.V.; Gabel, H.; Pavlichin, D.; Kao, A.; Clark, D.A.; Samuel, A.D. Neural circuits mediate electrosensory behavior in Caenorhabditis elegans. J. Neurosci. 2007, 27, 7586–7596. [Google Scholar] [CrossRef] [PubMed]
- Aubry, G.; Zhan, M.; Lu, H. Hydrogel-droplet microfluidic platform for high-resolution imaging and sorting of early larval Caenorhabditis elegans. Lab Chip 2015, 15, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Crane, M.M.; Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 2008, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Rohde, C.B.; Zeng, F.; Gonzalez-Rubio, R.; Angel, M.; Yanik, M.F. Microfluidic system for on-chip high-throughput whole-animal sorting and screening at subcellular resolution. Proc. Natl. Acad. Sci. USA 2007, 104, 13891–13895. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jiang, L.; Shi, W.; Qin, J.; Lin, B. A programmable microvalve-based microfluidic array for characterization of neurotoxin-induced responses of individual C. elegans. Biomicrofluidics 2009, 3, 44114. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Shevkoplyas, S.S.; McGuigan, A.P.; Apfeld, J.; Fontana, W.; Whitesides, G.M. Lifespan-on-a-chip: Microfluidic chambers for performing lifelong observation of C. elegans. Lab Chip 2010, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Shi, W.; Qin, J. Multiparameter evaluation of the longevity in C. elegans under stress using an integrated microfluidic device. Biomed. Microdevices 2012, 14, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Belfer, S.J.; Chuang, H.S.; Freedman, B.L.; Yuan, J.; Norton, M.; Bau, H.H.; Raizen, D.M. Caenorhabditis-in-drop array for monitoring C. elegans quiescent behavior. Sleep 2013, 36, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, E.; Tavernarakis, N. High-throughput and longitudinal analysis of aging and senescent decline in Caenorhabditis elegans. Methods Mol. Biol. 2013, 965, 485–500. [Google Scholar] [PubMed]
- Nghe, P.; Boulineau, S.; Gude, S.; Recouvreux, P.; van Zon, J.S.; Tans, S.J. Microfabricated polyacrylamide devices for the controlled culture of growing cells and developing organisms. PLoS ONE 2013, 8, e75537. [Google Scholar]
- Turek, M.; Besseling, J.; Bringmann, H. Agarose Microchambers for Long-term Calcium Imaging of Caenorhabditis elegans. J. Vis. Exp. 2015, 100, 52742. [Google Scholar] [CrossRef] [PubMed]
- Krajniak, J.; Lu, H. Long-term high-resolution imaging and culture of C. elegans in chip-gel hybrid microfluidic device for developmental studies. Lab Chip 2010, 10, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Yu, Y.; Zhu, G.; Jiang, L.; Qin, J. A droplet microchip with substance exchange capability for the developmental study of C. elegans. Lab Chip 2015, 15, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Cornaglia, M.; Krishnamani, G.; Mouchiroud, L.; Sorrentino, V.; Lehnert, T.; Auwerx, J.; Gijs, M.A. Automated longitudinal monitoring of in vivo protein aggregation in neurodegenerative disease C. elegans models. Mol. Neurodegener. 2016, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Dempsey, C.M.; Zoval, J.V.; Sze, J.Y.; Madou, M.J. Automated microfluidic compact disc (CD) cultivation system of Caenorhabditis elegans. Sens. Actuators B 2007, 122, 511–518. [Google Scholar] [CrossRef]
- Gilleland, C.L.; Rohde, C.B.; Zeng, F.; Yanik, M.F. Microfluidic immobilization of physiologically active Caenorhabditis elegans. Nat. Protoc. 2010, 5, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Kopito, R.B.; Levine, E. Durable spatiotemporal surveillance of Caenorhabditis elegans response to environmental cues. Lab Chip 2014, 14, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, T.V.; Ben-Yakar, A.; Chronis, N. CO2 and compressive immobilization of C. elegans on-chip. Lab Chip 2009, 9, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ranawade, A.; Gupta, B.P.; Selvaganapathy, P.R. A microfluidic device for C. elegans immobilization and long term imaging. In preparation.
