Digital Microfluidic System with Vertical Functionality
Abstract
:1. Introduction
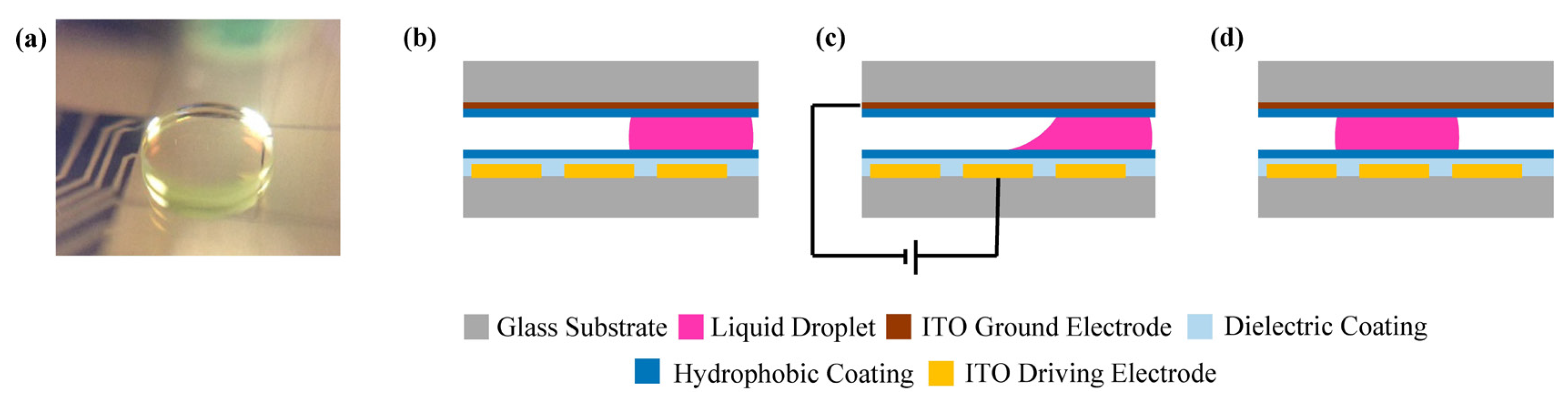
2. Experimental Section
2.1. Experimental Design and Configuration
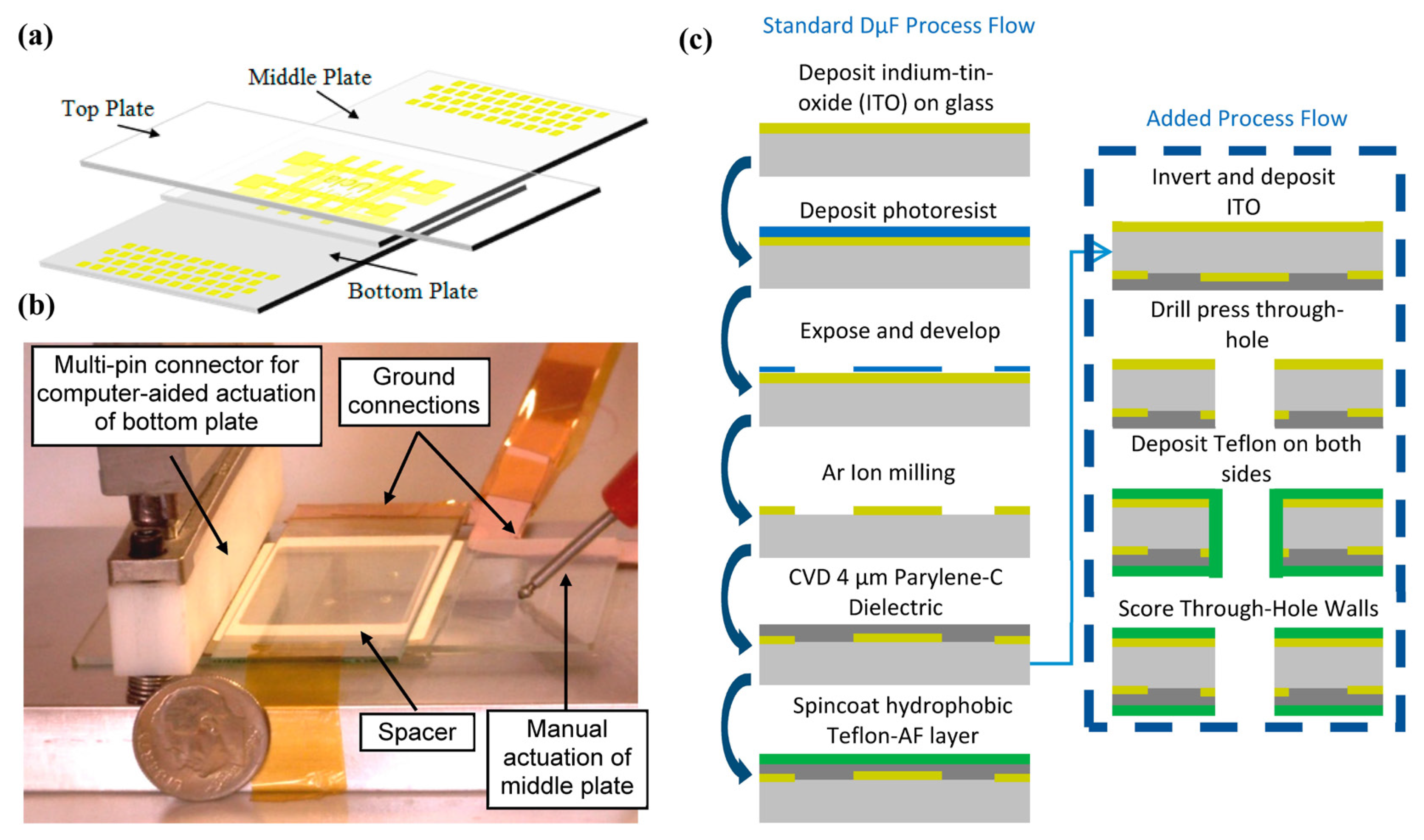
2.2. Bottom DµF Plate and Top Plate Fabrication
2.3. Middle DµF Plate Fabrication
2.4. DµF Experiments
2.4.1. Vertical Functionality
2.4.2. Calcium Alginate Hydrogel Particle Sieve
2.5. Calcium Alginate Hydrogel Fabrication and Characterization
3. Results and Discussion
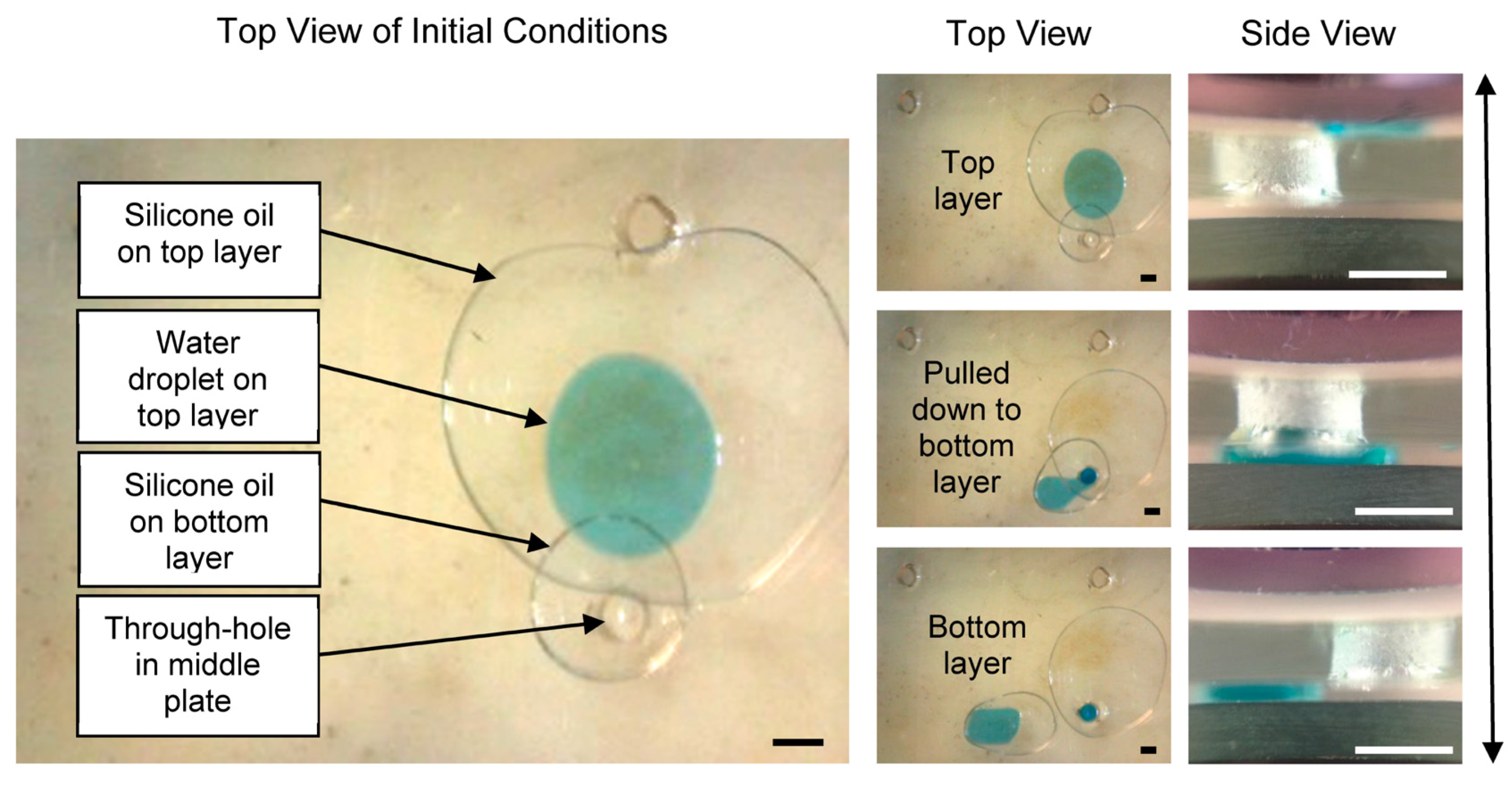
3.1. Vertical Functionality
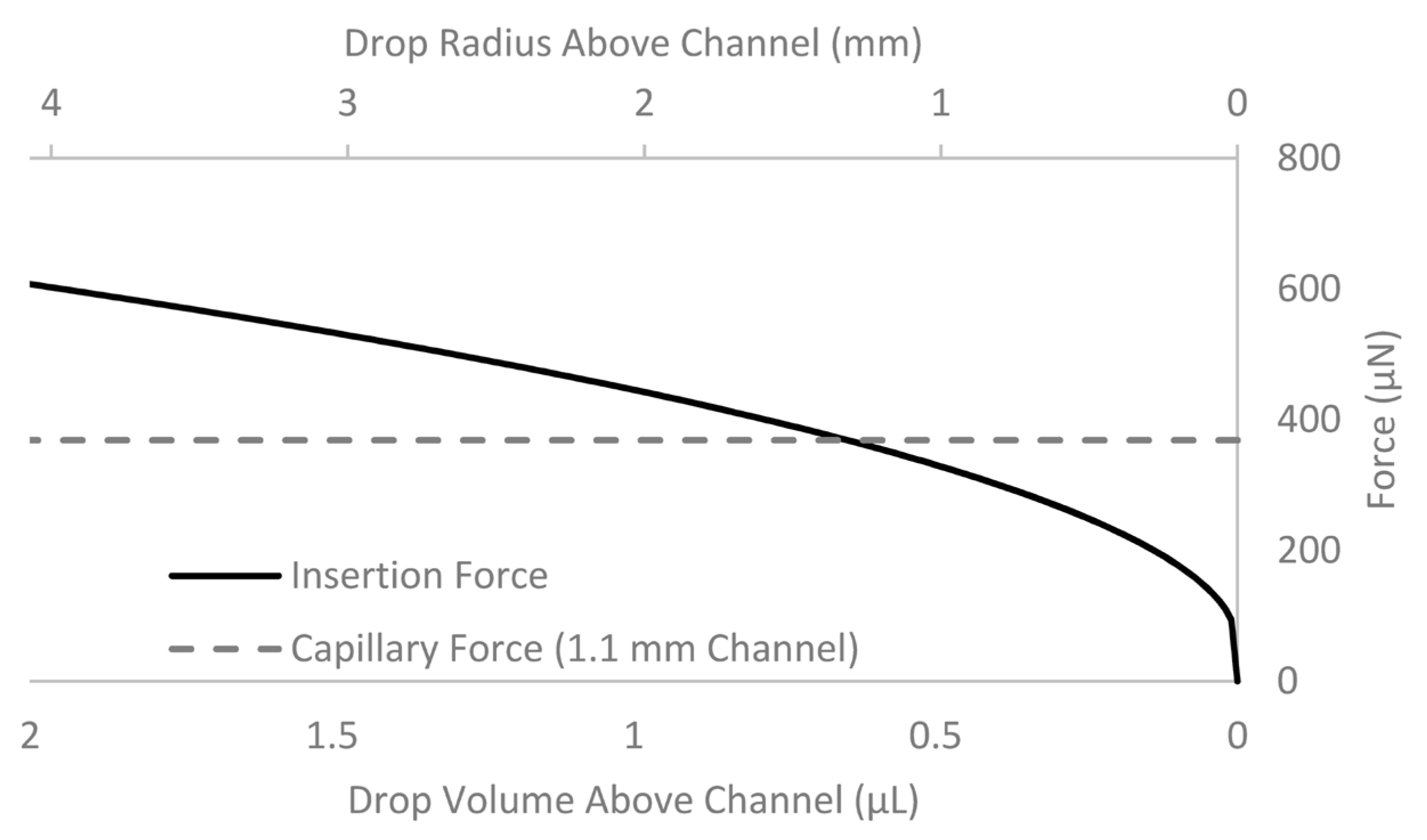
3.2. Characterization of Droplet Forces and Design Parameters

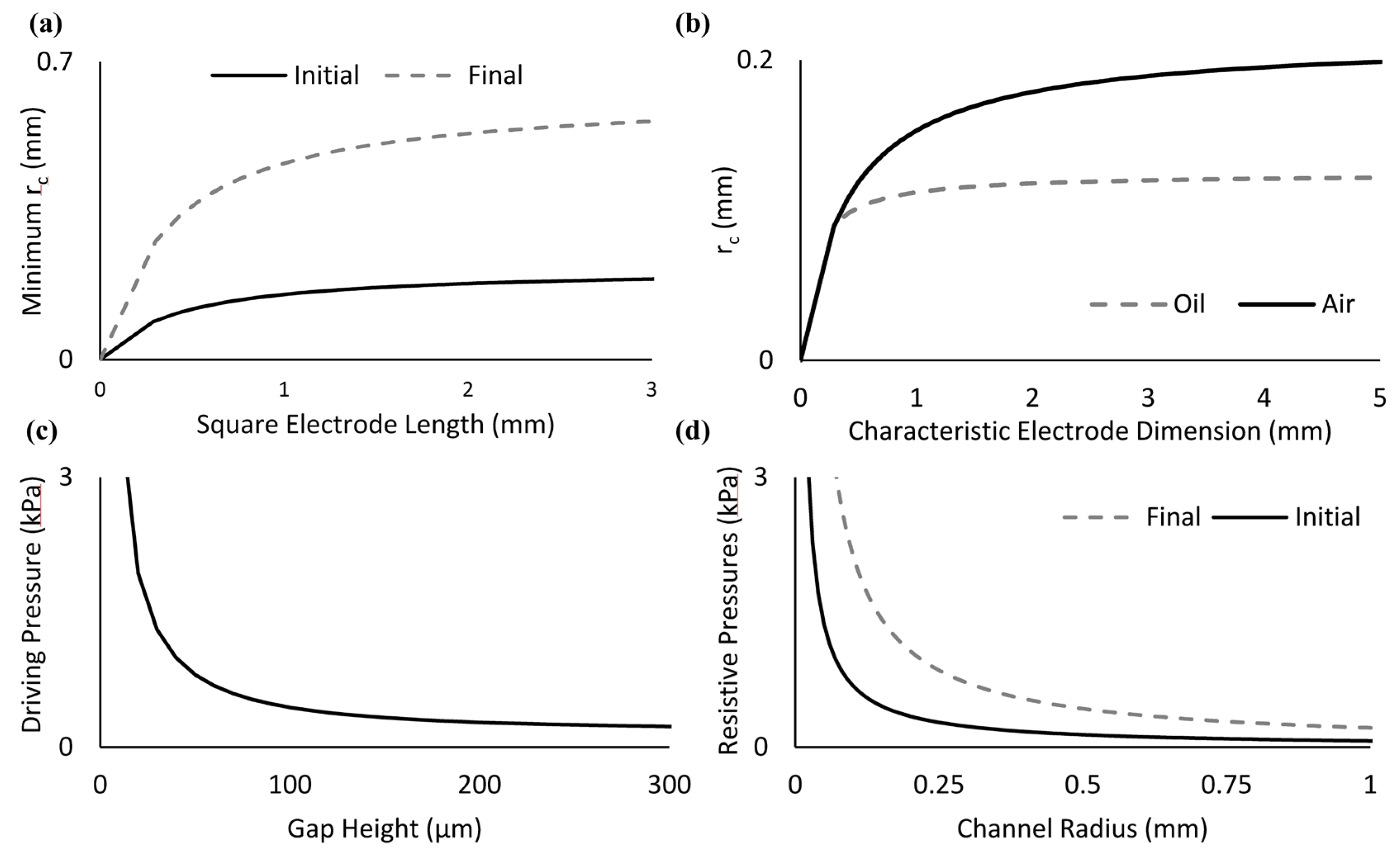
3.3. Applications
3.3.1. Sample-in-Sample Delivery with Spatiotemporal Control
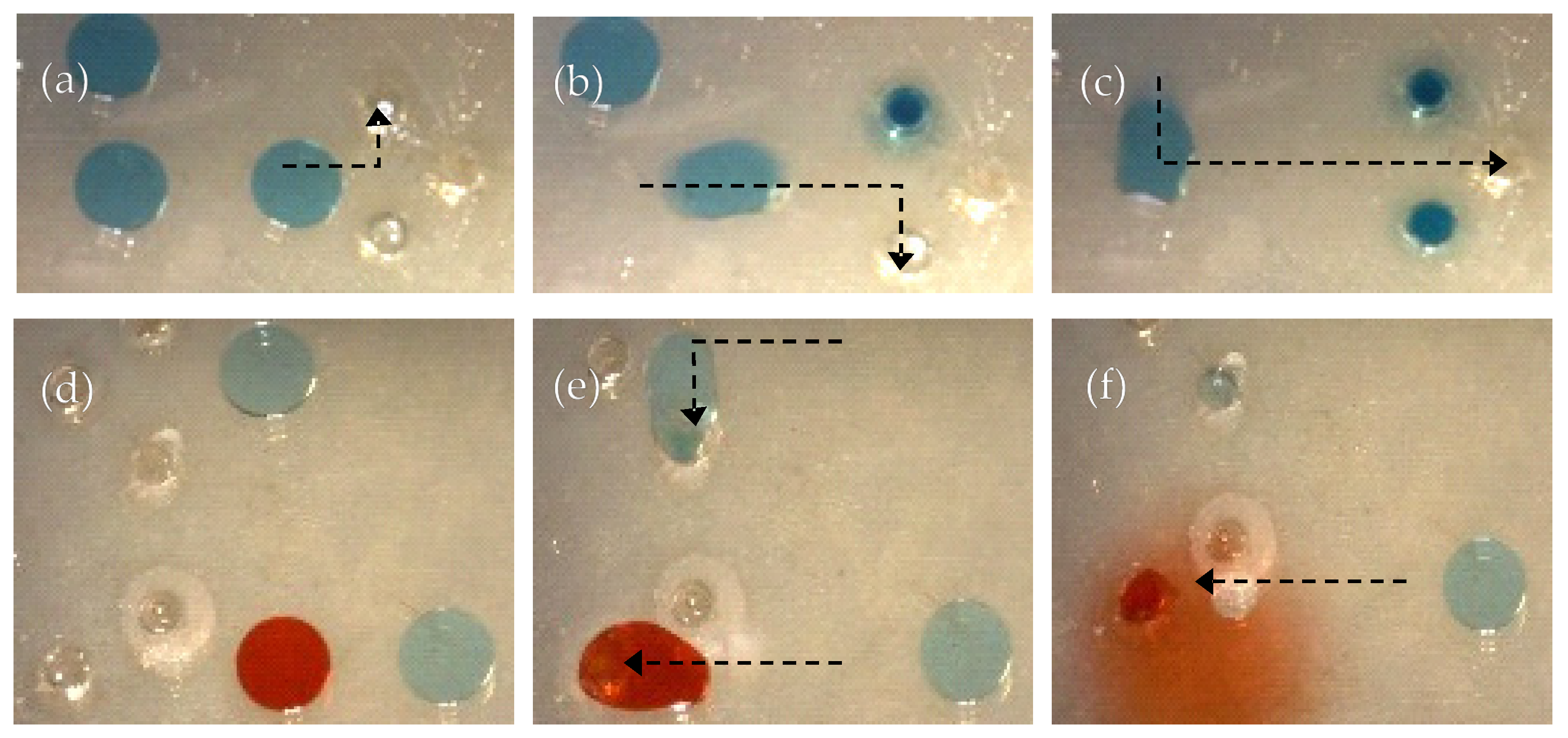
3.3.2. Calcium Alginate Hydrogel Crosslink Gradient

3.3.3. Embryoid Body (EB) Sample Retrieval
