Three-Dimensional Electro-Sonic Flow Focusing Ionization Microfluidic Chip for Mass Spectrometry †
Abstract
:1. Introduction
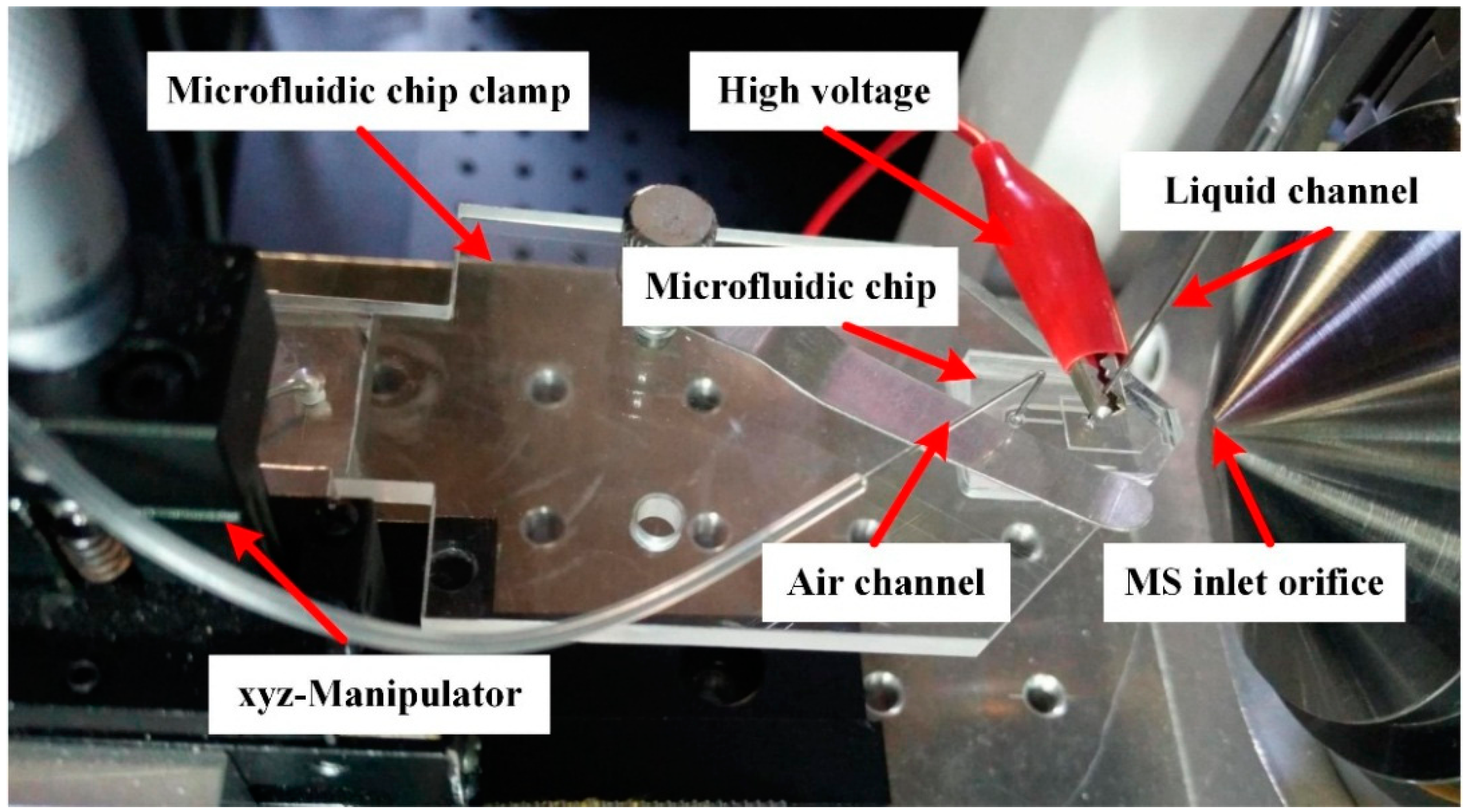
2. Materials and Methods
2.1. Materials and Equipment
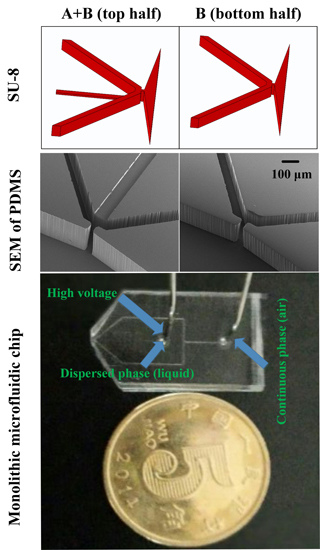
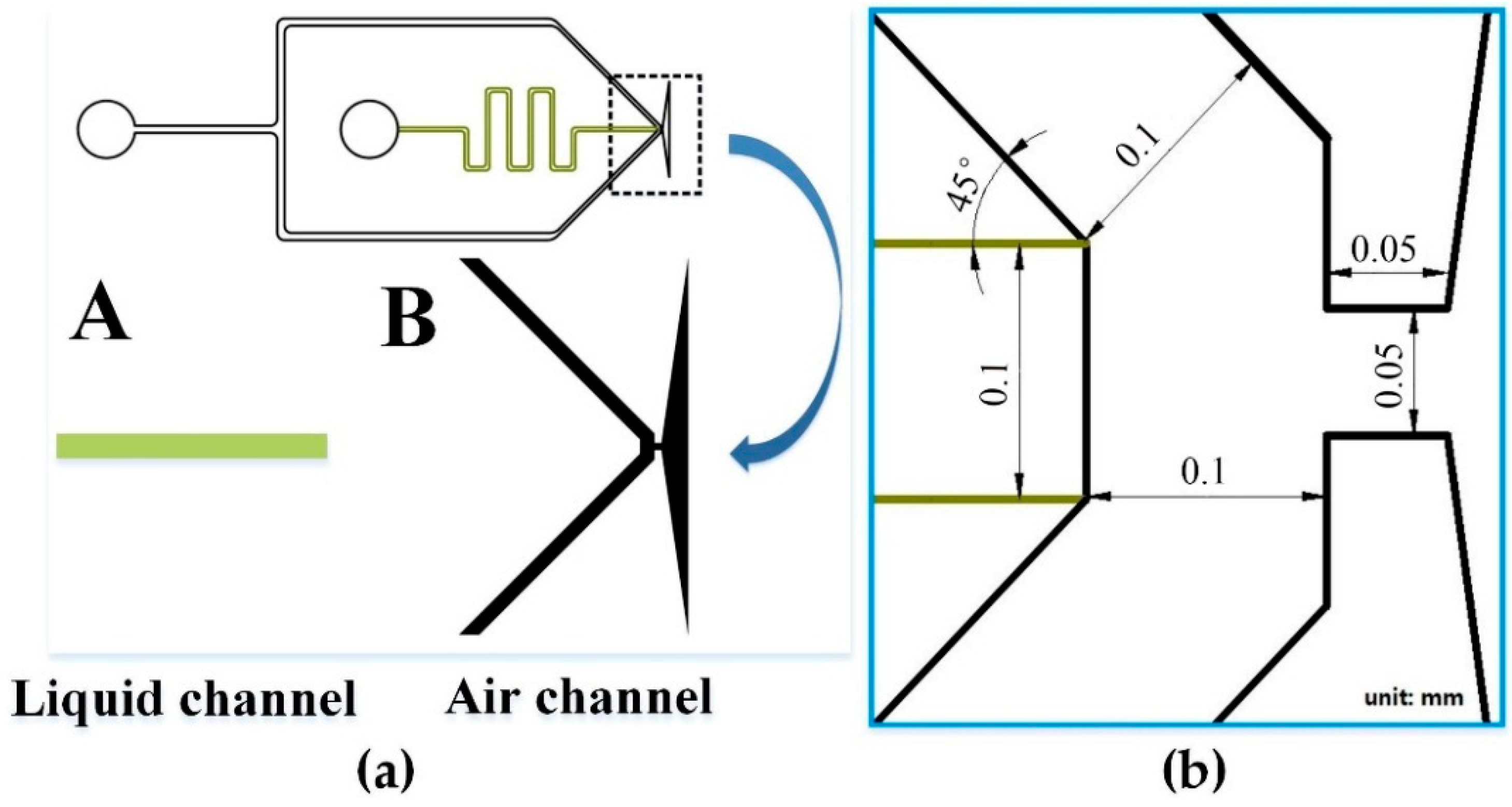
2.2. ESFFI Microfluidic Chip Design
2.3. Fabrication of the ESFFI Microfluidic Chip
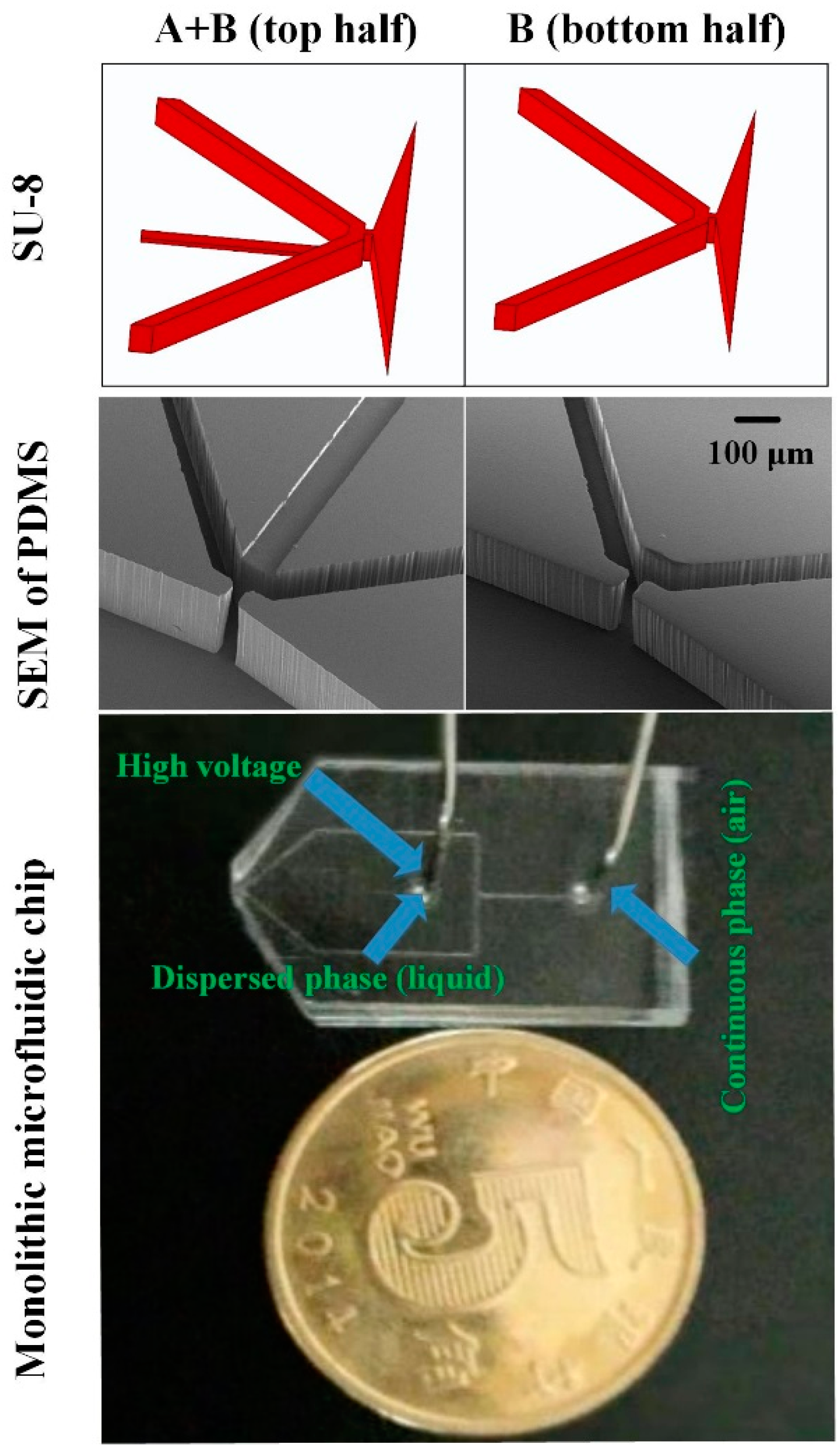
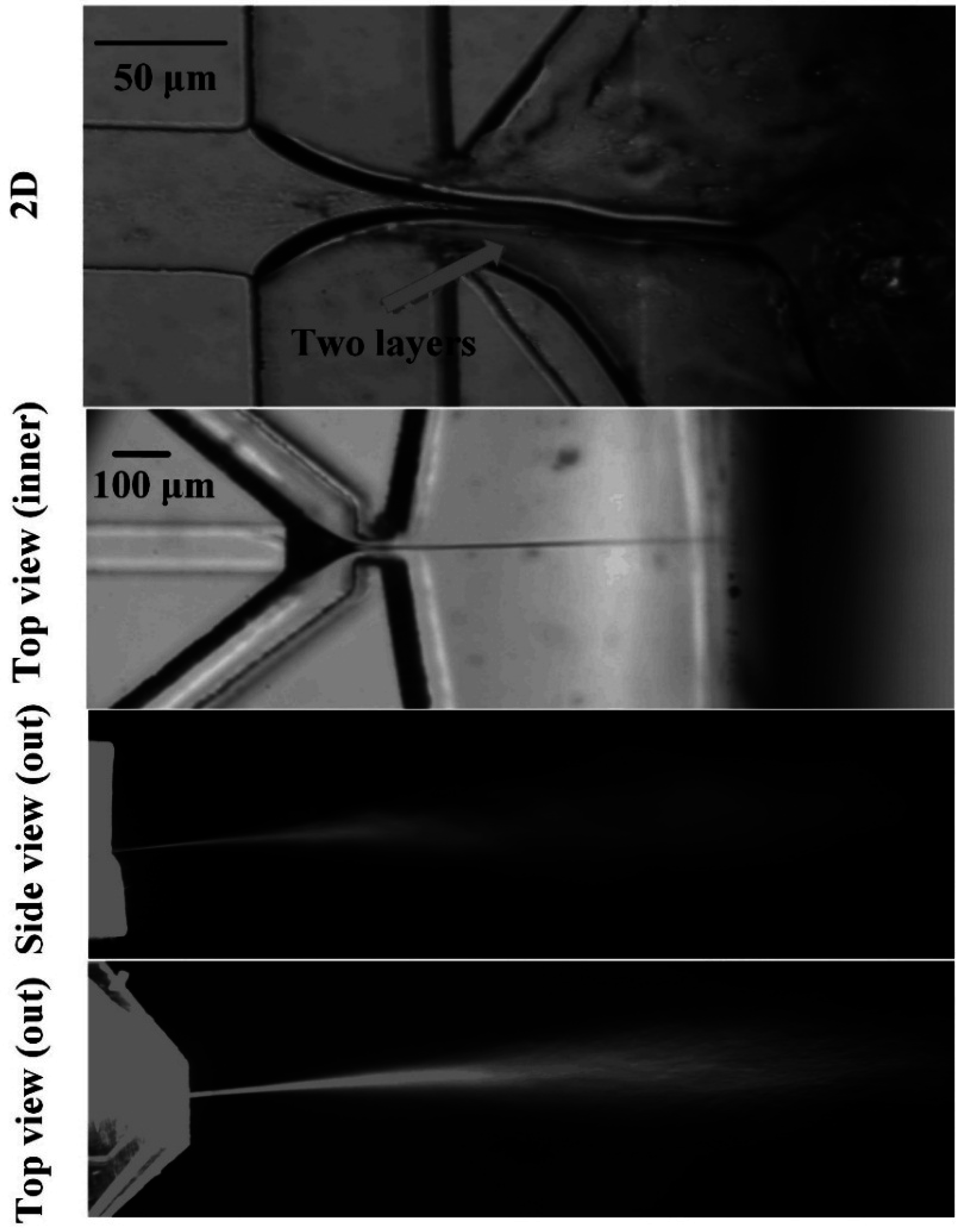
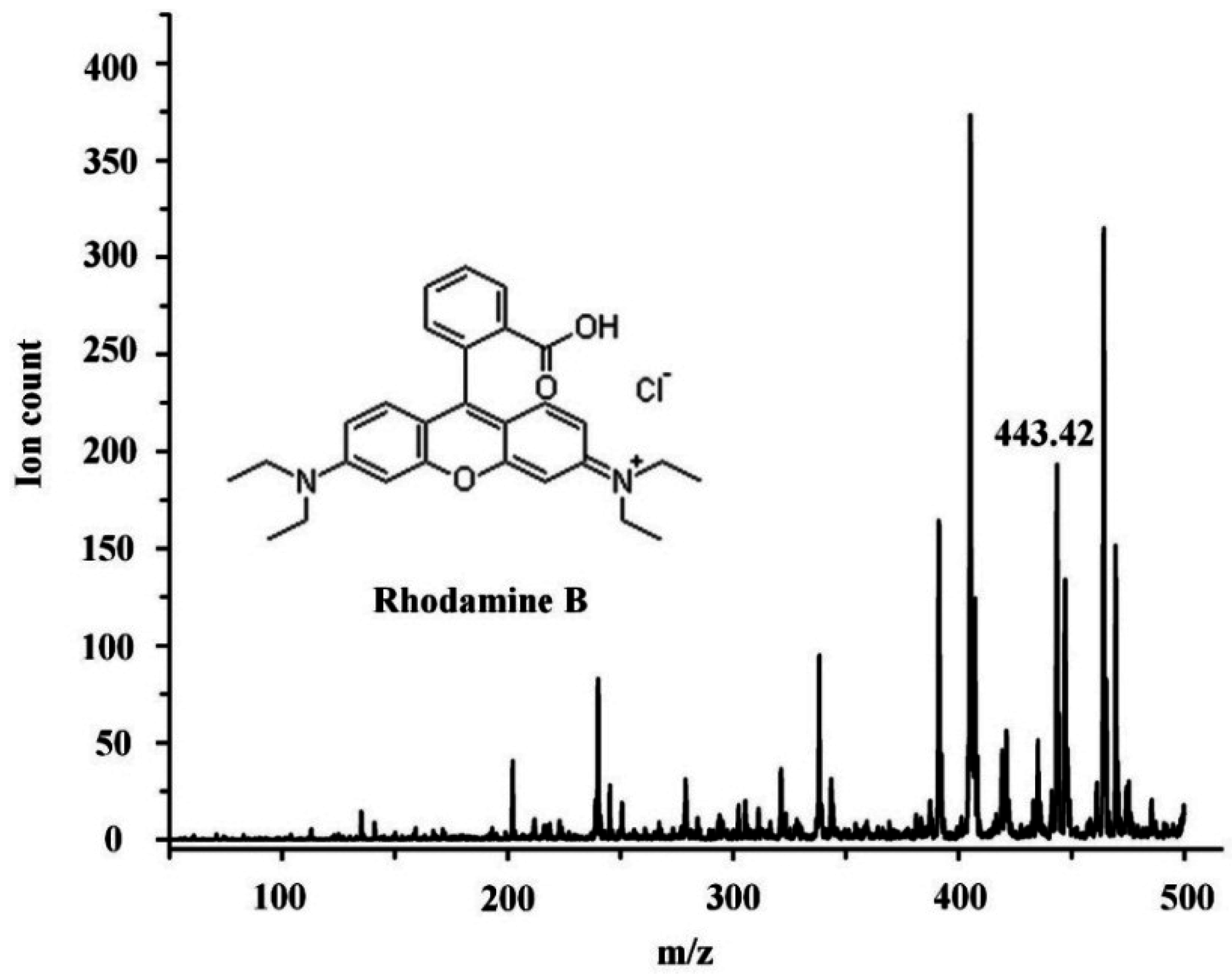
3. Results and Discussions
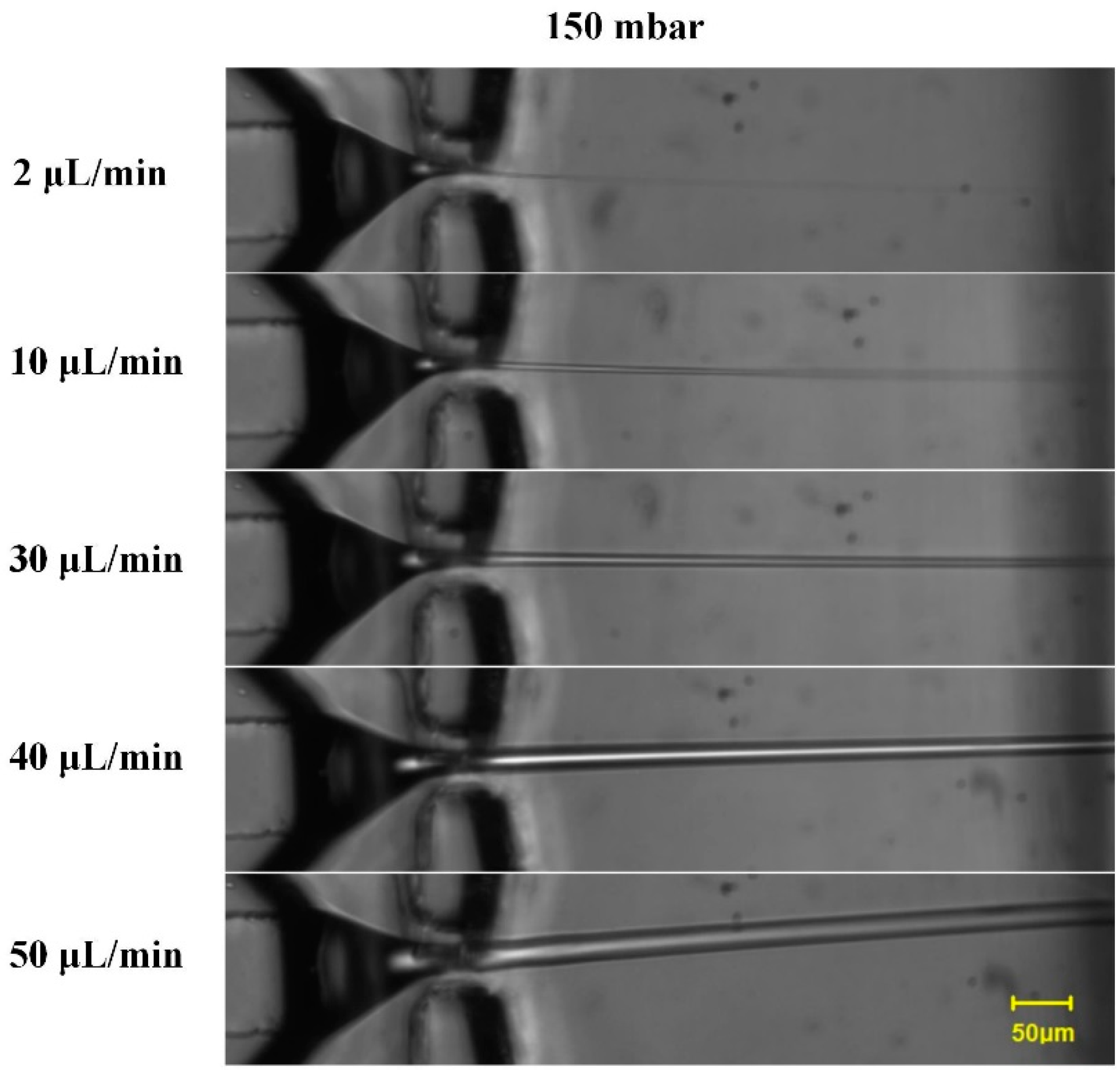
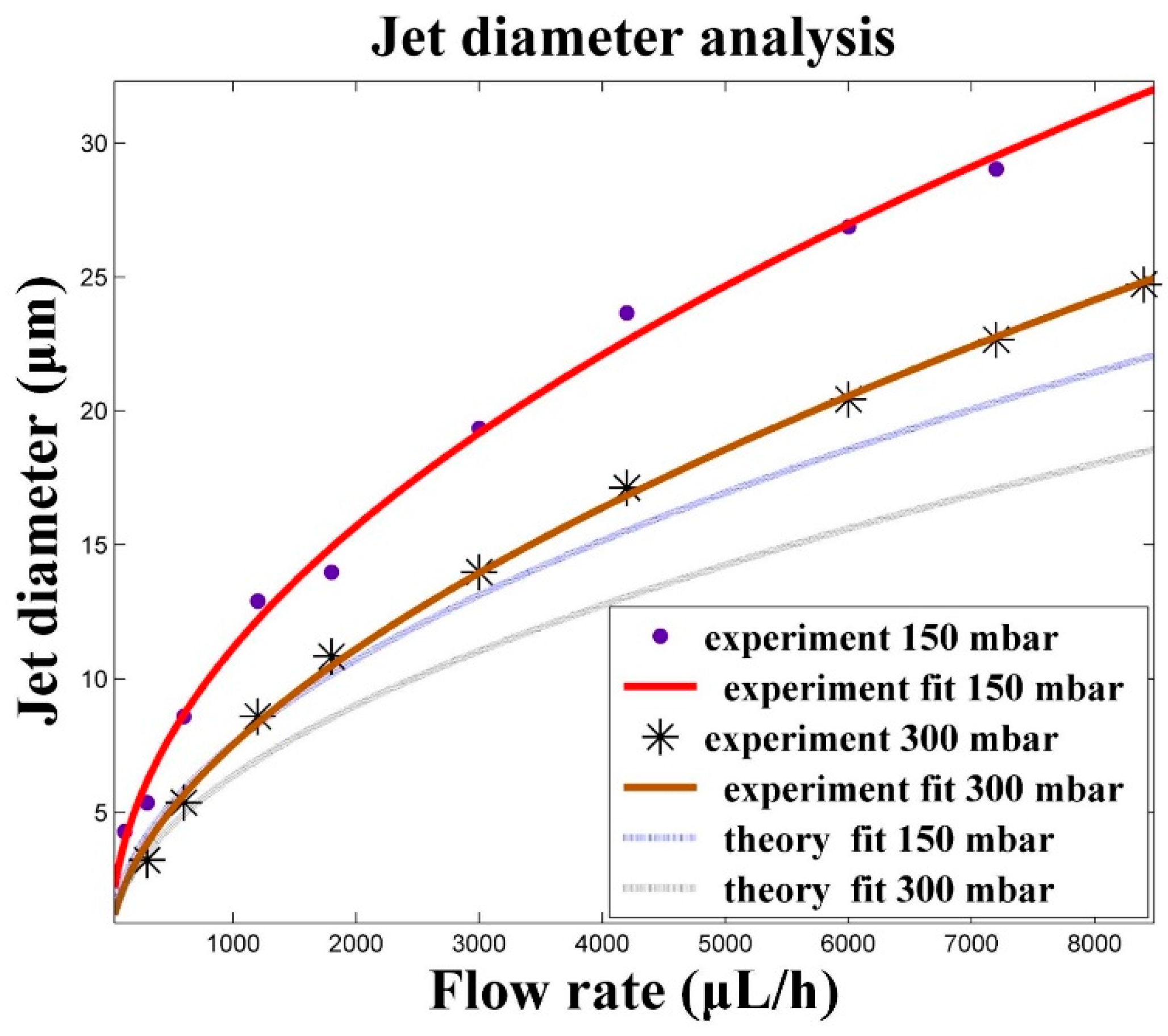

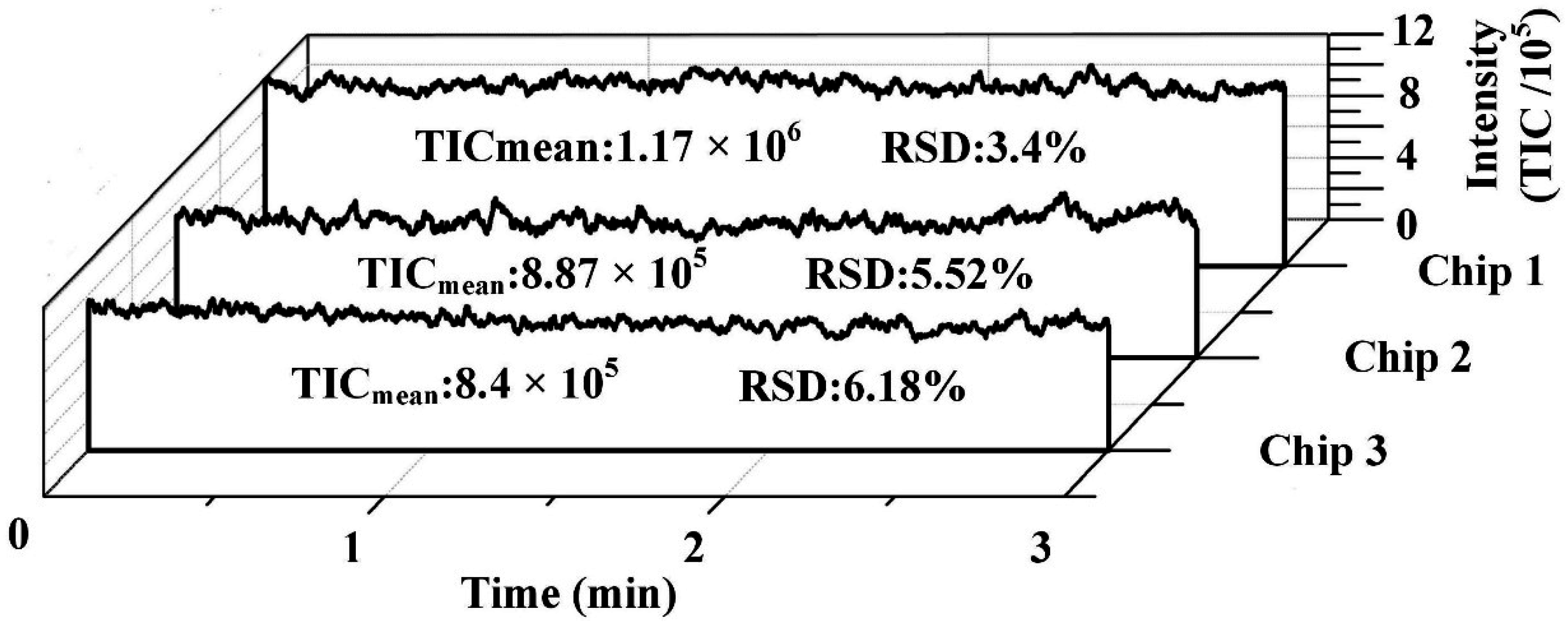

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, X.; Kelly, R.T.; Tang, K.; Smith, R.D. Membrane-Based Emitter for Coupling Microfluidics with Ultrasensitive Nanoelectrospray Ionization-Mass Spectrometry. Anal. Chem. 2011, 83, 5797–5803. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Belov, M.E.; Jaitly, N.; Qian, W.J.; Smith, R.D. Accurate mass measurements in proteomics. Chem. Rev. 2007, 107, 3621–3653. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, C.T.; Jacobson, S.C.; Ramsey, J.M. Microchip devices for high-efficiency separations. Anal. Chem. 2000, 72, 5814–5819. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.C.; Culbertson, C.T.; Daler, J.E.; Ramsey, J.M. Microchip structures for submillisecond electrophoresis. Anal. Chem. 1998, 70, 3476–3480. [Google Scholar] [CrossRef]
- Sun, X.; Kelly, R.T.; Tang, K.; Smith, R.D. Ultrasensitive nanoelectrospray ionization-mass spectrometry using poly(dimethylsiloxane) microchips with monolithically integrated emitters. Analyst 2010, 135, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wu, H.K.; Bhaya, D.; Grossman, A.; Granier, S.; Kobilka, B.K.; Zare, R.N. Counting low-copy number proteins in a single cell. Science 2007, 315, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.M.; Trisiripisal, P.; Sarvaiya, H.A. Microfluidic liquid chromatography system for proteomic applications and biomarker screening. Anal. Chem. 2006, 78, 5513–5524. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.Y.; Wang, H.T.; Huang, Y.; Zhong, W.; Lu, H.J.; Kong, J.L.; Yang, P.Y.; Liu, B.H. Stable microstructured network for protein patterning on a plastic microfluidic channel: Strategy and characterization of on-chip enzyme microreactors. Anal. Chem. 2004, 76, 6426–6433. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kato, K.; Kato, M.; Toyo'oka, T. Creation of an on-chip enzyme reactor by encapsulating trypsin in sol-gel on a plastic microchip. Anal. Chem. 2003, 75, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Oleschuk, R.D.; Harrison, D.J. Analytical microdevices for mass spectrometry. TrAC Trend. Anal. Chem. 2000, 19, 379–388. [Google Scholar] [CrossRef]
- Gao, D.; Liu, H.X.; Jiang, Y.Y.; Lin, J.M. Recent advances in microfluidics combined with mass spectrometry: Technologies and applications. Lab Chip 2013, 13, 3309–3322. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Bai, H.; Lin, T.; Fuh, M. Microfluidic chip-based liquid chromatography coupled to mass spectrometry for determination of small molecules in bioanalytical applications. Electrophoresis 2012, 33, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; Verpoorte, E. A decade of microfluidic analysis coupled with electrospray mass spectrometry: An overview. Lab Chip 2007, 7, 1394–1412. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.C.; Makamba, H.; Chen, S.H. Chip-based microfluidic devices coupled with electrospray ionization-mass spectrometry. Electrophoresis 2005, 26, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.F.; Foret, F.; Dunayevskiy, Y.M.; Zavracky, P.M.; McGruer, N.E.; Karger, B.L. Multichannel microchip electrospray mass spectrometry. Anal. Chem. 1997, 69, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, R.S.; Ramsey, J.M. Generating electrospray from microchip devices using electroosmotic pumping. Anal. Chem. 1997, 69, 1174–1178. [Google Scholar] [CrossRef]
