A Fast Colourimetric Assay for Lead Detection Using Label-Free Gold Nanoparticles (AuNPs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Gold Nanoparticle Preparation
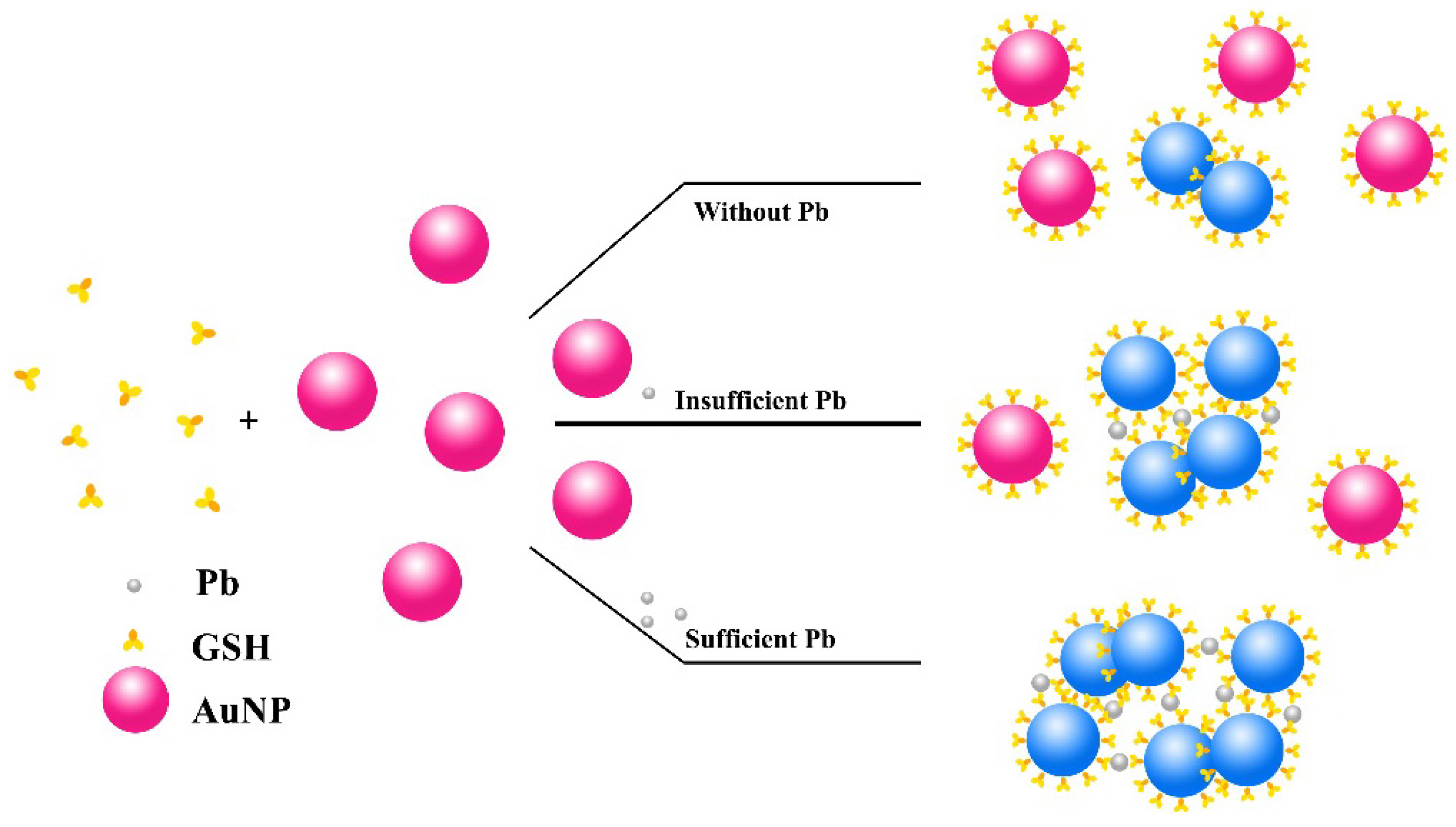
2.2. Assay
3. Results and Discussion
3.1. Gold Nanoparticles
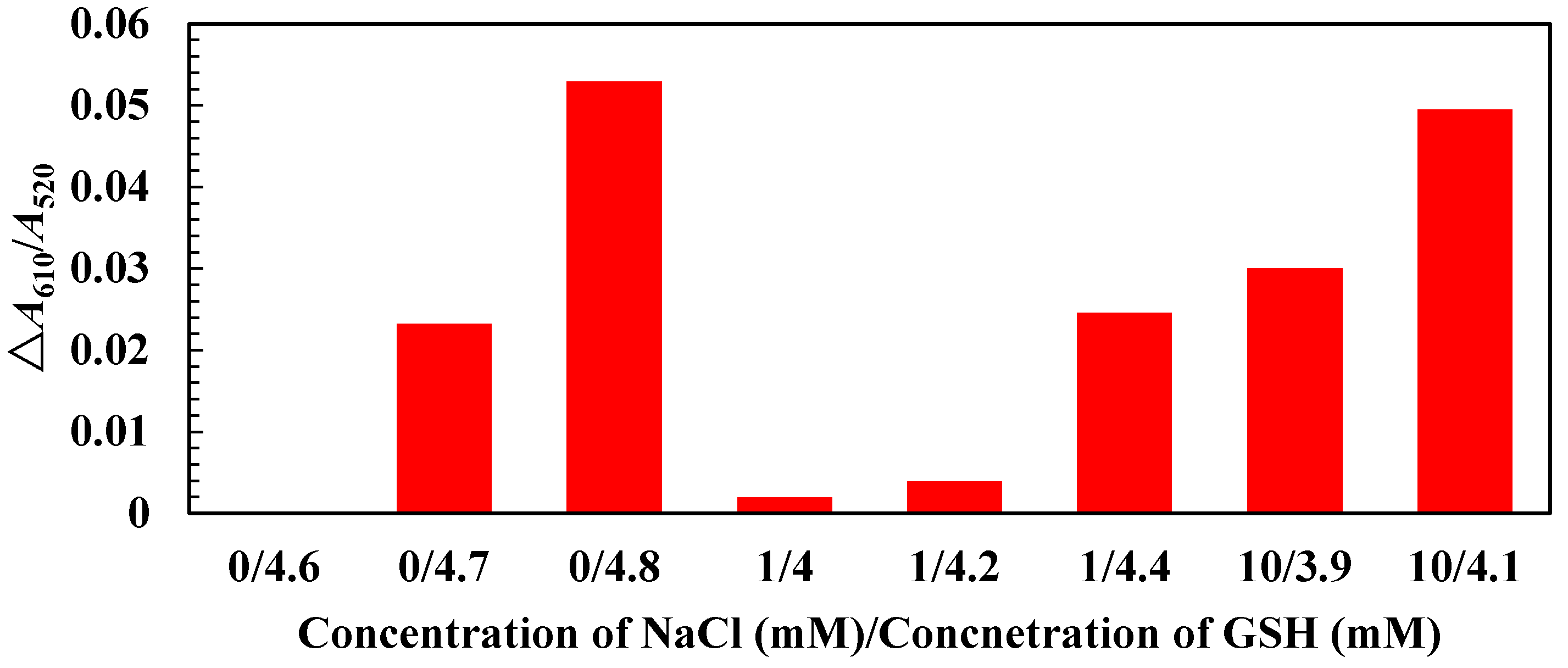