- Mondal, S.; Ahlawat, S.; Rau, K.; Venkataraman, V.; Koushika, S.P. Imaging in vivo neuronal transport in genetic model organisms using microfluidic devices. Traffic 2011, 12, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.S.; Chen, H.Y.; Chen, C.S.; Chiu, W.T. Immobilization of the nematode Caenorhabditis elegans with addressable light-induced heat knockdown (ALINK). Lab Chip 2013, 13, 2980–2989. [Google Scholar] [CrossRef] [PubMed]
- Cornaglia, M.; Mouchiroud, L.; Marette, A.; Narasimhan, S.; Lehnert, T.; Jovaisaite, V.; Auwerx, J.; Gijs, M.A. An automated microfluidic platform for C. elegans embryo arraying, phenotyping, and long-term live imaging. Sci. Rep. 2015, 5, 10192. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, R.; Tong, J.; Selvaganapathy, P.R.; Gupta, B.P. Microfluidic device for microinjection of Caenorhabditis elegans. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Freiburg, Germany, 27–31 October 2013; pp. 1821–1823.
- Ghaemi, R. Microfluidic device for microinjection of Caenorhabditis elegans. Available online: https://macsphere.mcmaster.ca/handle/11375/15339 (accessed on 18 July 2016).
- Zhao, X.; Xu, F.; Tang, L.; Du, W.; Feng, X.; Liu, B.F. Microfluidic chip-based C. elegans microinjection system for investigating cell-cell communication in vivo. Biosens. Bioelectron. 2013, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Dong, X.; Liu, X. A microfluidic device for automated, high-speed microinjection of Caenorhabditis elegans. Biomicrofluidics 2016, 10, 011912. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hwang, H.; Nam, S.W.; Martinez, F.; Austin, R.H.; Ryu, W.S. Enhanced Caenorhabditis elegans locomotion in a structured microfluidic environment. PLoS ONE 2008, 3, e2550. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A.; Lycke, R.; Carr, J.A.; Pandey, S. Amplitude-modulated sinusoidal microchannels for observing adaptability in C. elegans locomotion. Biomicrofluidics 2011, 5, 24112. [Google Scholar] [CrossRef] [PubMed]
- Johari, S.; Nock, V.; Alkaisi, M.M.; Wang, W. On-chip analysis of C. elegans muscular forces and locomotion patterns in microstructured environments. Lab Chip 2013, 13, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Tu, L.; Huang, L.; Zhu, T.; Nock, V.; Yu, E.; Liu, X.; Wang, W. An integrated platform enabling optogenetic illumination of Caenorhabditis elegans neurons and muscular force measurement in microstructured environments. Biomicrofluidics 2015, 9, 014123. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Barnes, D.E.; Matsunaga, Y.; Benian, G.M.; Ono, S.; Lu, H. Muscle contraction phenotypic analysis enabled by optogenetics reveals functional relationships of sarcomere components in Caenorhabditis elegans. Sci. Rep. 2016, 6, 19900. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.B.; Sgro, A.E.; Chao, D.L.; Doepker, B.E.; Scott Edgar, J.; Shen, K.; Chiu, D.T. Single-synapse ablation and long-term imaging in live C. elegans. J. Neurosci. Methods 2008, 173, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.A.; Coakley, S.; Mugno, P.; Hammarlund, M.; Hilliard, M.A.; Lu, H. A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans. Lab Chip 2014, 14, 4513–4522. [Google Scholar] [CrossRef] [PubMed]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Larsch, J.; Ventimiglia, D.; Bargmann, C.I.; Albrecht, D.R. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2013, 110, E4266–E4273. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, A. Fluorescence imaging of physiological activity in complex systems using GFP-based probes. Curr. Opin. Neurobiol. 2003, 13, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ardeshiri, R.; Murphy, B.; Zhen, M.; Rezai, P. A Hybrid Microfluidic Device for C. elegans On-Demand Orientation and Multidirectional Imaging. In Proceedings of the International Conference on Miniaturized Systems for Chemistry and Life Sciences, Gyengju, Korea, 25–29 October 2015; pp. 707–709.
