Progress of MEMS Scanning Micromirrors for Optical Bio-Imaging
Abstract
:1. Introduction
2. Scanning Micromirror Technologies
2.1. Thermoelectric Actuation
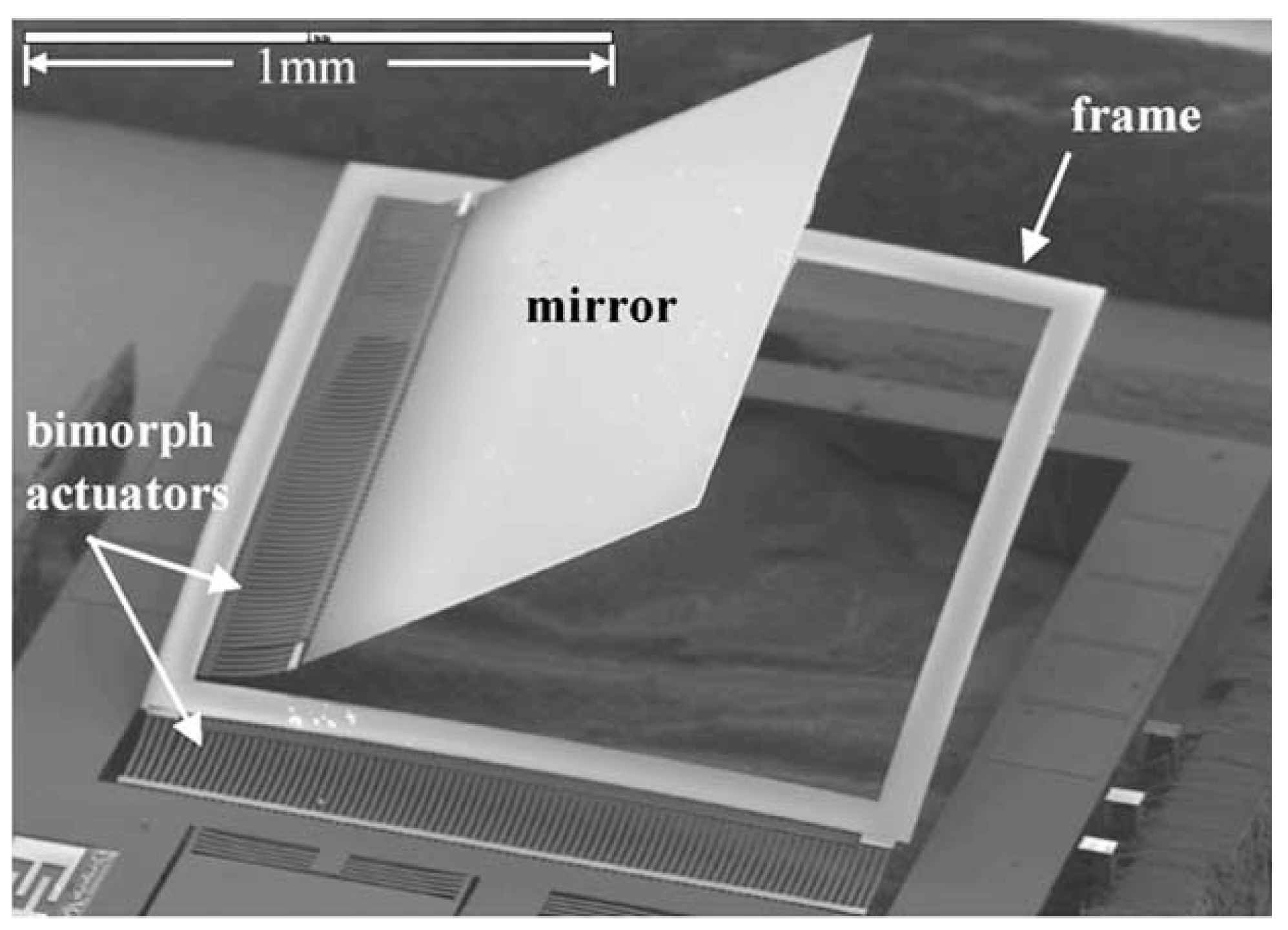
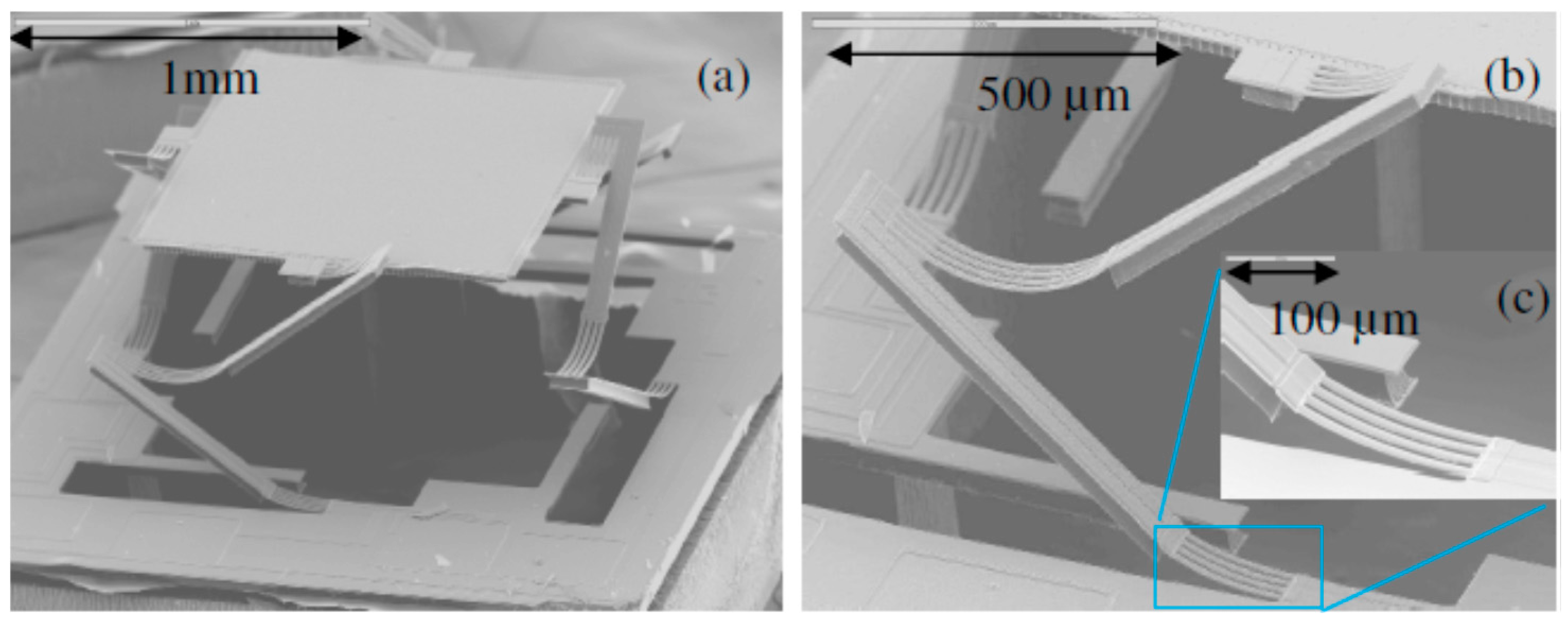
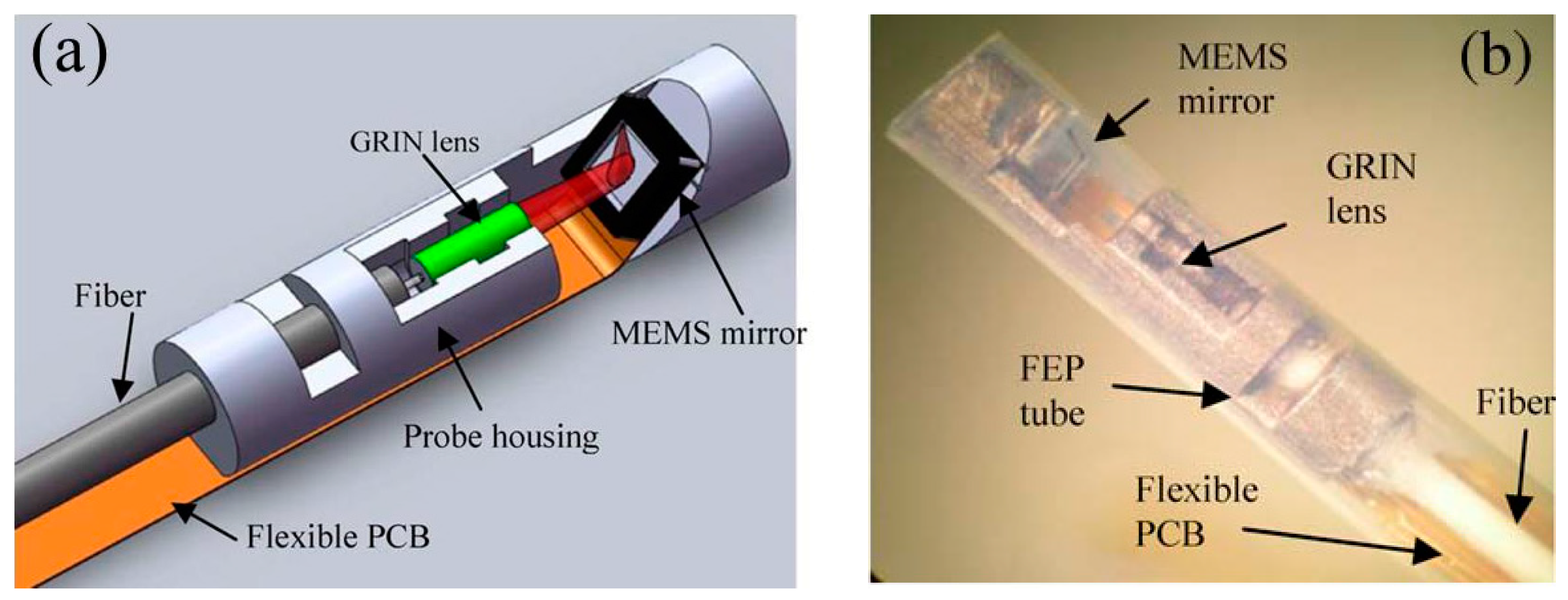

2.2. Electrostatic Actuation
2.2.1. Comb-Drive Actuation
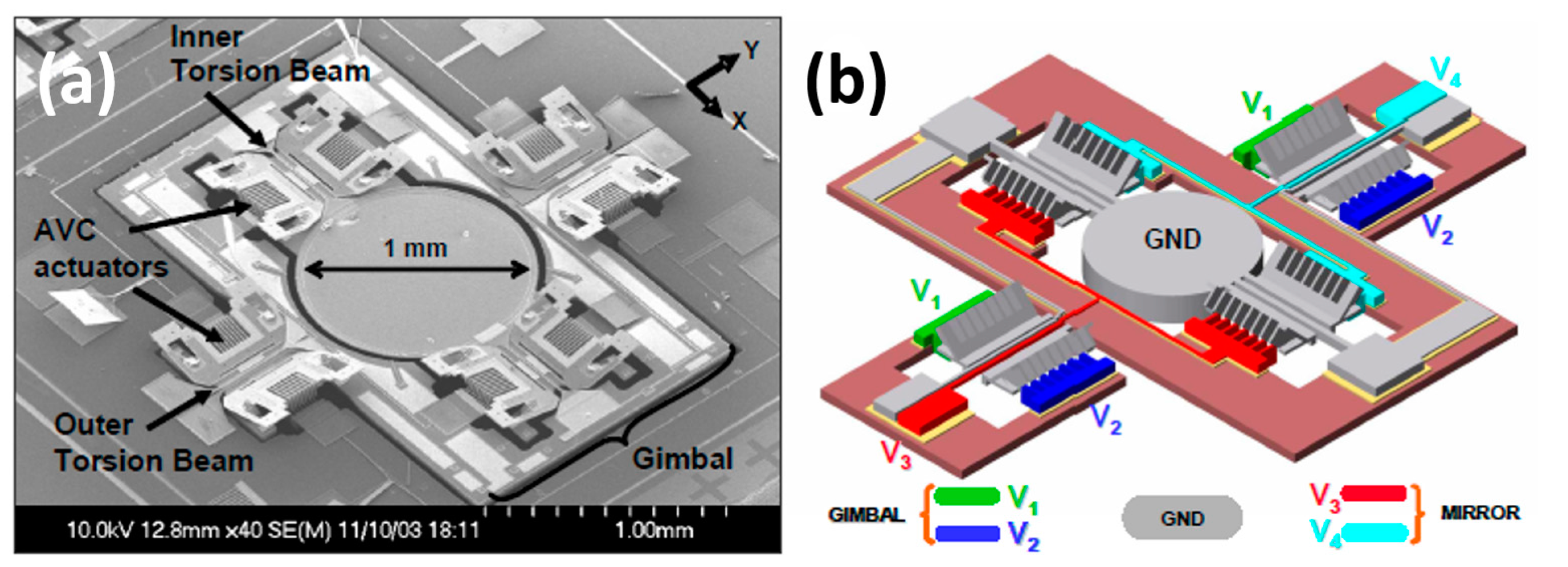
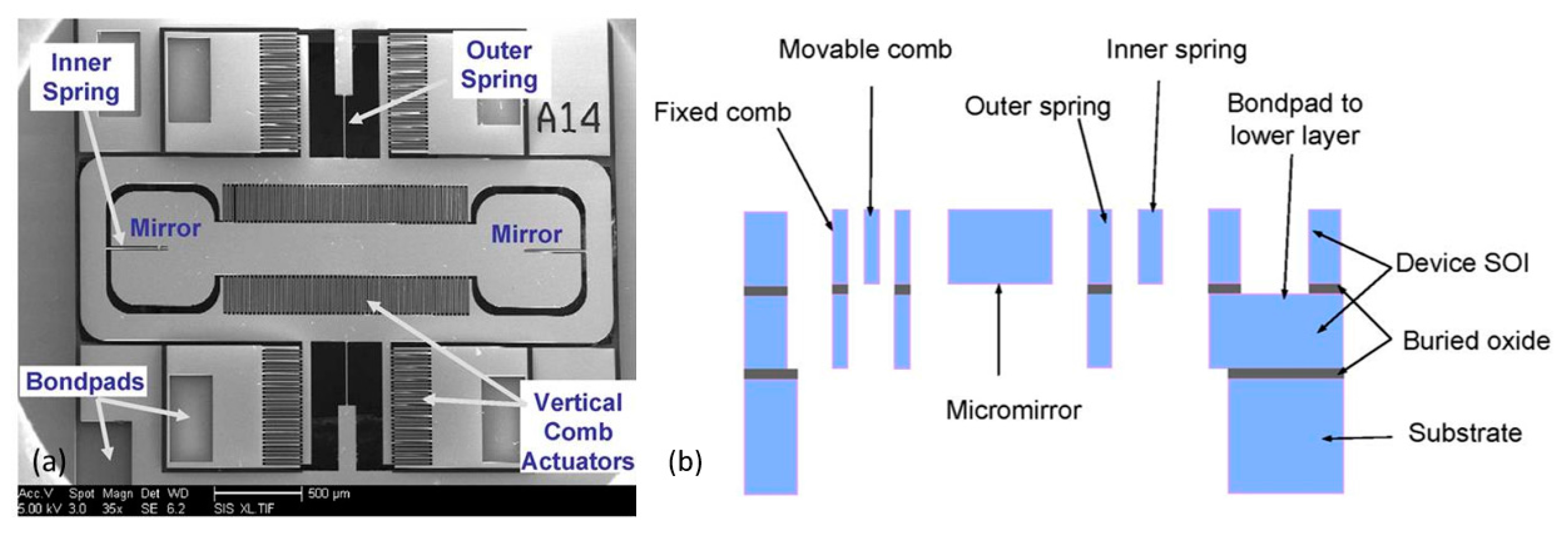

2.2.2. Gap-Closing Actuation
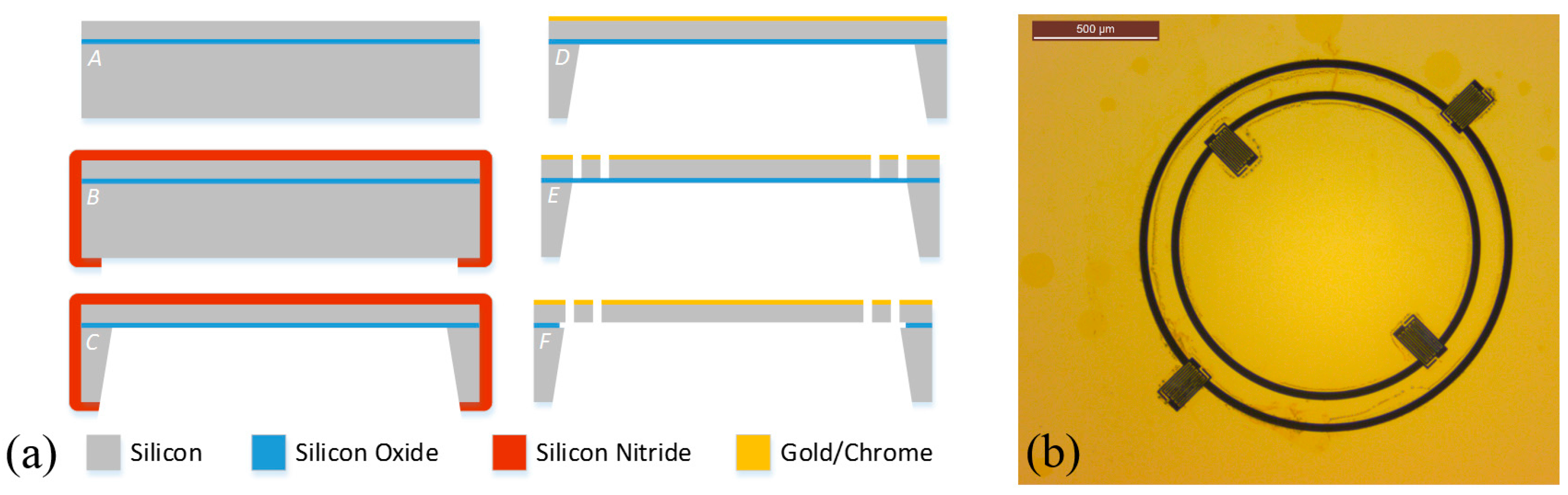
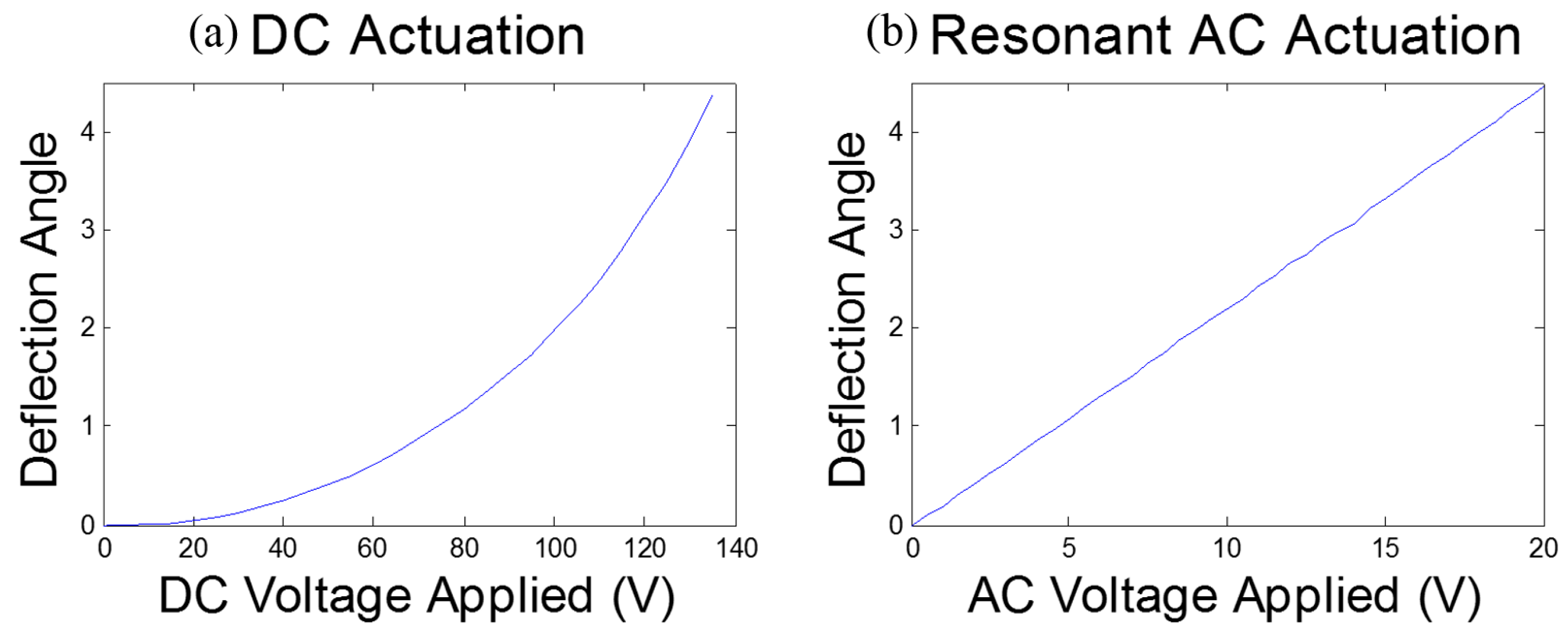

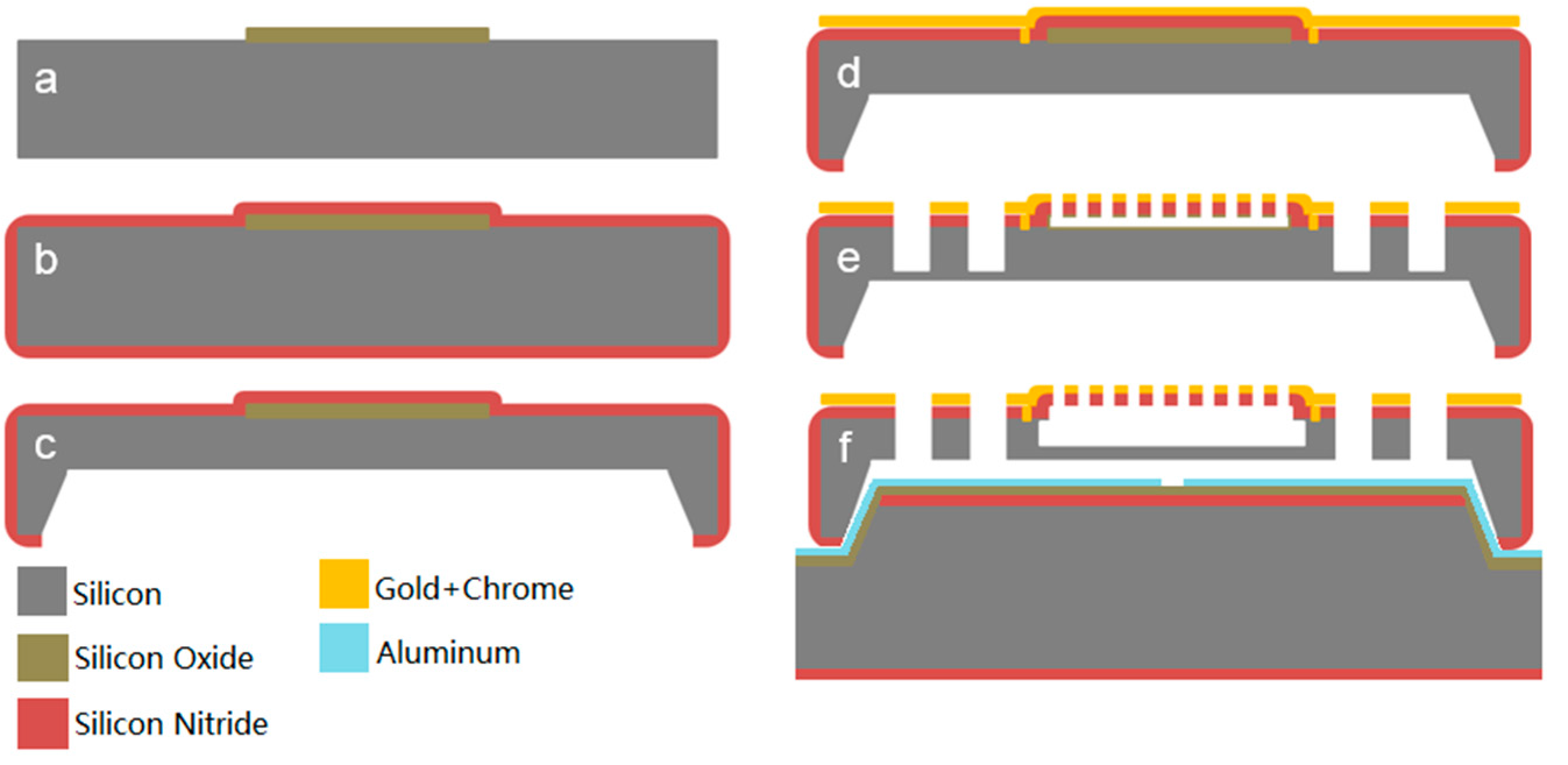
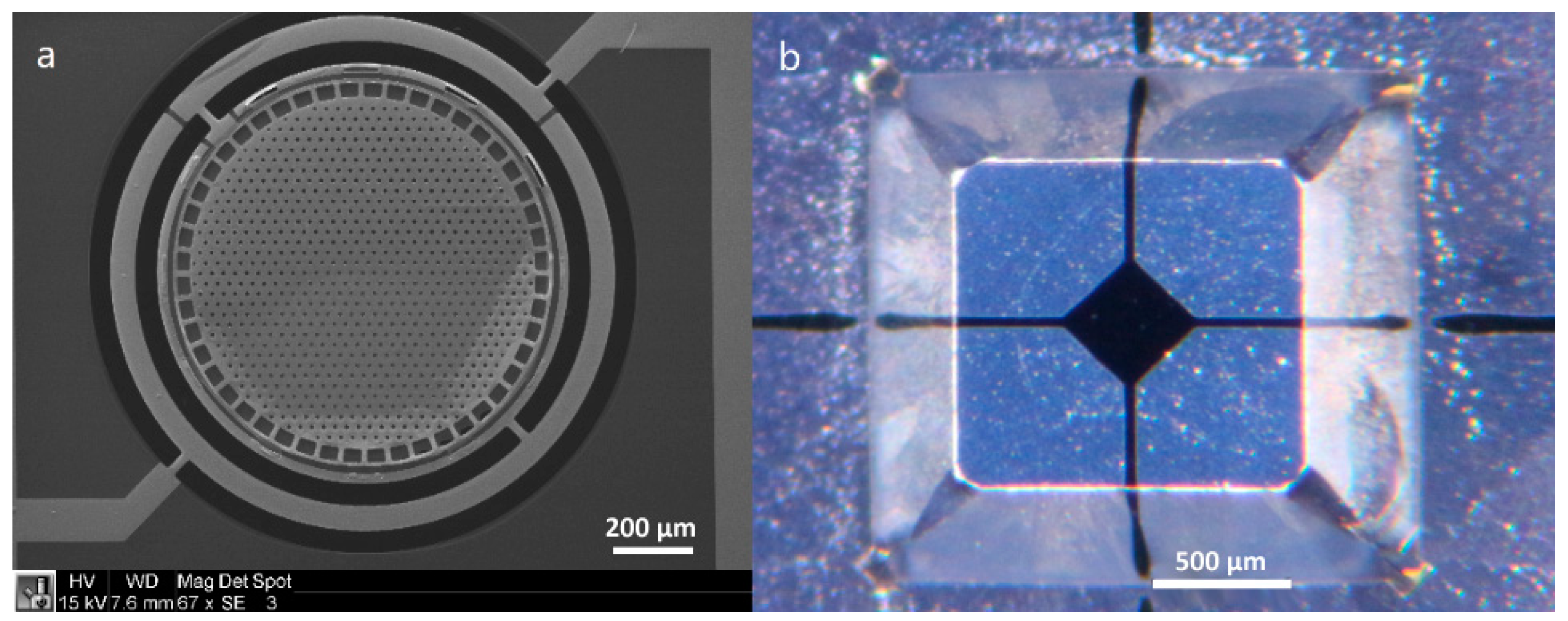
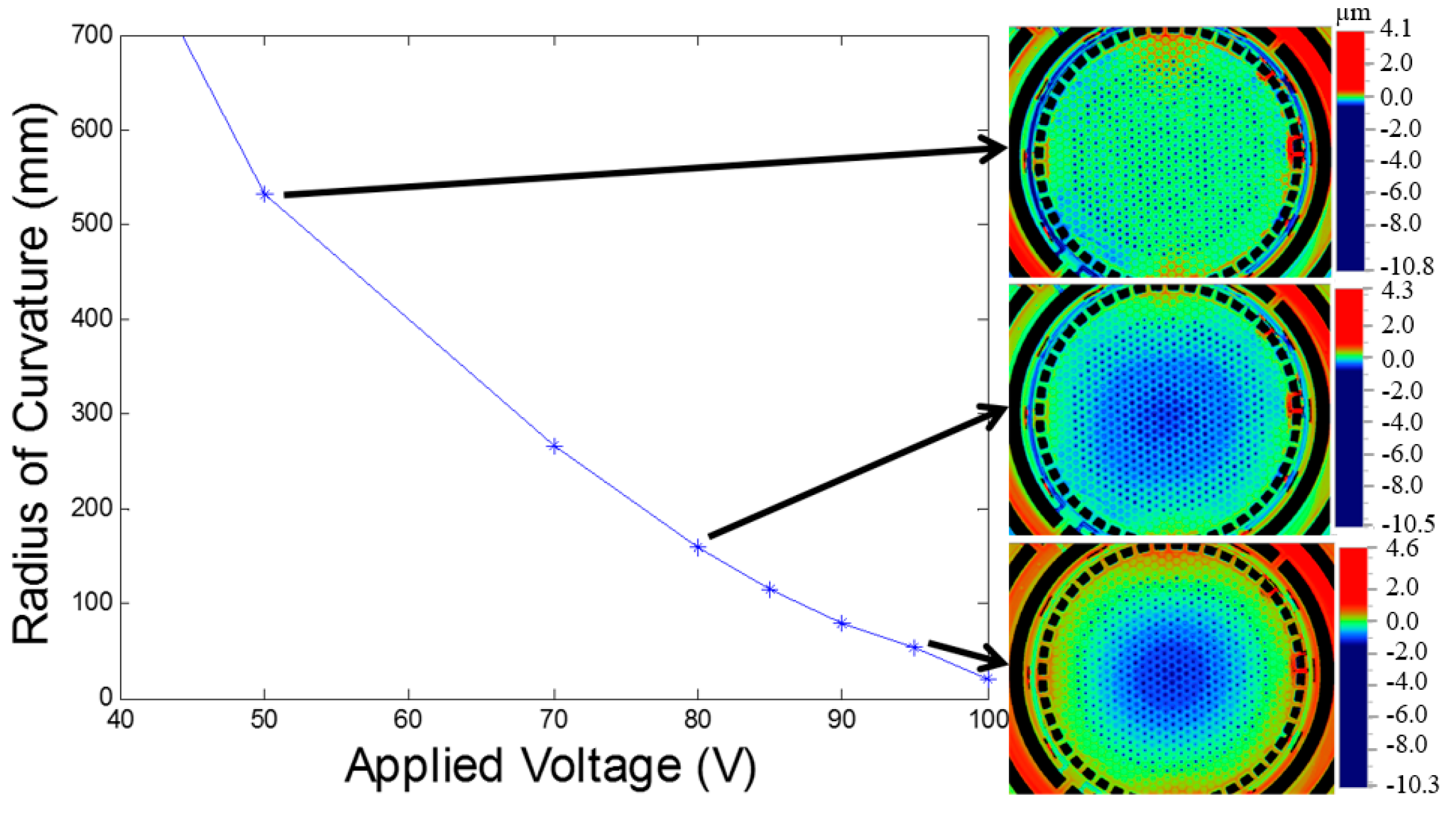
2.3. Electromagnetic Actuation
2.4. Piezoelectric Actuation
3. Image Correction
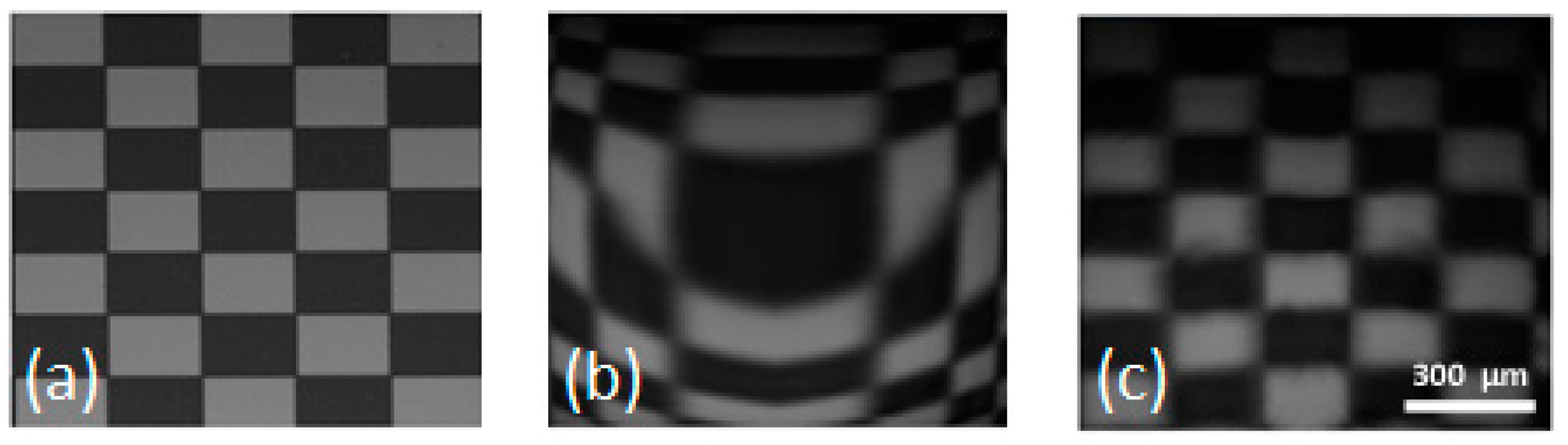
4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pawley, J.B. Handbook of Biological Confocal Microscopy; Plenum Press: New York, NY, USA, 1995. [Google Scholar]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Brezinski, M.E.; Bouma, B.E.; Boppart, S.A.; Pitris, C.; Southern, J.F.; Fujimoto, J.G. In vivo endoscopic optical biopsy with optical coherence tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Brezinski, M.E.; Fujimoto, J.G.; Weissman, N.J.; Boppart, S.A.; Bouma, B.E.; Southern, J.F. Scanning single-mode fiber optic catheter–endoscope for optical coherence tomography. Opt. Lett. 1996, 21, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cobb, M.J.; Chen, Y.; Kimmey, M.B.; Li, X. Rapid-scanning forward-imaging