- Li, J.; Thibault, P.; Bings, N.H.; Skinner, C.D.; Wang, C.; Colyer, C.; Harrison, J. Integration of Microfabricated Devices to Capillary Electrophoresis-Electrospray Mass Spectrometry Using a Low Dead Volume Connection: Application to Rapid Analyses of Proteolytic Digests. Anal. Chem. 1999, 71, 3036–3045. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Gomez-Sjoberg, R.; Wang, D. Multinozzle Emitter Array Chips for Small-Volume Proteomics. Anal. Chem. 2013, 85, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Wang, H.; Yang, P.; Wang, D. Multinozzle Emitter Arrays for Nanoelectrospray Mass Spectrometry. Anal. Chem. 2011, 83, 6082–6089. [Google Scholar] [CrossRef] [PubMed]
- Covey, T.R.; Thomson, B.A.; Schneider, B.B. Atmospheric pressure ion sources. Mass Spectrom. Rev. 2009, 28, 870–897. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Gibson, G.T.T.; Mugo, S.M.; Marecak, D.M.; Oleschuk, R.D. Microstructured Photonic Fibers as Multichannel Electrospray Emitters. Anal. Chem. 2009, 81, 7281–7287. [Google Scholar] [CrossRef] [PubMed]
- Mery, E.; Ricoul, F.; Sarrut, N.; Constantin, O.; Delapierre, G.; Garin, J.; Vinet, F. A silicon microfluidic chip integrating an ordered micropillar array separation column and a nano-electrospray emitter for LC/MS analysis of peptides. Sens. Actuator B Chem. 2008, 134, 438–446. [Google Scholar] [CrossRef]
- Arscott, S. SU-8 as a material for lab-on-a-chip-based mass spectrometry. Lab Chip 2014, 14, 3668–3689. [Google Scholar] [CrossRef] [PubMed]
