The Graphene/l-Cysteine/Gold-Modified Electrode for the Differential Pulse Stripping Voltammetry Detection of Trace Levels of Cadmium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Reagents
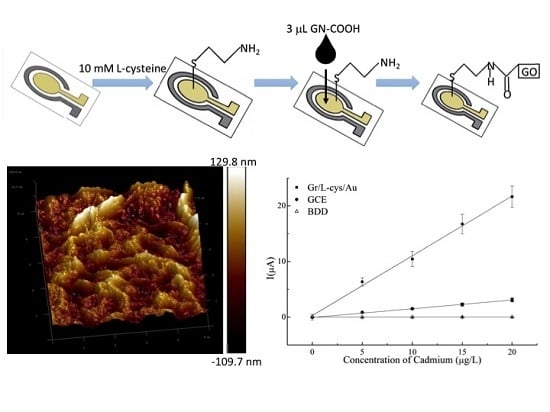
2.2. Fabrication and Modification of Microelectrode Chip
2.3. Electrochemical Methods of Differential Pulse Stripping Voltammetry (DPSV)
3. Discussion
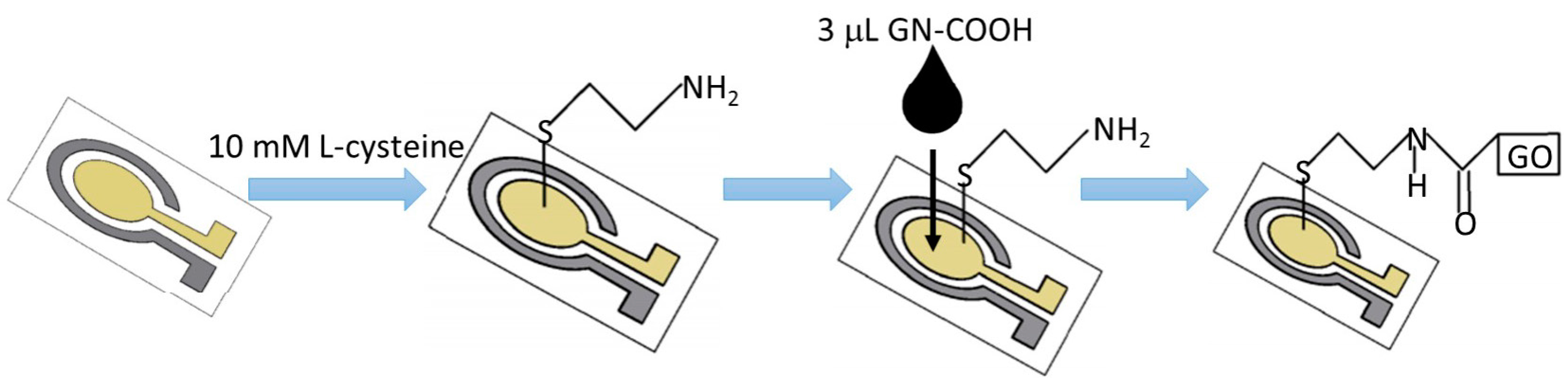
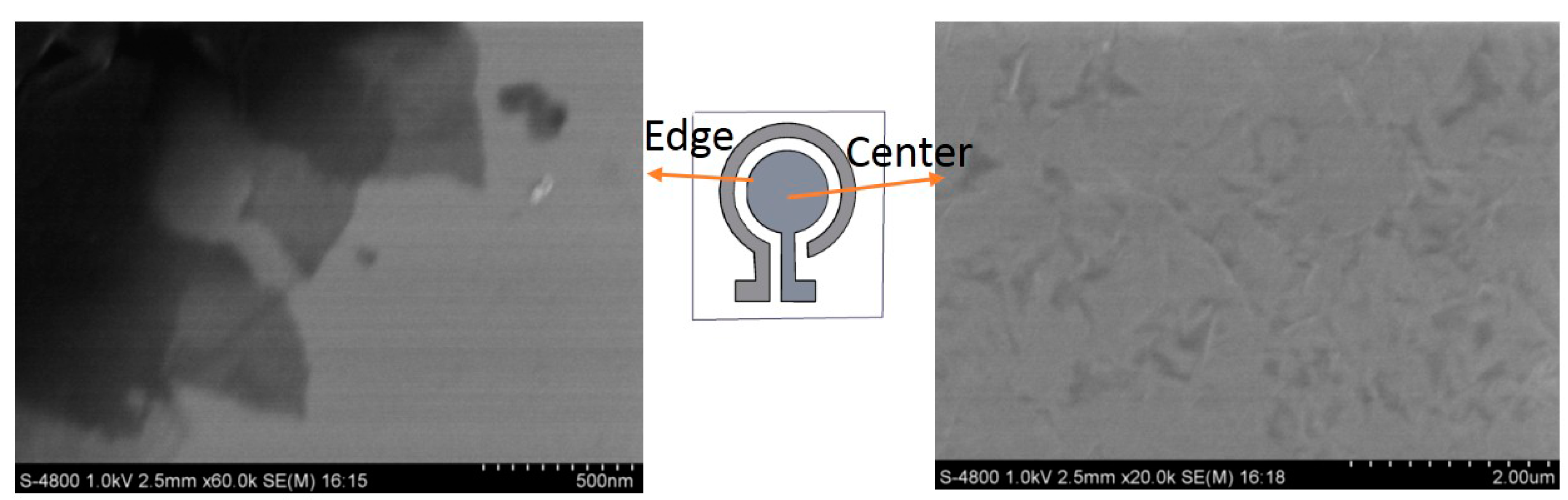
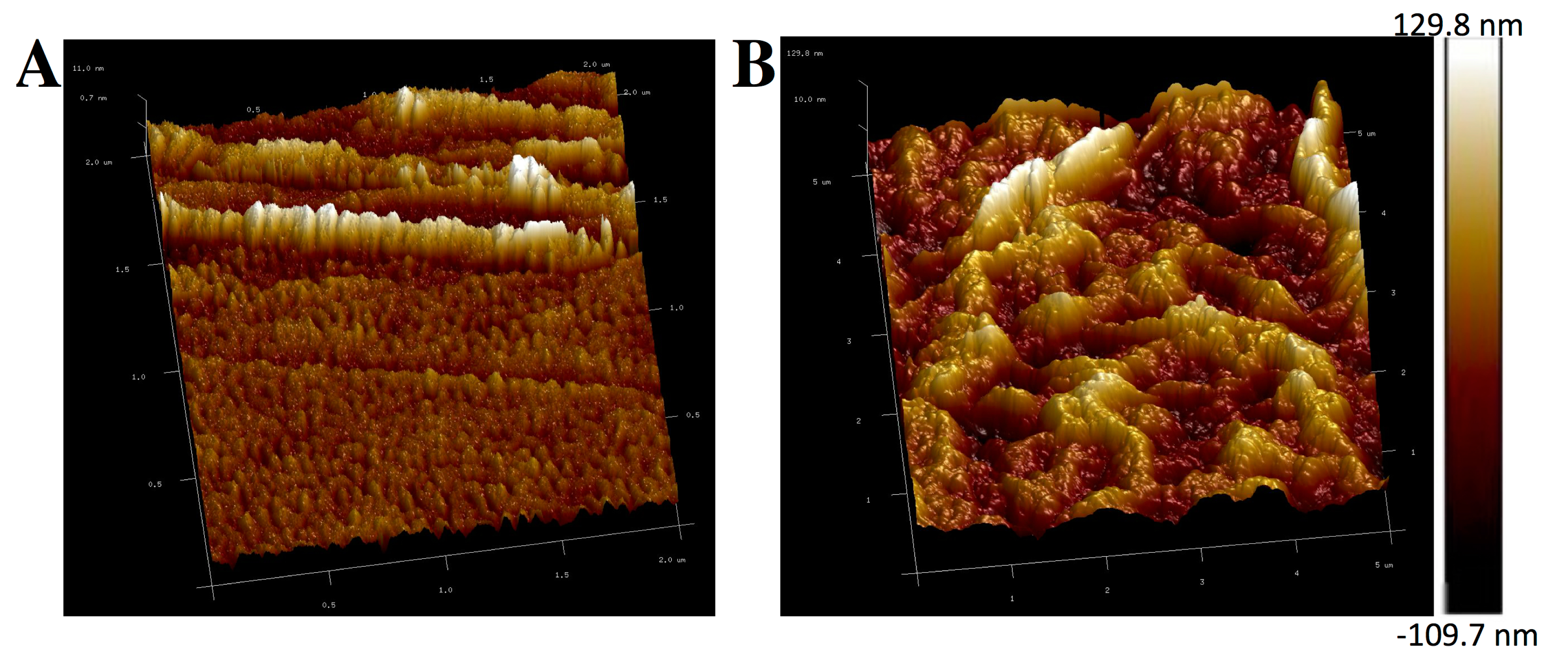
3.1. Characterization of Microelectrode Chip
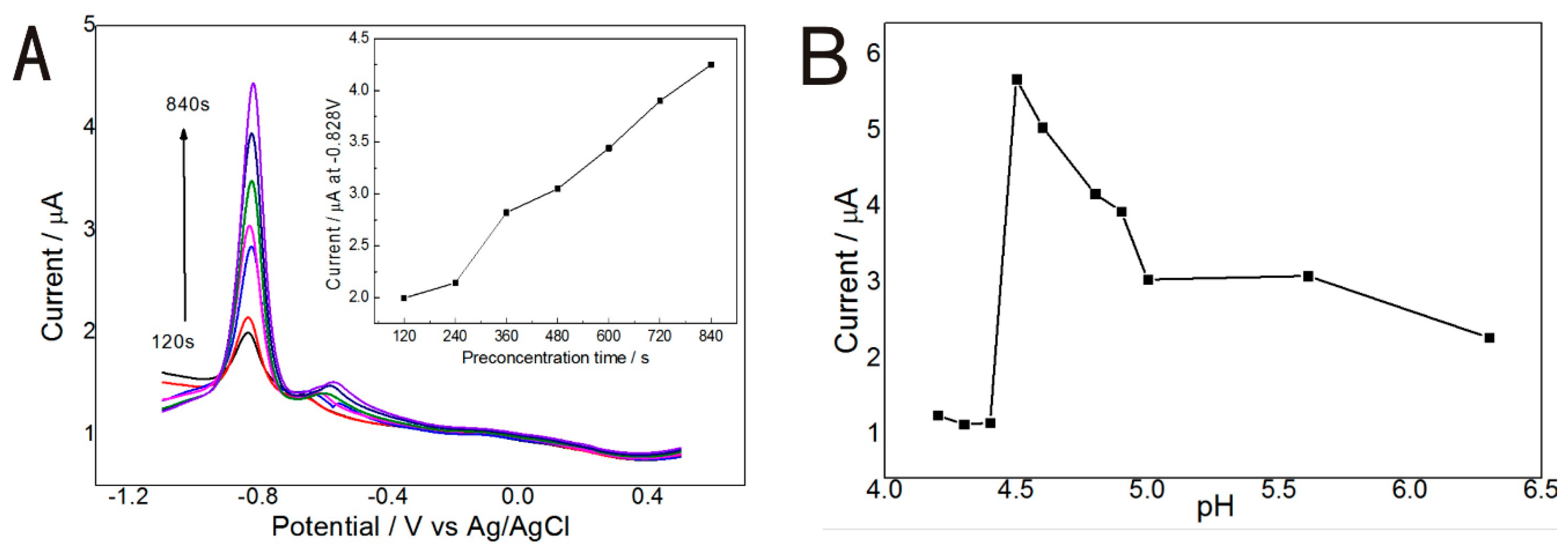
3.2. Optimazation of Depositon Time and pH Value of Detection Solution
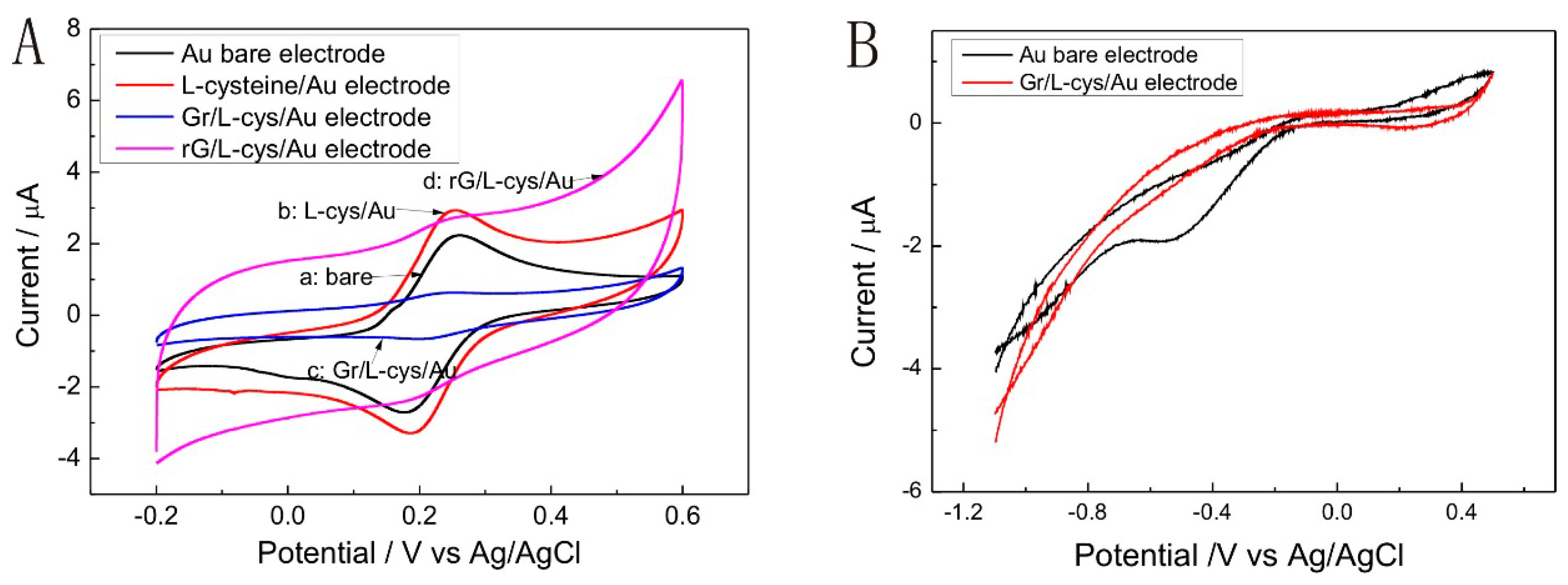
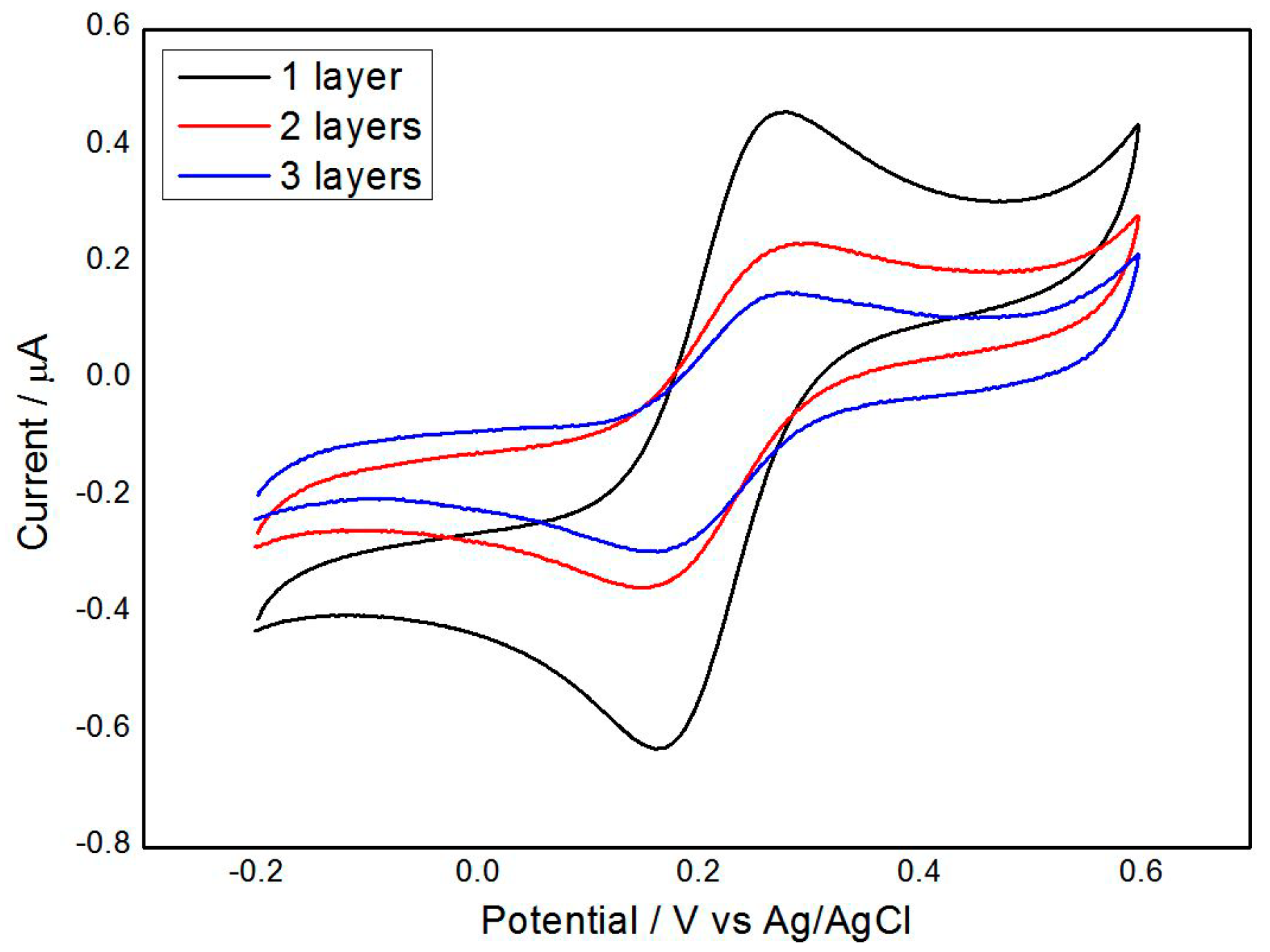
3.3. Electrochemical Characterization of the Gr/l-Cysteine Composite-Modified Electrode
3.4. Analytical Performance of the Gr/l-Cys/Au Electrode, the GCE, and the BDD Electrode for Cd(II) Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cd | cadmium |
| Gr | graphene |
| l-cys | l-cysteine |
| GCE | glass carbon electrode |
| BDD | boron-doped diamond |
| DPSV | differential pulse stripping voltammetry |
References
- Zang, Y. Cadmium: Toxicology. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 550–555. [Google Scholar]