miniature endoscope for real-time optical coherence tomography. Opt. Lett. 2004, 29, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Seibel, E.J.; Smithwick, Q.Y.J. Unique features of optical scanning, single fiber endoscopy. Lasers Surg. Med. 2002, 30, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Arrasmith, C.L.; Dickensheets, D.L.; Mahadevan-Jansen, A. Mems-based handheld confocal microscope for in vivo skin imaging. Opt. Express 2010, 18, 3805–3819. [Google Scholar] [CrossRef] [PubMed]
- Dickensheets, D.L.; Kino, G.S. Silicon-micromachined scanning confocal optical microscope. J. Microelectromech. Syst. 1998, 7, 38–47. [Google Scholar] [CrossRef]
- Miyajima, H.; Asaoka, N.; Isokawa, T.; Ogata, M.; Aoki, Y.; Imai, M.; Fujimori, O.; Katashiro, M.; Matsumoto, K. A mems electromagnetic optical scanner for a commercial confocal laser scanning microscope. J. Microelectromech. Syst. 2003, 12, 243–251. [Google Scholar] [CrossRef]
- Piyawattanametha, W.; Hyejun, R.; Mandella, M.J.; Loewke, K.; Wang, T.D.; Kino, G.S.; Solgaard, O.; Contag, C.H. 3D near-infrared fluorescence imaging using an mems-based miniature dual-axis confocal microscope. IEEE J. Sel. Top. Quantum Electron. 2009, 15, 1344–1350. [Google Scholar] [CrossRef]
- Piyawattanametha, W.; Ra, H.; Qiu, Z.; Friedland, S.; Liu, J.T.C.; Loewke, K.; Kino, G.S.; Solgaard, O.; Wang, T.D.; Mandella, M.J.; et al. In vivo near-infrared dual-axis confocal microendoscopy in the human lower gastrointestinal tract. J. Biomed. Opt. 2012, 17, 0211021–0211024. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, E.; Zhang, X.; Liang, W.; Li, X.; Xie, H. Mems-based 3D confocal scanning microendoscope using memsscanners for both lateral and axial scan. Sens. Actuators A 2014, 215, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, B.H.; Maguluri, G.N.; Lee, T.W.; Rogomentich, F.J.; Bancu, M.G.; Bouma, B.E.; de Boer, J.F.; Bernstein, J.J. Two-axis magnetically-driven mems scanning catheter for endoscopic high-speed optical coherence tomography. Opt. Express 2007, 15, 18130–18140. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Zhang, J.; Wang, L.; Wilder-Smith, P.; Chen, Z.; McCormick, D.T.; Tien, N.C. Three-dimensional optical coherence tomography employing a 2-axis microelectromechanical scanning mirror. IEEE J. Sel. Top. Quantum Electron. 2005, 11, 806–810. [Google Scholar] [CrossRef]
- Yang, V.X.D.; Mao, Y.; Standish, B.A.; Munce, N.R.; Chiu, S.; Burnes, D.; Wilson, B.C.; Vitkin, I.A.; Himmer, P.A.; Dickensheets, D.L. Doppler optical coherence tomography with a micro-electro-mechanical membrane mirror for high-speed dynamic focus tracking. Opt. Lett. 2006, 31, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Strathman, M.; Liu, Y.; Keeler, E.G.; Song, M.; Baran, U.; Xi, J.; Sun, M.-T.; Wang, R.; Li, X.; Lin, L.Y. Mems scanning micromirror for optical coherence tomography. Biomed. Opt. Express 2015, 6, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.D.; Hertz, P.R.; Chen, Y.; Fujimoto, J.G.; Piyawattanametha, W.; Fan, L.; Wu, M.C. Two-axis