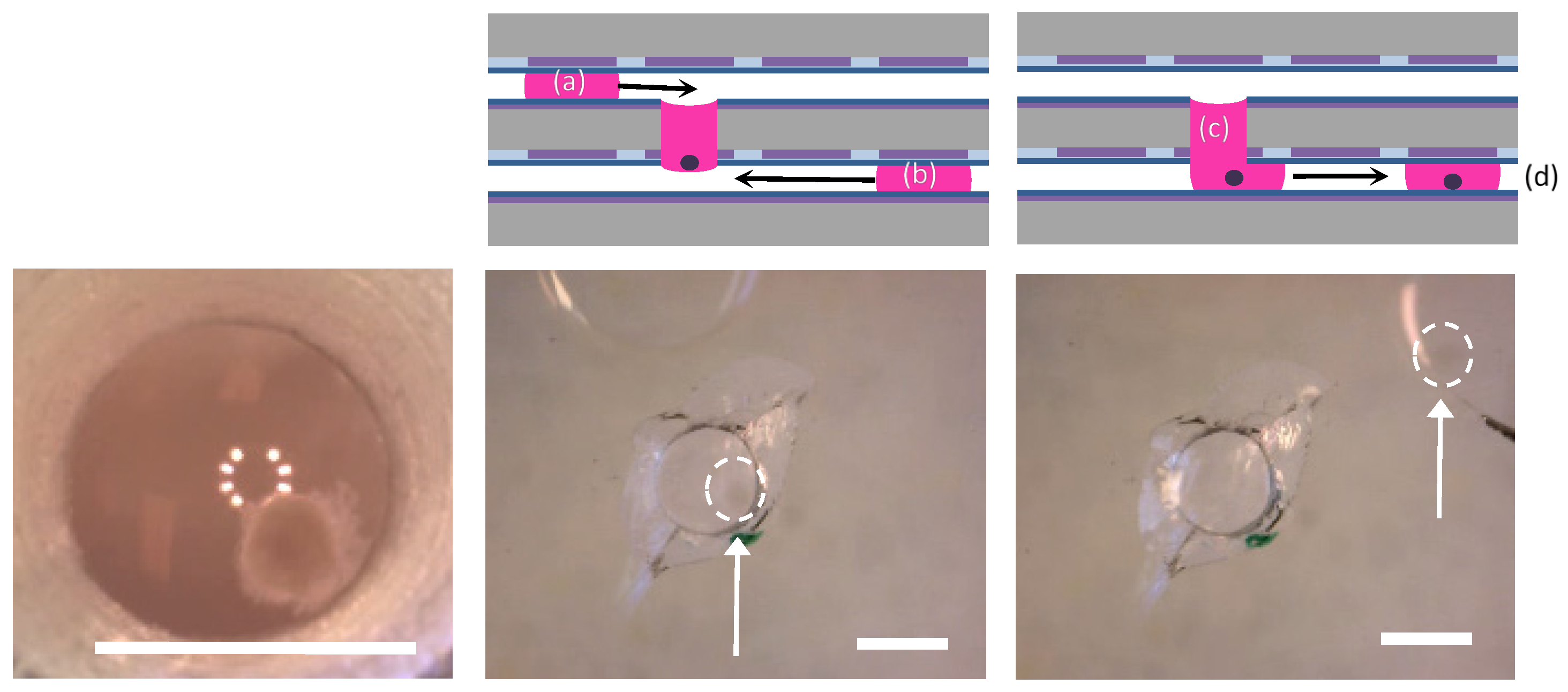
3.3.4. Particle Sieving

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mugele, F.; Baret, J.-C. Electrowetting: From basics to applications. J. Phys. Condens. Matter 2005, 17, R705–R775. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H.; Chang-Jin, K. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar] [CrossRef]
- Choi, K.; Ng, A.H.C.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef] [PubMed]
- Jebrail, M.J.; Bartschb, M.S.; Patel, K.D. Digital microfluidics: A versatile tool for applications in chemistry, biology and medicine. Lab Chip 2012, 12, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Fobel, R.; Fobel, C.; Wheeler, A.R. Dropbot: An open-source digital microfluidic control system with precise control of electrostatic driving force and instantaneous drop velocity measurement. Appl. Phys. Lett. 2013, 102, 193513. [Google Scholar] [CrossRef]