- Kovacik, J.; Klejdus, B.; Hedbavny, J.; Stork, F.; Backor, M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 2009, 320, 231–242. [Google Scholar] [CrossRef]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Lombaert, N.; Lison, D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ. Int. 2016, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- D’Agostino, F.; Oliveri, E.; Bagnato, E.; Falco, F.; Mazzola, S.; Sprovieri, M. Direct determination of total mercury in phosphate rock using alkaline fusion digestion. Anal. Chim. Acta 2014, 852, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.; Pessenda, L.C.R.; Ruzicka, J.; Hansen, E.H. Combination of flow-injection analysis with flame atomic-absorption spectrophotometry—Determination of trace amounts of heavy-metals in polluted seawater. Analyst 1983, 108, 905–917. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Kianfar, A.H.; Mirsadeghi, A.S.; Pourfarokhi, A.; Soylak, M. The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation-preconcentration on bis salicyl aldehyde, 1,3 propan diimine (BSPDI) loaded on activated carbon. J. Hazard. Mater. 2008, 154, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Namiesnik, J. The role of speciation in analytical chemistry. TrAC Trends Anal. Chem. 2000, 19, 69–79. [Google Scholar] [CrossRef]
- Yuan, C.G.; Shi, J.B.; He, B.; Liu, J.F.; Liang, L.N.; Jiang, G.B. Speciation of heavy metals in marine sediments from the East China sea by ICP-MS with sequential extraction. Environ. Int. 2004, 30, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Zougagh, M.; de Torres, A.G.A.; Cano Pavón, J.M. Determination of cadmium in water by ICP-AES with on-line adsorption preconcentration using DPTH-gel and TS-gel microcolumns. Talanta 2002, 56, 753–761. [Google Scholar] [CrossRef]
- Daskalova, N.; Boevski, I. Spectral interferences in the determination of trace elements in environmental materials by inductively coupled plasma atomic emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1999, 54, 1099–1122. [Google Scholar] [CrossRef]
- Sperling, M.; Xu, S.K.; Welz, B. Determination of chromium(III) and chromium(VI) in water using flow-injection online preconcentration with selective adsorption on activated alumina and flame atomic-absorption spectrometric detection. Anal. Chem. 1992, 64, 3101–3108. [Google Scholar] [CrossRef]
- Zu, W.; Wang, Z. Ultra-trace determination of methylmercuy in seafood by atomic fluorescence spectrometry coupled with electrochemical cold vapor generation. J. Hazard. Mater. 2016, 304, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, K.C.; Tatum, C.E.; Dansby-Sparks, R.N.; Chambers, J.Q.; Xue, Z.-L. Individual and simultaneous determination of lead, cadmium, and zinc by anodic stripping voltammetry at a bismuth bulk electrode. Talanta 2010, 82, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Navarro, C.; Meyer, J.C.; Sundaram, R.S.; Chuvilin, A.; Kurasch, S.; Burghard, M.; Kern, K.; Kaiser, U. Atomic