mems scanning catheter for ultrahigh resolution three-dimensional and en face imaging. Opt. Express 2007, 15, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kopa, A.; Pan, Y.; Fedder, G.K.; Xie, H. A two-axis electrothermal micromirror for endoscopic optical coherence tomography. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 636–642. [Google Scholar] [CrossRef]
- Sun, J.; Guo, S.; Wu, L.; Liu, L.; Choe, S.-W.; Sorg, B.S.; Xie, H. 3D in vivo optical coherence tomography based on a low-voltage, large-scan-range 2D mems mirror. Opt. Express 2010, 18, 12065–12075. [Google Scholar] [CrossRef] [PubMed]
- Piyawattanametha, W.; Barretto, R.P.J.; Ko, T.H.; Flusberg, B.A.; Cocker, E.D.; Ra, H.; Lee, D.; Solgaard, O.; Schnitzer, M.J. Fast-scanning two-photon fluorescence imaging based on a microelectromechanical systems two-dimensional scanning mirror. Opt. Lett. 2006, 31, 2018–2020. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Imboden, M.; Little, T.D.C.; Bishop, D.J. Electrothermally actuated tip-tilt-piston micromirror with integrated varifocal capability. Opt. Express 2015, 23, 9555–9566. [Google Scholar] [CrossRef] [PubMed]
- Dickensheets, D.L. Requirements of mems membrane mirrors for focus adjustment and aberration correction in endoscopic confocal and optical coherence tomography imaging instruments. J. Micro Nanolithogr. MEMS MOEMS 2008, 7, 021008. [Google Scholar] [CrossRef]