- Paik, P.; Pamula, V.K.; Fair, R.B. Rapid droplet mixers for digital microfluidic systems. Lab Chip 2003, 3, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Sukhatme, S.; Agarwal, A. Digital microfluidics: Techniques, their applications and advantages. J. Bioeng. Biomed. Sci. 2012, S8, 001. [Google Scholar]
- Nelson, W.C.; Kim, C.-J. Droplet actuation by electrowetting-on-dielectric (EWOD): A review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef]
- Aijian, A.P.; Chatterjee, D.; Garrell, R.L. Fluorinated liquid-enabled protein handling and surfactant-aided crystallization for fully in situ digital microfluidic MALDI-MS analysis. Lab Chip 2012, 12, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Hetayothin, B.; Wheeler, A.R.; King, D.J.; Garrell, R.L. Droplet-based microfluidics with nonaqueous solvents and solutions. Lab Chip 2005, 6, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Shepherd, H.; Garrell, R.L. Electromechanical model for actuating liquids in a two-plate droplet microfluidic device. Lab Chip 2009, 9, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Korsmeyer, T. Principles of droplet electrohydrodynamics for lab-on-a-chip. Lab Chip 2004, 4, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.; Watson, M.; Wheeler, A. Hybrid microfluidics: A digital-to-channel interface for in-line sample processing and chemical separations. Lab Chip 2009, 9, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jones, T.B. Moving droplets between closed and open microfluidic systems. Lab Chip 2015, 15, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Fair, R.B.; Pollack, M.G. Automated on-chip droplet dispensing with volume control by electro-wetting actuation and capacitance metering. Sens. Actuators B Chem. 2004, 98, 319–327. [Google Scholar] [CrossRef]