structure of reduced graphene oxide. Nano Lett. 2010, 10, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Kochmann, S.; Hirsch, T.; Wolfbeis, O.S. Graphenes in chemical sensors and biosensors. TrAC Trends Anal. Chem. 2012, 39, 87–113. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.H.; Adam, S.; Das Sarma, S. Carrier transport in two-dimensional graphene layers. Phys. Rev. Lett. 2007, 98, 186806. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.M.; Wang, Z.; Liu, X.; Chen, Z.; Liu, E. Glassy carbon electrode modified by graphene–gold nanocomposite coating for detection of trace lead ions in acetate buffer solution. Thin Solid Films 2015, 584, 85–89. [Google Scholar] [CrossRef]
- Xu, R.-X.; Yu, X.-Y.; Gao, C.; Jiang, Y.-J.; Han, D.-D.; Liu, J.-H.; Huang, X.-J. Non-conductive nanomaterial enhanced electrochemical response in stripping voltammetry: The use of nanostructured magnesium silicate hollow spheres for heavy metal ions detection. Anal. Chim. Acta 2013, 790, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Afkhami, A.; Khoshsafar, H.; Rezaei, M.; Sabounchei, S.J.; Sarlakifar, M. Simultaneous electrochemical sensing of thallium, lead and mercury using a novel ionic liquid/graphene modified electrode. Anal. Chim. Acta 2015, 870, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, W.; Wang, G.; Ren, J.; Guo, H.; Gao, J. A novel electrochemical sensor of bisphenol a based on stacked graphene nanofibers/gold nanoparticles composite modified glassy carbon electrode. Electrochim. Acta 2013, 98, 167–175. [Google Scholar] [CrossRef]