- Qi, B.; Himmer, P.A.; Gordon, M.L.; Yang, V.X.D.; Dickensheets, D.L.; Vitkin, I.A. Dynamic focus control in high-speed optical coherence tomography based on a microelectromechanical mirror. Opt. Commun. 2004, 232, 123–128. [Google Scholar] [CrossRef]
- Sasaki, T.; Hane, K. Initial deflection of silicon-on-insulator thin membrane micro-mirror and fabrication of varifocal mirror. Sens. Actuators A Phys. 2011, 172, 516–522. [Google Scholar] [CrossRef]
- Strathman, M.; Liu, Y.; Li, X.; Lin, L.Y. Dynamic focus-tracking mems scanning micromirror with low actuation voltages for endoscopic imaging. Opt. Express 2013, 21, 23934–23941. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Pan, Y.; Fedder, G.K. Endoscopic optical coherence tomographic imaging with a cmos-mems micromirror. Sens. Actuators A 2003, 103, 237–241. [Google Scholar] [CrossRef]
- Fu, L.; Jain, A.; Xie, H.; Cranfield, C.; Gu, M. Nonlinear optical endoscopy based on a double-clad photonic crystal fiber and a mems mirror. Opt. Express 2006, 14, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, S.R.; Wu, L.; Sun, J.; Choe, S.-W.; Sorg, B.S.; Xie, H. A 2.8-mm imaging probe based on a high-fill-factor mems mirror and wire-bonding-free packaging for endoscopic optical coherence tomography. J. Microelectromechanical Syst. 2012, 21, 1291–1302. [Google Scholar] [CrossRef]
- Liu, W.; Talghader, J.J. Current-controlled curvature of coated micromirrors. Opt. Lett. 2003, 28, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Teo, J.H.S.; Xu, Y.; Premachandran, C.S.; Chen, N.; Kotlanka, R.; Olivo, M.; Sheppard, C.J.R. A two axes scanning soi mems micromirror for endoscopic bioimaging. J. Micromech. Microeng. 2008, 18, 025001. [Google Scholar] [CrossRef]