- Bhargava, K.C.; Thompson, B.; Malmstadt, N. Discrete elements for 3D microfluidics. Proc. Natl. Acad. Sci. USA 2014, 111, 15013–15018. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fan, S.-K.; Lin, C.-P.; Wu, C.-T.; Hsu, W. 3D Droplet Transportations by EWOD Actuations on Flexible Polymer Films. In Proceedings of ASME 2005 International Mechanical Engineering Congress and Exposition, Orlando, FL, USA, 5–11 November 2005; pp. 249–252.
- George, S.M.; Moon, H. Three dimensional tissue based digital microfluidic screening platform. In Proceedings of 2011 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2011), Seattle, WA, USA, 2–6 October 2011; pp. 1545–1547.
- Ay, C.; Yeh, C.-C.; Hsu, M.-C.; Hurng, H.-Y.; Kwok, P.C.L.; Chang, H.-I. Evaluation of the correlation between focal adhesion kinase phosphorylation and cell adhesion force using “DEP” technology. Sensors 2012, 12, 5951–5965. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-K.; Huang, P.-W.; Wange, T.-T.; Peng, Y.-H. Cross-scale electric manipulations of cells and droplets by frequency-modulated dielectrophoresis and electrowetting. Lab Chip 2008, 8, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, X.B.; Becker, F.F.; Gascoyne, P.R.C. Introducing dielectrophoresis as a new force field for field-flow fractionation. Biophys. J. 1997, 73, 1118–1129. [Google Scholar] [CrossRef]
- Huang, Y.; Holzel, R.; Pethig, R.; Wang, X.-B. Differences in the ac electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Phys. Med. Biol. 1992, 37, 1499–1517. [Google Scholar] [CrossRef] [PubMed]
- Luk, V.N.; Fiddes, L.K.; Luk, V.M.; Kumacheva, E.; Wheeler, A.R. Digital microfluidic hydrogel microreactors for proteomics. Proteomics 2012, 12, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-Y.; Fan, S.-K. Electric manipulations of hydrogel on a digital microfluidic platform. In Proceedings of 2012 7th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Kyoto, Japan, 5–8 March 2012; pp. 407–410.