3.2. Mechanism, Assay Optimization and Performance


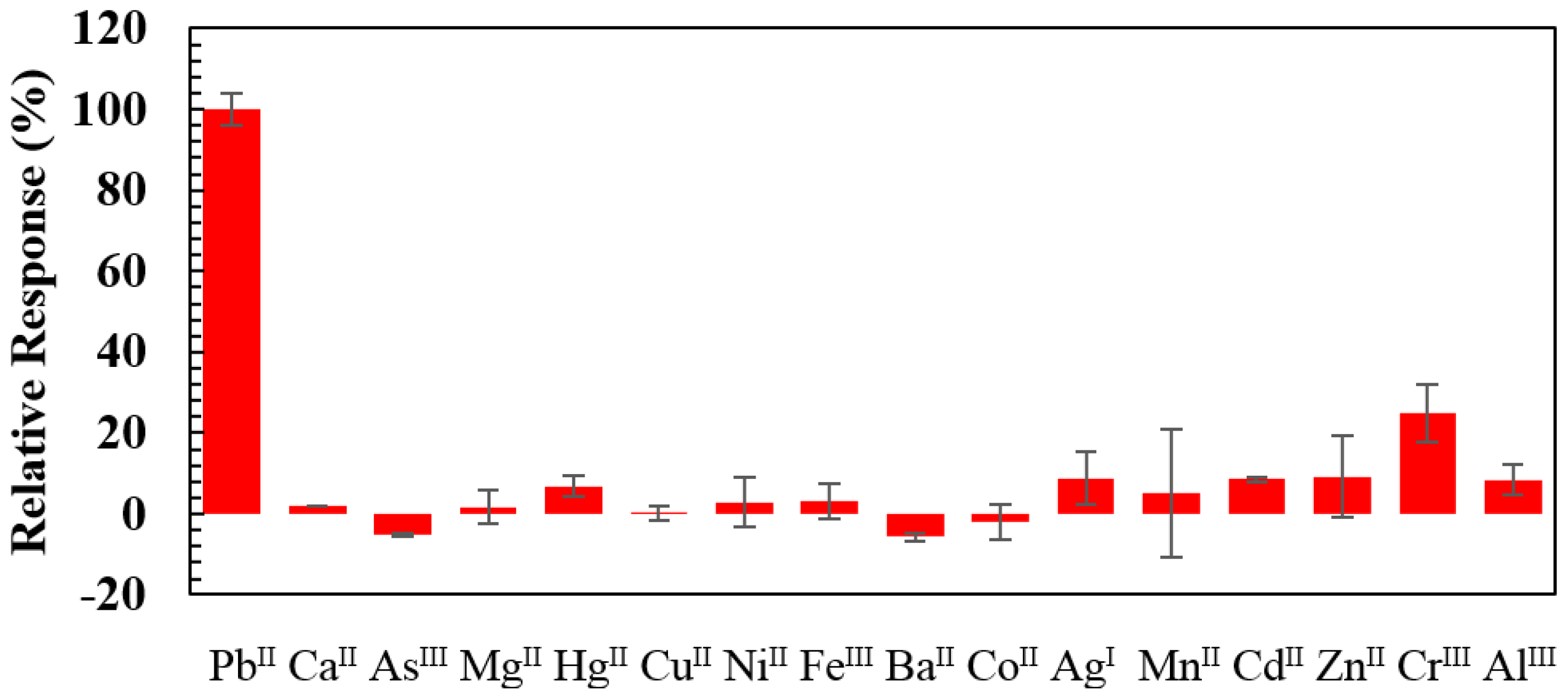
3.3. Selectivity Evaluation
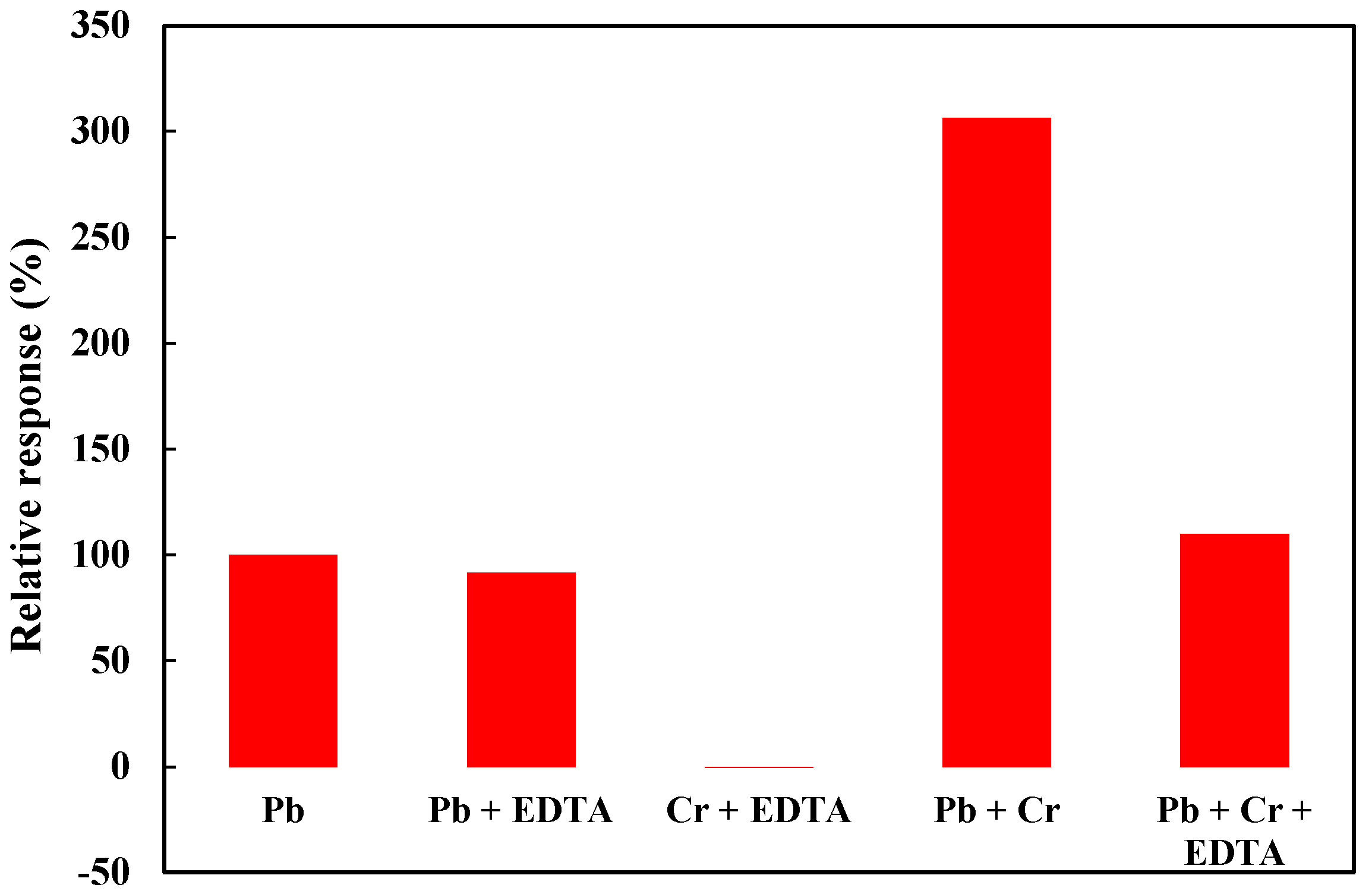
4. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References
- Madejón, P.; Murillo, J.M.; Marañón, T.; Cabrera, F.; López, R. Bioaccumulation of As, Cd, Cu, Fe and Pb in wild grasses affected by the aznalcóllar mine spill (SW Spain). Sci. Total Environ. 2002, 290, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H. Exposure to metal ions and susceptibility to dental caries. J. Dent. Educ. 2001, 65, 1046–1053. [Google Scholar] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 3rd ed.; WHO Press: Geneva, Switzerland, 2008; Volume 1. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality Summary Table; Health Canada: Ottawa, ON, Canada, 2012. [Google Scholar]
- U.S. Environmental Protection Agency. National Primary Drinking Water Regulations; EPA 816-F-09-004; U.S. EPA: Washington, DC, USA, 2009.