- Jung, W.; McCormick, D.T.; Zhang, J.; Wang, L.; Tien, N.C.; Chen, Z. Three-dimensional endoscopic optical coherence tomography by use of a two-axis microelectromechanical scanning mirror. Appl. Phys. Lett. 2006, 88, 163901. [Google Scholar] [CrossRef]
- Karthik, K.; Jonathan, C.C.; Austin, M.; Nate, J.K.; Kazunori, H.; Thomas, E.M.; Xiaojing, Z. Fast 3d in vivo swept-source optical coherence tomography using a two-axis mems scanning micromirror. J. Opt. A Pure Appl. Opt. 2008, 10, 044013. [Google Scholar] [CrossRef]
- Hyejun, R.; Piyawattanametha, W.; Taguchi, Y.; Lee, S.; Mandella, M.J.; Solgaard, O. Two-dimensional mems scanner for dual-axes confocal microscopy. J. Microelectromech. Syst. 2007, 16, 969–976. [Google Scholar] [CrossRef]
- Maitland, K.C.; Shin, H.J.; Ra, H.; Lee, D.; Solgaard, O.; Richards-Kortum, R. Single fiber confocal microscope with a two-axis gimbaled mems scanner for cellular imaging. Opt. Express 2006, 14, 8604–8612. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.C.; Mandella, M.J.; Ra, H.; Wong, L.K.; Solgaard, O.; Kino, G.S.; Piyawattanametha, W.; Contag, C.H.; Wang, T.D. Miniature near-infrared dual-axes confocal microscope utilizing a two-dimensional microelectromechanical systems scanner. Opt. Lett. 2007, 32, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Piyawattanametha, W.; Wang, T.D. Mems-based dual-axes confocal microendoscopy. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Mandella, M.J.; Contag, C.H.; Kino, G.S. Dual-axis confocal microscope for high-resolution in vivo imaging. Opt. Lett. 2003, 28, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Jae-Woong, J.; Sunwoo, K.; Solgaard, O. Split-frame gimbaled two-dimensional mems scanner for miniature dual-axis confocal microendoscopes fabricated by front-side processing. J. Microelectromech. Syst. 2012, 21, 308–315. [Google Scholar] [CrossRef]
- Yeow, J.T.W.; Yang, V.X.D.; Chahwan, A.; Gordon, M.L.; Qi, B.; Vitkin, I.A.; Wilson, B.C.; Goldenberg, A.A. Micromachined 2D scanner for 3D optical coherence tomography. Sens. Actuators A Phys. 2005, 117, 331–340. [Google Scholar] [CrossRef]
- Hokari, R.; Hane, K. Micro-mirror laser scanner combined with a varifocal mirror. Microsyst. Technol. 2012, 18, 475–480. [Google Scholar] [CrossRef]
- Shao, Y.; Dickensheets, D.L. Moems 3D scan mirror for single-point control of beam deflection and focus. MOEMS 2005, 4, 041502. [Google Scholar] [CrossRef]
- Beeby, S.P.; Blackburn, A.; White, N.M. Processing of PZT piezoelectric thick films on silicon for microelectromechanical systems. J. Micromech. Microeng. 1999, 9, 218–229. [Google Scholar] [CrossRef]
- Gilchrist, K.H.; McNabb, R.P.; Izatt, J.A.; Grego, S. Piezoelectric scanning mirrors for endoscopic optical coherence tomography. J. Micromech. Microeng. 2009, 19, 095012. [Google Scholar] [CrossRef]