- Pethig, R. Review article—Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 4–35. [Google Scholar]
- Gupta, S.; Alargova, R.G.; Kilpatrick, P.K.; Velev, O.D. On-chip dielectrophoretic coassembly of live cells and particles into responsive biomaterials. Langmuir 2009, 26, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Vlahovska, P.M.; Gracia, R.S.; Aranda-Espinoza, S.; Dimova, R. Electrohydrodynamic model of vesicle deformation in alternating electric fields. Biophys. J. 2009, 96, 4789–4803. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, L.A.; Buschmann, M.D.; Wertheimer, M.R. Mechanical properties of mammalian cells in suspension measured by electro-deformation. J. Micromech. Microeng. 2010, 20, 1–11. [Google Scholar] [CrossRef]
- Engelhardt, H.; Sackmann, E. On the measurement of shear elastic moduli and viscosities of erythrocyte plasma membranes by transient deformation in high frequency electric fields. Biophys. J. 1988, 54, 495–508. [Google Scholar] [CrossRef]
- Li, H.; Ye, T.; Lam, K.Y. Qualitative and quantitative analysis of dynamic deformation of a cell in nonuniform alternating electric field. J. Appl. Phys. 2011, 110, 1–6. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H. Electrowetting on dielectric (EWOD): New tool for bio/micro fluids handling. Biochip. J. 2008, 2, 79–96. [Google Scholar]
- Fair, R.B.; Khlystov, A.; Tailor, T.D.; Ivanov, V.; Evans, R.D.; Griffin, P.B.; Vijay, S.; Pamula, V.K.; Pollack, M.G.; Zhou, J. Chemical and biological applications of digital-microfluidic devices. IEEE Des. Test Comput. 2007, 24, 10–24. [Google Scholar] [CrossRef]
- Fiddes, L.K.; Luk, V.N.; Au, S.H.; Ng, A.H.C.; Luk, V.; Kumacheva, E.; Wheeler, A.R. Hydrogel discs for digital microfluidics. Biomicrofluidics 2012, 6, 014112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; Alici, G.; Nguyen, N.-T.; Di Carlo, D.; Li, W. Real-time control of inertial focusing in microfluidics using dielectrophoresis (dep). RSC Adv. 2014, 4, 62076–62085. [Google Scholar] [CrossRef]
- Emaminejad, S.; Dutton, R.W.; Davis, R.W.; Javanmard, M. Multiplexed actuation using ultra dielectrophoresis for proteomics applications: A comprehensive electrical and electrothermal design methodology. Lab Chip 2014, 14, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-W.; Prieto, J.L.; Voldman, J. Rapid dielectrophoretic characterization of single cells using the dielectrophoretic spring. Lab Chip 2013, 13, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Barbulovic-Nad, I.; Auab, S.H.; Wheeler, A.R. A microfluidic platform for complete mammalian cell culture. Lab Chip 2010, 10, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Aijian, A.P.; Garrell, R.L. Digital microfluidics for hanging drop cell spheroid culture. J. Lab. Autom. 2014, 20, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Fobel, R.; Desai, S.P.; Voldman, J.; Wheeler, A.R. Cellular bias on the microscale: Probing the effects of digital microfluidic actuation on mammalian cell health, fitness and phenotype. Integr. Biol. 2013, 5, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Hancock, M.J.; Donnelly, J.P.; Iyer, D.; Khademhosseini, A. Biomimetic gradient hydrogels for tissue engineering. Can. J. Chem. Eng. 2010, 88, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review or recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Mikos, A.G.; Fisher, J.P.; Jansen, J.A. Strategic directions in tissue engineering. Tissue Eng. 2007, 13, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Thampatty, B.P. Mechanobiology of adult and stem cells. Int. Rev. Cell Mol. Biol. 2008, 271, 301–334. [Google Scholar] [PubMed]
- Sheehy, S.P.; Grosberg, A.; Parker, K.K. The contribution of cellular mechanotransduction to cardiomyocyte form and function. Biomech. Model. Mechanobiol. 2012, 11, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Moon, H. Digital microfluidic three-dimensional cell culture and chemical screening platform using alginate hydrogels. Biomicrofluidics 2015, 9, 024116. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Mooney, D.J. Alginate type and rgd density control myoblast phenotype. J. Biomed. Mat. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Arany, P.R.; Wang, Y.-S.; Mooney, D.J. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials 2009, 30, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Primiceri, E.; Chiriaco, M.S.; Rinaldi, R.; Maruccio, G. Cell chips as new tools for cell biology—Results, perspectives and opportunities. Lab Chip 2013, 13, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Ying, L.; Zhang, P.-C.; Zhuo, R.-X.; Kang, E.-T.; Leong, K.W.; Mao, H.-Q. High density of immobilized galactose ligand enhances hepatocyte attachment and function. J. Biomed. Mater. Res. 2003, 67, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dong, Z.; Wejinya, U.C.; Jin, S.; Ye, K. Determination of mechanical properties of soft tissue scaffolds by atomic force microscopy nanoindentation. J. Biomech. 2011, 44, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, M.; Dietler, G.; Schlapbach, L. Elastic deformations of tip and sample during atomic force microscope measurements. J. Vac. Sci. Technol. B 1996, 14, 1250–1254. [Google Scholar] [CrossRef]
- Yeung, J.H.C.; Young, E.F.Y.; Choy, C.S. In Reducing pin count on cross-referencing digital microfluidic biochip. In Proceedings of 2014 IEEE International Symposium on Circuits and Systems (ISCAS), Melbourne, Australia, 1–5 June 2014; pp. 790–793.