- Sikanen, T.; Tuomikoski, S.; Ketola, R.A.; Kostiainen, R.; Franssila, S.; Kotiaho, T. Analytical characterization of microfabricated SU-8 emitters for electrospray ionization mass spectrometry. J. Mass Spectrom. 2008, 43, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Sikanen, T.; Heikkilä, L.; Tuomikoski, S.; Ketola, R.A.; Kostiainen, R.; Franssila, S.; Kotiaho, T. Performance of SU-8 Microchips as Separation Devices and Comparison with Glass Microchips. Anal. Chem. 2007, 79, 6255–6263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Soper, S.A.; Murray, K.K. Development of an efficient on-chip digestion system for protein analysis using MALDI-TOF MS. Analyst 2009, 134, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Batz, N.G.; Mellors, J.S.; Alarie, J.P.; Ramsey, J.M. Chemical vapor deposition of aminopropyl silanes in microfluidic channels for highly efficient microchip capillary electrophoresis-electrospray ionization-mass spectrometry. Anal. Chem. 2014, 86, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Mellors, J.S.; Black, W.A.; Chambers, A.G.; Starkey, J.A.; Lacher, N.A.; Ramsey, J.M. Hybrid Capillary/Microfluidic System for Comprehensive Online Liquid Chromatography-Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry. Anal. Chem. 2013, 85, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.G.; Ramsey, J.M. Microfluidic Dual Emitter Electrospray Ionization Source for Accurate Mass Measurements. Anal. Chem. 2012, 84, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Mellors, J.S.; Jorabchi, K.; Smith, L.M.; Ramsey, J.M. Integrated Microfluidic Device for Automated Single Cell Analysis Using Electrophoretic Separation and Electrospray Ionization Mass Spectrometry. Anal. Chem. 2010, 82, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Tang, K.Q.; Smith, R.D.; Kelly, R.T. Controlled dispensing and mixing of pico- to nanoliter volumes using on-demand droplet-based microfluidics. Microfluid. Nanofluid. 2013, 15, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.T.; Tang, K.; Irimia, D.; Toner, M.; Smith, R.D. Elastomeric microchip electrospray emitter for stable cone-jet mode operation in the nanoflow regime. Anal. Chem. 2008, 80, 3824–3831. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Haeusig, U.; Schulze, P.; Belder, D. Microfluidic glass chips with an integrated nanospray emitter for coupling to a mass spectrometer. Angew. Chem. Int. Ed. 2007, 46, 4913–4916. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.E.; Roper, M.G.; Jeffery, E.D.; Easley, C.J.; Balchunas, C.; Landers, J.P.; Ferrance, J.P. Glass microfluidic devices with thin membrane voltage junctions for electrospray mass spectrometry. Lab Chip 2005, 5, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Barbier, V.; Tatoulian, M.; Li, H.; Arefi-Khonsari, F.; Ajdari, A.; Tabeling, P. Stable modification of PDMS surface properties by plasma polymerization: Application to the formation of double emulsions in microfluidic systems. Langmuir 2006, 22, 5230–5232. [Google Scholar] [CrossRef] [PubMed]
- Rohner, T.C.; Rossier, J.S.; Girault, H.H. Polymer microspray with an integrated thick-film microelectrode. Anal. Chem. 2001, 73, 5353–5357. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xu, J.; Yu, C.; Chen, Y.; Yu, Q.; Ni, K.; Wang, X. A Reliable and Simple Method for Fabricating a Poly(Dimethylsiloxane) Electrospray Ionization Chip with a Corner-Integrated Emitter. Sensors 2015, 15, 8931–8944. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, P.; Dahlin, A.P.; Bergstrom, S.K.; Thorslund, S.; Andren, P.E.; Nikolajeff, F.; Bergquist, J. Sample pretreatment on a microchip with an integrated electrospray emitter. Electrophoresis 2006, 27, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, A.P.; Wetterhall, M.; Liljegren, G.; Bergstrom, S.K.; Andren, P.; Nyholm, L.; Markides, K.E.; Bergquist, J. Capillary electrophoresis coupled to mass spectrometry from a polymer modified poly(dimethylsiloxane) microchip with an integrated graphite electrospray tip. Analyst 2005, 130, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, B. Controlled formation of double-emulsion drops in sudden expansion channels. J. Colloid