- Qiu, Z.; Rhee, C.-H.; Choi, J.; Wang, T.D.; Oldham, K.R. Large stroke verticle pzt microactuator with high-speed rotational scanning. J. Microelectromech. Syst. 2014, 23, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Siedlecki, D.; Remon, L.; Marcos, S. Optical coherence tomography for quantitative surface topography. Appl. Opt. 2009, 48, 6708–6715. [Google Scholar] [CrossRef] [PubMed]
- Podoleanu, A.; Charalambous, I.; Plesea, L.; Dogariu, A.; Rosen, R. Correction of distortions in optical coherence tomography imaging of the eye. Phys. Med. Biol. 2004, 49, 1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, P.; Samuelson, S.; Jia, H.; Ma, J.; Xie, H. Correction of image distortions in endoscopic optical coherence tomography based on two-axis scanning mems mirrors. Biomed. Opt. Express 2013, 4, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, Y.; Kang, J.U. Distortion-free freehand-scanning OCT implemented with real-time scanning speed variance correction. Opt. Express 2012, 20, 16567–16583. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.Y.; Keeler, E.G. Progress of MEMS Scanning Micromirrors for Optical Bio-Imaging. Micromachines 2015, 6, 1675-1689. https://doi.org/10.3390/mi6111450
Lin LY, Keeler EG. Progress of MEMS Scanning Micromirrors for Optical Bio-Imaging. Micromachines. 2015; 6(11):1675-1689. https://doi.org/10.3390/mi6111450
Chicago/Turabian StyleLin, Lih Y., and Ethan G. Keeler. 2015. "Progress of MEMS Scanning Micromirrors for Optical Bio-Imaging" Micromachines 6, no. 11: 1675-1689. https://doi.org/10.3390/mi6111450