- Yang, Z.; Chakrabarty, K. Pin-count-aware online testing of digital microfluidic biochips. In Proceedings of 2010 28th VLSI Test Symposium (VTS), Santa Cruz, CA, USA, 19–22 April 2010; pp. 111–116.
- Lee, J.; Moon, H.; Fowler, J.; Schoelhammer, T.; Kim, C.-J. Electrowetting and electrowetting-on-dielectric for microscale liquid handling. Sens. Actuators A Phys. 2002, 95, 259–268. [Google Scholar] [CrossRef]
- Schertzer, M.J.; Gubarenko, S.I.; Ben-Mrad, R.; Sullivan, P.E. An empirically validated analytical model of droplet dynamics in electrowetting on dielectric devices. Langmuir 2010, 26, 19230–19238. [Google Scholar] [CrossRef] [PubMed]
- Tenan, M.A.; Hackwood, S.; Beni, G. Friction in capillary systems. J. Appl. Phys. 1982, 53, 6687–6692. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Gong, J.; Kim, C.J. All-electronic droplet generation on-chip with real-time feedback control for EWOD digital microfluidics. Lab Chip 2008, 8, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.C.; Kim, C.-J. Monolithic fabrication of EWOD chips for picoliter droplets. J. Microelectromech. Syst. 2011, 20, 1419–1427. [Google Scholar] [CrossRef]
- Brassard, D.; Malic, L.; Normandin, F.; Tabrizianc, M.; Veres, T. Water-oil core-shell droplets for electrowetting-based digital microfluidic devices. Lab Chip 2008, 8, 1342–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolley, C.F.; Hayes, M.A. Recent developments in emerging microimmunoassays. Bioanalysis 2013, 5, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wang, H.; Wang, H.; Wang, L.; Qiao, M.; Wu, S.; Liu, T. Investigation of the effective action distance between hematopoietic stem/progenitor cells and human adipose-derived stem cells during their in vitro co-culture. Appl. Biochem. Biotechnol. 2011, 165, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Tay, S. Automated co-culture system for spatiotemporal analysis of cell-to-cell communication. Lab Chip 2015, 15, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Singh, S.S.; Task, K.; Kumta, P.N.; Banerjee, I. Early differentiation patterning of mouse embryonic stem cells in response to variations in alginate substrate stiffness. J. Biol. Eng. 2013, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Wong, E.; Mooney, D.J. Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules 2003, 36, 4582–4588. [Google Scholar] [CrossRef]
- Dean, D.A.; Ramanathan, T.; Machado, D.; Sundararajan, R. Electrical impedance spectroscopy study of biological tissues. J. Electrost. 2009, 66, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.F.; Scott, C.W.; Ochalski, R.; Dragon, Y.P. Evalulation of cellular impedance measures of cardiomyocyte cultures for drug screening applications. Assay Drug Dev. Technol. 2012, 10, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.C.C.; Barbulovic-Nad, I.; Yang, X.; Fobel, R.; Wheeler, A.R. Digital microfluidics with impedance sensing for integrated cell culture and analysis. Biosens. Bioelectron. 2013, 42, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Arshi, A.; Nakashima, Y.; Nakano, H.; Eaimkhong, S.; Evseenko, D.; Reed, J.; Stieg, A.Z.; Gimzewski, J.K.; Nakano, A. Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells. Sci. Technol. Adv. Mater 2013, 14, 025003. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Javed, M.R.; Kim, H.-K.; Lei, J.; Lazari, M.; Shah, G.J.; Dooraghi, A.A.; Chatziioannou, A.F.; Dam, R.M.; Keng, P.-Y.; et al. Electrowetting-driven chemical synthesis and radioisotope purification of positron emission tomography (pet) radiotracers. In Proceedings of the 9th International Meeting on Electrowetting and Related Micro/Electrofluidic Science and Technology, Cincinnati, OH, USA, 23–25 June 2014.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, B.F.; Garrell, R.L. Digital Microfluidic System with Vertical Functionality. Micromachines 2015, 6, 1655-1674. https://doi.org/10.3390/mi6111448
Bender BF, Garrell RL. Digital Microfluidic System with Vertical Functionality. Micromachines. 2015; 6(11):1655-1674. https://doi.org/10.3390/mi6111448
Chicago/Turabian StyleBender, Brian F., and Robin L. Garrell. 2015. "Digital Microfluidic System with Vertical Functionality" Micromachines 6, no. 11: 1655-1674. https://doi.org/10.3390/mi6111448