Interface Sci. 2014, 415, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, Z.; Wu, Y.; Deng, Y.; Liu, Y.; Liu, G. Hydrodynamic particle focusing design using fluid-particle interaction. Biomicrofluidics 2013, 7, 54104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv. Drug Deliver. Rev. 2013, 65, 1420–1446. [Google Scholar] [CrossRef] [PubMed]
- Wörner, M. Numerical modeling of multiphase flows in microfluidics and micro process engineering: A review of methods and applications. Microfluid. Nanofluid. 2012, 12, 841–886. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Kobayashi, I.; Nakajima, M. Production of uniform droplets using membrane, microchannel and microfluidic emulsification devices. Microfluid. Nanofluid. 2012, 13, 151–178. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Middelberg, A.P.J. Two-phase microfluidic flows. Chem. Eng. Sci. 2011, 66, 1394–1411. [Google Scholar] [CrossRef]
- Park, J.I.; Nie, Z.H.; Kumachev, A.; Kumacheva, E. A microfluidic route to small CO2 microbubbles with narrow size distribution. Soft Matter 2010, 6, 630–634. [Google Scholar] [CrossRef]
- Taylor, J.K.; Ren, C.L.; Stubley, G.D. Numerical and experimental evaluation of microfluidic sorting devices. Biotechnol. Prog. 2008, 24, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Huang, Z.X.; Yang, R.J. Three-dimensional hydrodynamic focusing in two-layer polydimethylsiloxane (PDMS) microchannels. J. Micromech. Microeng. 2007, 17, 1479–1486. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.; Montanero, J. Revision of capillary cone-jet physics: Electrospray and flow focusing. Phys. Rev. E 2009, 79, 066305. [Google Scholar]
- Gañán-Calvo, A. Unconditional jetting. Phys. Rev. E 2008, 78, 026304. [Google Scholar] [CrossRef] [PubMed]
