Block V RTX Domain of Adenylate Cyclase from Bordetella pertussis: A Conformationally Dynamic Scaffold for Protein Engineering Applications
Abstract
:1. Introduction
2. Repeat Proteins as Scaffolds for Protein Engineering
2.1. Designed Ankyrin Repeat Proteins (DARPins)
2.2. Leucine Rich Repeats (LRRs)
2.3. Other Repeat Proteins
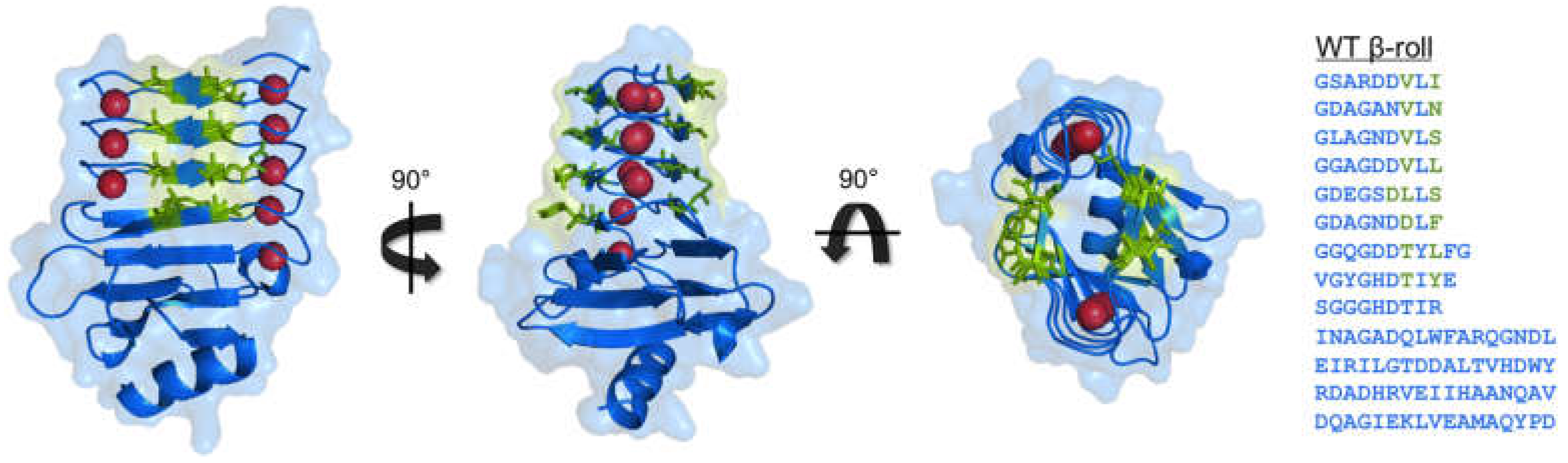
3. Block V RTX Domain of Adenylate Cyclase from Bordatella pertussis
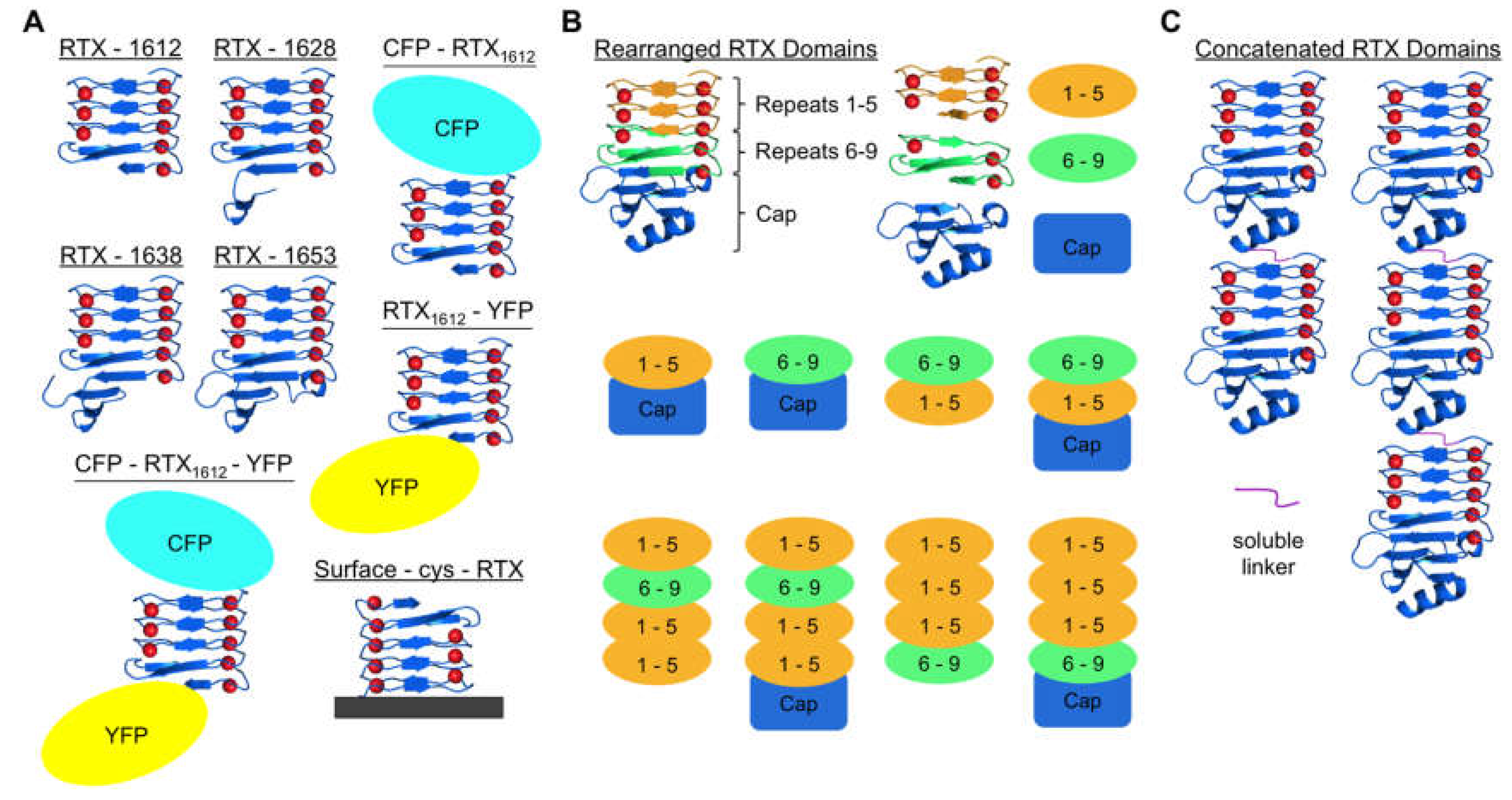
4. Native RTX Domain Insertions for Introducing Calcium-Mediated Function
5. Exploring the Order of RTX Domain Repeat Sequence Lead to Useful Precipitation for Bioseparations
6. Engineered β-Roll Domains with Hydrophobic Faces for Self-Assembly and Protein Hydrogel Formation
7. Evolution of β-Roll Domains Exhibiting Calcium-Dependent Biomolecular Recognition
8. Summary and Conclusions
Acknowledgments
Conflicts of Interest
References
- Bordet, I.J.; Gengou, O. Le microbe de la coqueluche. Ann. Inst. Pasteur 1906, 20, 731–741. [Google Scholar]
- Cherry, J.D.; Grimprel, E.; Guiso, N.; Heininger, U.; Mertsola, J. Defining Pertussis epidemiology: Clinical, microbiological and serologic perspectives. Pediatr. Infect. Dis. J. 2005, 24, S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.A.; Hewlett, E.L. Virulence factors of Bordetella pertussis. Ann. Rev. Microbiol. 1986, 40, 661–686. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, S.; Foreman-Wykert, A.K.; Cotter, P.A.; Miller, J.F. Mechanisms of Bordetella pathogenesis. Front. Biosci. 2001, 6, e168–e186. [Google Scholar] [CrossRef] [PubMed]
- Locht, C.; Antoine, R.; Jacob-Dubuisson, F. Bordetella pertussis: Molecular pathogenesis under multiple aspects. Curr. Opin. Microbiol. 2011, 4, 82–89. [Google Scholar]
- Ladant, D.; Ullmann, A. Bordetella pertussis adenylate cyclase: A toxin with multiple talents. Trends Microbiol. 1999, 7, 172–176. [Google Scholar] [CrossRef]
- Karst, J.C.; Sotomayor-Pérez, A.C.; Guijarro, J.I.; Raynal, B.; Chenal, A.; Ladant, D. Calmodulin-induced conformational and hydrodynamic changes in the catalytic domain of Bordetella pertussis adenylate cyclase toxin. Biochemistry 2010, 49, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Khelef, N.; Blouin, E.; Rieu, P.; Ricciardi-Castagnoli, P.; Guiso, N.; Ladant, D.; Leclerc, C. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αMβ2 integrin (Cd11b/Cd18). J. Exp. Med. 2001, 193, 1025–1044. [Google Scholar] [CrossRef]
- Welch, R.A. RTX toxin structure and function: A story of numerous anomalies and few analogies in toxin biology. In Pore-Forming Toxins; Springer: Berlin/Heidelberg, Germany, 2001; pp. 85–111. [Google Scholar]