- Liang, Z.; Zhai, H.; Chen, Z.; Wang, H.; Wang, S.; Zhou, Q.; Huang, X. A simple, ultrasensitive sensor for gallic acid and uric acid based on gold microclusters/sulfonate functionalized graphene modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 224, 915–925. [Google Scholar] [CrossRef]
- Yang, J.; Deng, S.; Lei, J.; Ju, H.; Gunasekaran, S. Electrochemical synthesis of reduced graphene sheet–AuPd alloy nanoparticle composites for enzymatic biosensing. Biosens. Bioelectron. 2011, 29, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Shervedani, R.K.; Hatefi-Mehrjardi, A. Comparative electrochemical behavior of glucose oxidase covalently immobilized on mono-, di- and tetra-carboxylic acid functional Au-thiol SAMs via anhydride-derivatization route. Sens. Actuators B Chem. 2009, 137, 195–204. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Saepudin, E.; Wardah, H.; Dewangga, N.; Einaga, Y. Development of a biochemical oxygen demand sensor using gold-modified boron doped diamond electrodes. Anal. Chem. 2012, 84, 9825–9832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Chung, T.-S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, X.-J. Voltammetric determination of mercury(II). TrAC Trends Anal. Chem. 2013, 51, 1–12. [Google Scholar] [CrossRef]