- Ahmed, D.; Ozcelik, A.; Bojanala, N.; Nama, N.; Upadhyay, A.; Chen, Y.; Hanna-Rose, W.; Huang, T.J. Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 2016, 7, 11085. [Google Scholar] [CrossRef] [PubMed]
- Chronis, N.; Zimmer, M.; Bargmann, C.I. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat. Methods 2007, 4, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, S.H.; Chronis, N.; Tsunozaki, M.; Gray, J.M.; Ramot, D.; Goodman, M.B.; Bargmann, C.I. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 2007, 450, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.R.; Bargmann, C.I. High-content behavioral analysis of Caenorhabditis elegans in precise spatiotemporal chemical environments. Nat. Methods 2011, 8, 599–605. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.E.; Gaertner, B.E.; Sottile, M.; Phillips, P.C.; Lockery, S.R. Microfluidic devices for analysis of spatial orientation behaviors in semi-restrained Caenorhabditis elegans. PLoS ONE 2011, 6, e25710. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, J.; Tan, H.; Ge, A.; Tang, L.; Feng, X.; Du, W.; Liu, B.F. Quantitative analysis of Caenorhabditis elegans chemotaxis using a microfluidic device. Anal. Chim. Acta 2015, 887, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.J.; Feng, X.J.; Du, W.; Liu, B.F. Microfluidic device for analysis of gas-evoked neuronal sensing in C. elegans. Sens. Actuators B Chem. 2015, 209, 109–115. [Google Scholar] [CrossRef]
- Vidal-Gadea, A.; Ward, K.; Beron, C.; Ghorashian, N.; Gokce, S.; Russell, J.; Truong, N.; Parikh, A.; Gadea, O.; Ben-Yakar, A.; et al. Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. Elife 2015, 4, e07493. [Google Scholar] [CrossRef] [PubMed]
- Sukul, N.C.; Croll, N.A. Influence of Potential Difference and Current on the Electrotaxis of Caenorhaditis elegans. J. Nematol. 1978, 10, 314–317. [Google Scholar] [PubMed]
- Caveness, F.E.; Panzer, J.D. Nemic galvanotaxis. Proc. Helminthol. Soc. Wash. 1960, 27, 73–74. [Google Scholar]
- Gupta, S.P. Galvanotactic reaction of infective larvae of Trichostrongylus retortaeformis. Exp. Parasitol. 1962, 12, 118–119. [Google Scholar] [CrossRef]
- Rezai, P.; Salam, S.; Selvaganapathy, P.R.; Gupta, B.P. Microfluidic systems to study the biology of human diseases and identify potential therapeutic targets in Caenorhabditis elegans. In Integrated Microsystems; Iniewski, K., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 581–608. [Google Scholar]
- Rezai, P.; Salam, S.; Selvaganapathy, P.R.; Gupta, B.P. Effect of pulse direct current signals on electrotactic movement of nematodes Caenorhabditis elegans and Caenorhabditis briggsae. Biomicrofluidics 2011, 5, 044116. [Google Scholar] [CrossRef] [PubMed]
- Rezai, P.; Siddiqui, A.; Selvaganapathy, P.R.; Gupta, B.P. Behavior of Caenorhabditis elegans in alternating electric field and its application to their localization and control. Appl. Phys. Lett. 2010, 96, 153702. [Google Scholar] [CrossRef]
- Liu, D.; Gupta, B.; Selvaganapathy, P.R. An automated microfluidic system for screening Caenorhabditis elegans behaviors using electrotaxis. Biomicrofluidics 2016, 10, 014117. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Hagerbaumer, A.; Hoss, S.; Heininger, P.; Traunspurger, W. Experimental studies with nematodes in ecotoxicology: An overview. J. Nematol. 2015, 47, 11–27. [Google Scholar] [PubMed]
- Jung, J.; Nakajima, M.; Kojima, M.; Ooe, K.; Fukuda, T. Microchip device for measurement of body volume of C. elegans as bioindicator application. J. Micro Nano Mechatron. 2011, 7, 3–11. [Google Scholar] [CrossRef]
- Jung, J.; Nakajima, M.; Tajima, H.; Huang, Q.; Fukuda, T. A microfluidic device for the continuous culture and analysis of Caenorhabditis elegans in a toxic aqueous environment. J. Micromech. Microeng. 2013, 23, 1–8. [Google Scholar] [CrossRef]
- Salam, S.; Ansari, A.; Amon, S.; Rezai, P.; Selvaganapathy, P.R.; Mishra, R.K.; Gupta, B.P. A microfluidic phenotype analysis system reveals function of sensory and dopaminergic neuron signaling in C. elegans electrotactic swimming behavior. Worm 2013, 2, e24558. [Google Scholar] [CrossRef] [PubMed]
- Salam, S.; Gupta, B.P. Unpublished observations.