- Guo, Q.; Shen, Y.; Lee, Y.-S.; Gibbs, C.S.; Mrksich, M.; Tang, W.-J. Structural basis for the interaction of Bordetella pertussis adenylyl cyclase toxin with calmodulin. EMBO J. 2005, 24, 3190–3201. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gray, M.C.; Hewlett, E.L.; Maynard, J.A. The Bordetella adenylate cyclase repeat-in-toxin (RTX) domain is immunodominant and elicits neutralizing antibodies. J. Biol. Chem. 2015, 290, 3576–3591. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Guzmán, C.A.; Walker, M.J. The virulence factors of Bordetella pertussis: A matter of control. FEMS Microbiol. Rev. 2001, 25, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Chenal, A.; Karst, J.C.; Sotomayor-Pérez, A.C.; Wozniak, A.K.; Baron, B.; England, P.; Ladant, D. Calcium-induced folding and stabilization of the intrinsically disordered RTX domain of the CyaA toxin. Biophys. J. 2010, 99, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Bejerano, M.; Nisan, I.; Ludwig, A.; Goebel, W.; Hanski, E. Characterization of the C-terminal domain essential fortoxic activity of adenylate cyclase toxin. Mol. Microbiol. 1999, 31, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Ullmann, A.; Sebo, P. Identification by in vitro complementation of regions required for cell-invasive activity of Bordetella pertussis adenylate cyclase toxin. Mol. Microbiol. 1995, 17, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Bauche, C.; Chenal, A.; Knapp, O.; Bodenreider, C.; Benz, R.; Chaffotte, A.; Ladant, D. Structural and functional characterization of an essential RTX subdomain of Bordetella pertussis adenylate cyclase toxin. J. Biol. Chem. 2006, 281, 16914–16926. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.P.; Hernandez, B.; Durand, D.; Hourdel, V.; Sotomayor-Pérez, A.C.; Vachette, P.; Ghomi, M.; Chamot-Rooke, J.; Ladant, D.; Brier, S. Structural models of intrinsically disordered and calcium-bound folded states of a protein adapted for secretion. Sci. Rep. 2015, 5, 14223–14234. [Google Scholar] [CrossRef] [PubMed]
- Bumba, L.; Masin, J.; Macek, P.; Wald, T.; Motlova, L.; Bibova, I.; Klimova, N.; Bednarova, L.; Veverka, V.; Kachala, M.; et al. Calcium-driven folding of RTX domain β-rolls ratchets translocation of RTX proteins through type I secretion ducts. Mol. Cell 2016, 62, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Cannella, S.E.; Ntsogo Enguene, V.Y.; Davi, M.; Malosse, C.; Sotomayor-Pérez, A.C.; Chamot-Rooke, J.; Vachette, P.; Durand, D.; Ladant, D.; Chenal, A. Stability, structural and functional properties of a monomeric, calcium-loaded adenylate cyclase toxin, CyaA, from Bordetella pertussis. Sci. Rep. 2017, 7, 42065–42082. [Google Scholar] [CrossRef] [PubMed]