- Argun, A.A.; Banks, A.M.; Merlen, G.; Tempelman, L.A.; Becker, M.F.; Schuelke, T.; Dweik, B.M. Highly sensitive detection of urinary cadmium to assess personal exposure. Anal. Chim. Acta 2013, 773, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, M. Electrochemical sensor based on graphene doped gold nanoparticles modified electrode for detection of diethylstilboestrol. Sens. Actuators B Chem. 2015, 215, 445–450. [Google Scholar] [CrossRef]
- Serrano, N.; González-Calabuig, A.; del Valle, M. Crown ether-modified electrodes for the simultaneous stripping voltammetric determination of Cd(II), Pb(II) and Cu(II). Talanta 2015, 138, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Hu, S.; Fei, J.; Bai, W. Mercury-free simultaneous determination of cadmium and lead at a glassy carbon electrode modified with multi-wall carbon nanotubes. Anal. Chim. Acta 2003, 489, 215–221. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Nitrogen-doped porous carbon derived from metal-organic gel for electrochemical analysis of heavy-metal ion. ACS Appl. Mater. Interfaces 2014, 6, 16210–16216. [Google Scholar] [CrossRef] [PubMed]
- Ruas de Souza, A.P.; Foster, C.W.; Kolliopoulos, A.V.; Bertotti, M.; Banks, C.E. Screen-printed back-to-back electroanalytical sensors: Heavy metal ion sensing. Analyst 2015, 140, 4130–4136. [Google Scholar] [CrossRef] [PubMed]