- Zhang, B.; Li, Y.; He, Q.; Qin, J.; Yu, Y.; Li, X.; Zhang, L.; Yao, M.; Liu, J.; Chen, Z. Microfluidic platform integrated with worm-counting setup for assessing manganese toxicity. Biomicrofluidics 2014, 8, 054110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Ching, P.; Shi, Q.; Li, X. An integrated microfluidic platform for evaluating in vivo antimicrobial activity of natural compounds using a whole-animal infection model. Lab Chip 2013, 13, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Saeki, S.; Yamamoto, M.; Iino, Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J. Exp. Biol. 2001, 204, 1757–1764. [Google Scholar] [PubMed]
- Wen, J.Y.; Kumar, N.; Morrison, G.; Rambaldini, G.; Runciman, S.; Rousseau, J.; van der Kooy, D. Mutations that prevent associative learning in C. elegans. Behav. Neurosci. 1997, 111, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, H.; Bargmann, C.I. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005, 438, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wheeler, A.R. Maze exploration and learning in C. elegans. Lab Chip 2007, 7, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Xian, B.; Shen, J.; Chen, W.; Sun, N.; Qiao, N.; Jiang, D.; Yu, T.; Men, Y.; Han, Z.; Pang, Y.; et al. WormFarm: A quantitative control and measurement device toward automated Caenorhabditis elegans aging analysis. Aging Cell 2013, 12, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Stone, H.A.; Murphy, C.T. A microfluidic device and automatic counting system for the study of C. elegans reproductive aging. Lab Chip 2015, 15, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.S.; Kuo, W.J.; Lee, C.L.; Chu, I.H.; Chen, C.S. Exercise in an electrotactic flow chamber ameliorates age-related degeneration in Caenorhabditis elegans. Sci. Rep. 2016, 6, 28064. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Whitesides, G.M. Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew. Chem. Int. Ed. Engl. 2011, 50, 4774–4807. [Google Scholar] [CrossRef] [PubMed]
- Ghorashian, N.; Gokce, S.K.; Guo, S.X.; Everett, W.N.; Ben-Yakar, A. An automated microfluidic multiplexer for fast delivery of C. elegans populations from multiwells. PLoS ONE 2013, 8, e74480. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.M.; Chung, K.; Lu, H. Computer-enhanced high-throughput genetic screens of C. elegans in a microfluidic system. Lab Chip 2009, 9, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.M.; Stirman, J.N.; Ou, C.Y.; Kurshan, P.T.; Rehg, J.M.; Shen, K.; Lu, H. Autonomous screening of C. elegans identifies genes implicated in synaptogenesis. Nat. Methods 2012, 9, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Caceres Ide, C.; Valmas, N.; Hilliard, M.A.; Lu, H. Laterally orienting C. elegans using geometry at microscale for high-throughput visual screens in neurodegeneration and neuronal development studies. PLoS ONE 2012, 7, e35037. [Google Scholar]
- Yan, Y.; Boey, D.; Ng, L.T.; Gruber, J.; Bettiol, A.; Thakor, N.V.; Chen, C.H. Continuous-flow C. elegans fluorescence expression analysis with real-time image processing through microfluidics. Biosens. Bioelectron. 2016, 77, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, T.V.; Bazopoulou, D.; Chronis, N. An automated microfluidic platform for calcium imaging of chemosensory neurons in Caenorhabditis elegans. Lab Chip 2010, 10, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Lockery, S.R.; Hulme, S.E.; Roberts, W.M.; Robinson, K.J.; Laromaine, A.; Lindsay, T.H.; Whitesides, G.M.; Weeks, J.C. A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans. Lab Chip 2012, 12, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Raizen, D.; Song, B.M.; Trojanowski, N.; You, Y.J. Methods for Measuring Pharyngeal Behaviors. Available online: http://www.ncbi.nlm.nih.gov/books/NBK126648/ (accessed on 13 July 2016).