- Ganan-Calvo, A.M. Electro-flow focusing: The high-conductivity low-viscosity limit. Phys. Rev. Lett. 2007, 98, 239904. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M.; López-Herrera, J.M.; Riesco-Chueca, P. The combination of electrospray and flow focusing. J. Fluid Mech. 2006, 566, 421–445. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M. Generation of Steady Liquid Microthreads and Micron-Sized Monodisperse Sprays in Gas Streams. Phys. Rev. Lett. 1998, 80, 285–288. [Google Scholar] [CrossRef]
- Lee, W.; Walker, L.M.; Anna, S.L. Competition Between Viscoelasticity and Surfactant Dynamics in Flow Focusing Microfluidics. Macromol. Mater. Eng. 2011, 296, 203–213. [Google Scholar] [CrossRef]
- Stone, H.A. Dynamics of Drop Deformation and Breakup in Viscous Fluids. Annu. Rev. Fluid Mech. 1994, 26, 65–102. [Google Scholar] [CrossRef]
- Debruijn, R.A. Tipstreaming of drops in simple shear flows. Chem. Eng. Sci. 1993, 48, 277–284. [Google Scholar] [CrossRef]
- Bentley, B.J.; Leal, L.G. An experimental investigation of drop deformation and breakup in steady, two-dimensional linear flows. J. Fluid Mech. 1986, 167, 241–283. [Google Scholar] [CrossRef]
- Taylor, G.I. The formation of emulsions in definable fields of flow. Proc. R. Soc. Lond. Ser. A 1934, 146, 501–523. [Google Scholar] [CrossRef]
- Jeong, W.C.; Lim, J.M.; Choi, J.H.; Kim, J.H.; Lee, Y.J.; Kim, S.H.; Lee, G.; Kim, J.D.; Yi, G.R.; Yang, S.M. Controlled generation of submicron emulsion droplets via highly stable tip-streaming mode in microfluidic devices. Lab Chip 2012, 12, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.P.; Kim, J.S.; Erickson, J.S.; Hilliard, L.R.; Howell, P.B.; Anderson, G.P.; Nasir, M.; Ligler, F.S. Multi-wavelength microflow cytometer using groove-generated sheath flow. Lab Chip 2009, 9, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Trebbin, M.; Krüger, K.; DePonte, D.; Roth, S.V.; Chapman, H.N.; Förster, S. Microfluidic liquid jet system with compatibility for atmospheric and high-vacuum conditions. Lab Chip 2014, 14, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, W.G. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Santos, V.G.; Regiani, T.; Dias, F.F.G.; Romao, W.; Jara, J.L.P.; Klitzke, C.F.; Coelho, F.; Eberlins, M.N. Venturi Easy Ambient Sonic-Spray Ionization. Anal. Chem. 2011, 83, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, A.; Sakairi, M.; Koizumi, H. Sonic spray ionization method for atmospheric-pressure ionization mass-spectrometry. Anal. Chem. 1994, 66, 4557–4559. [Google Scholar] [CrossRef]
- Takats, Z.; Nanita, S.C.; Cooks, R.G.; Schlosser, G.; Vekey, K. Amino acid clusters formed by sonic spray ionization. Anal. Chem. 2003, 75, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Electrosonic Spray Ionization. A Gentle Technique for Generating Folded Proteins and Protein Complexes in the Gas Phase and for Studying Ion-Molecule Reactions at Atmospheric Pressure. Anal. Chem. 2004, 76, 4050–4058. [Google Scholar] [CrossRef] [PubMed]
- Kanaki, K.; Pergantis, S.A. Use of 3-nitrobenzonitrile as an additive for improved sensitivity in sonic-spray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, G. Alleviation of ion suppression effect in sonic spray ionization with induced alternating current voltage. J. Mass Spectrom. 2014, 49, 639–645. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Qian, X.; Chen, Y.; Yu, Q.; Ni, K.; Wang, X. Three-Dimensional Electro-Sonic Flow Focusing Ionization Microfluidic Chip for Mass Spectrometry. Micromachines 2015, 6, 1890-1902. https://doi.org/10.3390/mi6121463
Yu C, Qian X, Chen Y, Yu Q, Ni K, Wang X. Three-Dimensional Electro-Sonic Flow Focusing Ionization Microfluidic Chip for Mass Spectrometry. Micromachines. 2015; 6(12):1890-1902. https://doi.org/10.3390/mi6121463
Chicago/Turabian StyleYu, Cilong, Xiang Qian, Yan Chen, Quan Yu, Kai Ni, and Xiaohao Wang. 2015. "Three-Dimensional Electro-Sonic Flow Focusing Ionization Microfluidic Chip for Mass Spectrometry" Micromachines 6, no. 12: 1890-1902. https://doi.org/10.3390/mi6121463