- Xia, N.; Shi, Y.F.; Zhang, R.C.; Zhao, F.; Liu, F.; Liu, L. Simple, rapid and label-free colourimetric assay for arsenic based on unmodified gold nanoparticles and a phytochelatin-like peptide. Anal. Methods 2012, 4, 3937–3941. [Google Scholar] [CrossRef]
- Gao, A.R.; Liu, X.; Li, T.; Zhou, P.; Wang, Y.L.; Yang, Q.; Wang, L.H.; Fan, C.H. Digital microfluidic chip for rapid portable detection of mercury(II). IEEE Sens. J. 2011, 11, 2820–2824. [Google Scholar] [CrossRef]
- Guidotti, T.L.; Calhoun, T.; Davies-Cole, J.O.; Knuckles, M.E.; Stokes, L.; Glymph, C.; Lum, G.; Moses, M.S.; Goldsmith, D.F.; Ragain, L. Elevated lead in drinking water in Washington, DC, 2003–2004: The public health response. Environ. Health Perspect. 2007, 115, 695–701. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Elevated Lead in D.C. Drinking Water: A Study of Potential Causative Events; EPA 810-F-07-002; U.S. EPA: Washington, DC, USA, 2007.
- Amendola, V.; Meneghetti, M. Size evaluation of gold nanoparticles by UV−Vis spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Future Med. 2007, 2, 681–693. [Google Scholar]
- Bao, Q.Y.; Geng, D.D.; Xue, J.W.; Zhou, G.; Gu, S.Y.; Ding, Y.; Zhang, C. Glutathione-mediated drug release from tiopronin-conjugated gold nanoparticles for acute liver injury therapy. Int. J. Pharm. 2013, 446, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y. Functional DNA directed assembly of nanomaterials for biosensing. J. Mater. Chem. 2009, 19, 1788–1798. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y. A highly sensitive and selective catalytic DNA biosensor for lead ions. J. Am. Chem. Soc. 2000, 122, 10466–10467. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.Q.; Ye, B.C. Colourimetric assay for parallel detection of Cd2+, Ni2+ and Co2+ using peptide-modified gold nanoparticles. Analyst 2012, 137, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Darbha, G.K.; Singh, A.K.; Rai, U.S.; Yu, E.; Yu, H.; Chandra Ray, P. Selective detection of mercury (II) ion using nonlinear optical properties of gold nanoparticles. J. Am. Chem. Soc. 2008, 130, 8038–8043. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Niu, C.; Ruan, M.; Wang, X.; Zeng, G.; Deng, C. Highly sensitive strategy for Hg2+ detection in environmental water samples using long lifetime fluorescence quantum dots and gold nanoparticles. Environ. Sci. Technol. 2013, 47, 4392–4398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, J.H.; Lu, Y. Label-free colourimetric detection of lead ions with a nanomolar detection limit and tunable dynamic range by using gold nanoparticles and dnazyme. Adv. Mater. 2008, 20, 3263–3267. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Adenosine-dependent assembly of aptazyme-functionalized gold nanoparticles and its application as a colourimetric biosensor. Anal. Chem. 2004, 76, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Bingling, L.; Jing, L.; Shaojun, D.; Erkang, W. Dnazyme-based colourimetric sensing of lead (Pb2+) using unmodified gold nanoparticle probes. Nanotechnology 2008, 19, 095501. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Taglietti, A.; Bassi, B.; Donà, A.; Pallavicini, P. A naked eye aggregation assay for Pb2+ detection based on glutathione-coated gold nanostars. J. Nanopart. Res. 2014, 10, 1–11. [Google Scholar]
- Guo, Y.; Wang, Z.; Qu, W.; Shao, H.; Jiang, X. Colourimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens. Bioelectron. 2011, 26, 4064–4069. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Huang, C.-C.; Chang, H.-T. Gold nanoparticle probes for the detection of mercury, lead and copper ions. Analyst 2011, 136, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Yu, C.J.; Tseng, W.L. Sensitivity enhancement in the colourimetric detection of lead(II) ion using gallic acid-capped gold nanoparticles: Improving size distribution and minimizing interparticle repulsion. Biosens. Bioelectron. 2010, 25, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colourimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Li, Z.-P.; Tang, Z.-Y. One pot synthesis of monodispersed l-glutathione stabilized gold nanoparticles for the detection of Pb2+ ions. Front. Mater. Sci. 2010, 5, 322–328. [Google Scholar] [CrossRef]