- Hu, C.; Dillon, J.; Kearn, J.; Murray, C.; O’Connor, V.; Holden-Dye, L.; Morgan, H. NeuroChip: A microfluidic electrophysiological device for genetic and chemical biology screening of Caenorhabditis elegans adult and larvae. PLoS ONE 2013, 8, e64297. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Zhan, M.; Srinivasan, J.; Sternberg, P.W.; Gong, E.; Schroeder, F.C.; Lu, H. Microfluidic chamber arrays for whole-organism behavior-based chemical screening. Lab Chip 2011, 11, 3689–3697. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Parashar, A.; Gibson, R.; Robertson, A.P.; Martin, R.J.; Pandey, S. A microfluidic platform for high-sensitivity, real-time drug screening on C. elegans and parasitic nematodes. Lab Chip 2011, 11, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Bourgeois, F.; Chokshi, T.; Durr, N.J.; Hilliard, M.A.; Chronis, N.; Ben-Yakar, A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat. Methods 2008, 5, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Rohde, C.B.; Yanik, M.F. Sub-cellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond-laser manipulation. Lab Chip 2008, 8, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Lu, H. Automated high-throughput cell microsurgery on-chip. Lab Chip 2009, 9, 2764–2766. [Google Scholar] [CrossRef] [PubMed]
- Samara, C.; Rohde, C.B.; Gilleland, C.L.; Norton, S.; Haggarty, S.J.; Yanik, M.F. Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 18342–18347. [Google Scholar] [CrossRef] [PubMed]
- Gokce, S.K.; Guo, S.X.; Ghorashian, N.; Everett, W.N.; Jarrell, T.; Kottek, A.; Bovik, A.C.; Ben-Yakar, A. A fully automated microfluidic femtosecond laser axotomy platform for nerve regeneration studies in C. elegans. PLoS ONE 2014, 9, e113917. [Google Scholar] [CrossRef] [PubMed]



| Device | Method | Throughput | Sorting Capabilities | References |
|---|---|---|---|---|
| PDMS single channel device | Electrotaxis | 78 worms per minute | Specific developmental stages and adults; mutants from wild type | Rezai et al., 2010 and 2012 [9,10] |
| Agarose gel box containing a single long channel | Electrotaxis | Unknown | Adults of different age; mutants from wild type | Maniere et al., 2011 [11] |
| PDMS device containing interconnected mazes | Flow filtration | 200–300 worms per minute | Larvae from adults | Solvas et al., 2011 [12] |
| PDMS multichannel device | Electrotaxis | 4 worms per minute | All stages including adults | Han et al., 2012 [13] |
| PDMS micro-pillar device | Flow filtration | 130–180 worms per minute | All stages including adults | Ai et al., 2014 [14] |
| PDMS device containing optical fiber and laminar flow switch | Fluorescence filtration | 12 worms per minute | Fluorescent animals | Yan et al., 2014 [15] |
| PDMS-Agarose fan-shaped device | Electrotaxis | 56 worms per minute | L2–L4 larvae and adult; size-based separation | Wang et al., 2015 [16] |
| PDMS device containing adjustable filter | Pressure-based filtration | 200 worms per minute | Certain developmental stages | Dong et al., 2016 [17] |
| Immobilization Method | Throughput | Operation Performed | References |
|---|---|---|---|
| Pressure-based | 60 worm per hour | Neuroaxotomy and nerve regeneration | Guo et al. [97] |
| Pressure-based | One worm every few seconds | Neuroaxotomy | Zeng et al. [98] |
| Cooling | 120 worms per hour | Cell ablation | Chung and Lu [99] |
| Suction- and pressure-based | 180 worms per hour | Neuroaxotomy and nerve regeneration | Samara et al. [100] |
| Pressure-based | 210 worms per hour | Neuroaxotomy | Gokce et al. [101] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, B.P.; Rezai, P. Microfluidic Approaches for Manipulating, Imaging, and Screening C. elegans. Micromachines 2016, 7, 123. https://doi.org/10.3390/mi7070123
Gupta BP, Rezai P. Microfluidic Approaches for Manipulating, Imaging, and Screening C. elegans. Micromachines. 2016; 7(7):123. https://doi.org/10.3390/mi7070123
Chicago/Turabian StyleGupta, Bhagwati P., and Pouya Rezai. 2016. "Microfluidic Approaches for Manipulating, Imaging, and Screening C. elegans" Micromachines 7, no. 7: 123. https://doi.org/10.3390/mi7070123
APA StyleGupta, B. P., & Rezai, P. (2016). Microfluidic Approaches for Manipulating, Imaging, and Screening C. elegans. Micromachines, 7(7), 123. https://doi.org/10.3390/mi7070123