| Electrode | Metal Ion | Sensitivity (nA·mm−2·μg−1·L) | Linearity Range (μg/L) | Correlation Coefficient | LOD (μg/L) | Reference |
|---|---|---|---|---|---|---|
| Crown/GCE | Cd(II) | 16 | 7.9–191 | 0.99 | 2.4 | [33] |
| MWNTs/GCE | Cd(II) | 135 | 2.8–110 | 0.99 | 0.67 | [34] |
| N@MOG-C/GCE | Cd(II) | 89 | 2.8–55 | 0.99 | 0.25 | [35] |
| b2SPE | Pb(II) | 60 | 5–110 | 0.99 | 1.10 | [36] |
| GCE | Cd(II) | 21.69 | 5–20 | 0.998 | 0.30 | This work |
| BDD | Cd(II) | 0.18 | 5–20 | 0.950 | 1.15 | This work |
| Gr/l-cys/Au | Cd(II) | 152.0 | 5–20 | 0.997 | 1.42 | This work |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Bian, C.; Tong, J.; Li, Y.; Xia, S. The Graphene/l-Cysteine/Gold-Modified Electrode for the Differential Pulse Stripping Voltammetry Detection of Trace Levels of Cadmium. Micromachines 2016, 7, 103. https://doi.org/10.3390/mi7060103
Song Y, Bian C, Tong J, Li Y, Xia S. The Graphene/l-Cysteine/Gold-Modified Electrode for the Differential Pulse Stripping Voltammetry Detection of Trace Levels of Cadmium. Micromachines. 2016; 7(6):103. https://doi.org/10.3390/mi7060103
Chicago/Turabian StyleSong, Yu, Chao Bian, Jianhua Tong, Yang Li, and Shanghong Xia. 2016. "The Graphene/l-Cysteine/Gold-Modified Electrode for the Differential Pulse Stripping Voltammetry Detection of Trace Levels of Cadmium" Micromachines 7, no. 6: 103. https://doi.org/10.3390/mi7060103