- Chu, W.; Zhang, Y.; Li, D.; Barrow, C.J.; Wang, H.; Yang, W. A biomimetic sensor for the detection of lead in water. Biosens. Bioelectron. 2015, 67, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nie, Z.; He, K.; Xu, X.; Li, Y.; Huang, Y.; Yao, S. Simple, rapid and label-free colourimetric assay for Zn2+ based on unmodified gold nanoparticles and specific Zn2+ binding peptide. Chem. Commun. 2011, 47, 4412–4414. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Preparation of aptamer-linked gold nanoparticle purple aggregates for colourimetric sensing of analytes. Nat. Protoc. 2006, 1, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Z.M. Complexation Equilibria of Zn(II), Pb(II) and Cd(II) with Reduced Glutathione (GSH) and Biologically Important Zwitterionic Buffers; Chemical Society: Taipei, Taiwan, 2005; Volume 52, p. 9. [Google Scholar]
- Lim, I.I.S.; Mott, D.; Ip, W.; Njoki, P.N.; Pan, Y.; Zhou, S.; Zhong, C.-J. Interparticle interactions in glutathione mediated assembly of gold nanoparticles. Langmuir 2008, 24, 8857–8863. [Google Scholar] [CrossRef] [PubMed]
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colourimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water samples. ACS Appl. Mater. Interfaces 2011, 3, 668–673. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Definition and Procedure for the Determination of the Method Detection Limit-Revision 1.11; 40 CFR Appendix B, Part 136; U.S. EPA: Washington, DC, USA, 2000.
- Burns, C.; Spendel, W.U.; Puckett, S.; Pacey, G.E. Solution ionic strength effect on gold nanoparticle solution colour transition. Talanta 2006, 69, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Pal, T. Glutathione-induced aggregation of gold nanoparticles: Electromagnetic interactions in a closely packed assembly. J. Nanosci. Nanotechnol. 2007, 7, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, H.J.; Ottenwälder, H.; Bolt, H.M. The formation of glutathione-chromium complexes and their possible role in chromium disposition. In Receptors and Other Targets for Toxic Substances; Chambers, P., Cholnoky, E., Chambers, C., Eds.; Springer: Berlin, Germany, 1986; Volume 8, pp. 319–321. [Google Scholar]
- Pietrzyk, D.J.; Frank, C.W. Analytical Chemistry; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Penciner, J.S. Stability Constants of Metal Complexes of 5-Sulfosalicylic Acid; University of Toronto: Toronto, ON, Canada, 1962. [Google Scholar]
- Sigel, H. Metal Ions in Biological Systems. Volume 18: Circulation of the Metals in the Environment; Taylor & Francis: New York, NY, USA, 1984. [Google Scholar]
- Harris, D.C. Quantitative Chemical Analysis; W.H. Freeman: New York, NY, USA, 1999. [Google Scholar]
- Krezel, A.; Bal, W. Coordination chemistry of glutathione. Acta Biochim. Polonica 1999, 46, 567–580. [Google Scholar]
- Zhao, C.; Zhong, G.; Kim, D.-E.; Liu, J.; Liu, X. A portable lab-on-a-chip system for gold-nanoparticle-based colourimetric detection of metal ions in water. Biomicrofluidics 2014, 8, 052107. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, G.; Liu, J.; Liu, X. A Fast Colourimetric Assay for Lead Detection Using Label-Free Gold Nanoparticles (AuNPs). Micromachines 2015, 6, 462-472. https://doi.org/10.3390/mi6040462
Zhong G, Liu J, Liu X. A Fast Colourimetric Assay for Lead Detection Using Label-Free Gold Nanoparticles (AuNPs). Micromachines. 2015; 6(4):462-472. https://doi.org/10.3390/mi6040462
Chicago/Turabian StyleZhong, Guowei, Jinxia Liu, and Xinyu Liu. 2015. "A Fast Colourimetric Assay for Lead Detection Using Label-Free Gold Nanoparticles (AuNPs)" Micromachines 6, no. 4: 462-472. https://doi.org/10.3390/mi6040462