- Boersma, Y.L.; Plückthun, A. DARPins and other repeat protein scaffolds: Advances in engineering and applications. Curr. Opin. Biotechnol. 2011, 22, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Banta, S.; Dooley, K.; Shur, O. Replacing antibodies: Engineering new binding proteins. Annu. Rev. Biomed. Eng. 2013, 15, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Grove, T.Z.; Cortajarena, A.L.; Regan, L. Ligand binding by repeat proteins: Natural and designed. Curr. Opin. Struct. Biol. 2008, 18, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, F.; Pellarin, R.; Larsen, A.P.; Varadamsetty, G.; Stumpp, M.T.; Zerbe, O.; Caflisch, A.; Plückthun, A. Designed armadillo repeat proteins as general peptide-binding scaffolds: Consensus design and computational optimization of the hydrophobic core. J. Mol. Biol. 2008, 376, 1282–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Main, E.R.; Phillips, J.J.; Millership, C. Repeat protein engineering: Creating functional nanostructures/biomaterials from modular building blocks. Biochem. Soc. Trans. 2013, 41, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Bork, P. Hundreds of ankyrin-like repeats in functionally diverse proteins: Mobile modules that cross phyla horizontally? Proteins 1993, 17, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mahajan, A.; Tsai, M.-D. Ankyrin repeat: A unique motif mediating protein–protein interactions. Biochemistry 2006, 45, 15168–15178. [Google Scholar] [CrossRef] [PubMed]
- Plückthun, A. Designed ankyrin repeat proteins (DARPins): Binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, S.G.; Smerdon, S.J. The ankyrin repeat: A diversity of interactions on a common structural framework. Trends Biochem. Sci. 1999, 24, 311–316. [Google Scholar] [CrossRef]
- Binz, H.K.; Stumpp, M.T.; Forrer, P.; Amstutz, P.; Plückthun, A. Designing repeat proteins: Well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J. Mol. Biol. 2003, 332, 489–503. [Google Scholar] [CrossRef]
- Steiner, D.; Forrer, P.; Plückthun, A. Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display. J. Mol. Biol. 2008, 382, 1211–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plückthun, A. Ribosome display: A perspective. Methods Mol. Biol. 2012, 805, 3–28. [Google Scholar] [PubMed]
- Kummer, L.; Parizek, P.; Rube, P.; Millgramm, B.; Prinz, A.; Mittl, P.R.E.; Kaufholz, M.; Zimmermann, B.; Herberg, F.W.; Plückthun, A. Structural and functional analysis of phosphorylation-specific binders of the kinase ERK from designed ankyrin repeat protein libraries. Proc. Nat. Acad. Sci. USA 2012, 109, E2248–E2257. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, C.; Pecorari, F.; Straumann, N.; Wyler, E.; Plückthun, A. Selection and characterization of Her2 binding-designed ankyrin repeat proteins. J. Biol. Chem. 2006, 281, 35167–35175. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Martin-Killias, P.; Plückthun, A.; Zangemeister-Wittke, U. EpCAM-targeted delivery of nanocomplexed siRNA to tumor cells with designed ankyrin repeat proteins. Mol. Cancer Ther. 2009, 8, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Stumpp, M.T.; Schlegel, A.; Ekawardhani, S.; Lehrling, C.; Martin, G.; Gulotti-Georgieva, M.; Villemagne, D.; Forrer, P.; Agostini, H.T.; et al. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis 2013, 16, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kobe, B.; Kajava, A.V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 2011, 11, 725–732. [Google Scholar] [CrossRef]
- Buchanan, S.G.C.; Gay, N.J. Structural and functional diversity in the leucine-rich repeat family of proteins. Prog. Biophys. Mol. Biol. 1996, 65, 1–44. [Google Scholar] [CrossRef]
- Kajava, A.V. Structural diversity of leucine-rich repeat proteins. J. Mol. Biol. 1998, 277, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Pancer, Z.; Cooper, M.D. The evolution of adaptive immunity. Annu. Rev. Immunol. 2006, 24, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Mariuzza, R.A.; Velikovsky, C.A.; Deng, L.; Xu, G.; Pancer, Z. Structural insights into the evolution of the adaptive immune system: The variable lymphocyte receptors of jawless vertebrates. Biol. Chem. 2010, 391, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Alder, M.N.; Rogozin, I.B.; Iyer, L.M.; Glazko, G.V.; Cooper, M.D.; Pancer, Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science 2005, 310, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.M.; Povelones, M.; Christophides, G.K. Sequence-structure-function relations of the mosquito leucine-rich repeat immune proteins. BMC Genom. 2010, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, K.; Blanvillain-Baufume, S.; Parker, J.E. Molecular and spatial constraints on NB-LRR receptor signaling. Curr. Opin. Plant Biol. 2012, 15, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Baabur-Cohen, H.; Dayalan, S.; Shumacher, I.; Cohen-Luria, R.; Ashkenasy, G. Artificial leucine rich repeats as new scaffolds for protein design. Bioorg. Med. Chem. Lett. 2011, 21, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Park, K.; Han, J.; Lee, J.J.; Kim, H.J.; Hong, S.; Heu, W.; Kim, Y.J.; Ha, J.S.; Lee, S.G.; et al. Design of a binding scaffold based on variable lymphocyte receptors of jawless vertebrates by module engineering. Proc. Nat. Acad. Sci. USA 2012, 109, 3299–3304. [Google Scholar] [CrossRef] [PubMed]
- Rämisch, S.; Weininger, U.; Martinsson, J.; Akke, M.; André, I. Computational design of a leucine-rich repeat protein with a predefined geometry. Proc. Nat. Acad. Sci. USA 2014, 111, 17875–17880. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Armadillo repeat proteins: Beyond the animal kingdom. Trends Cell Biol. 2003, 13, 463–471. [Google Scholar] [CrossRef]
- D’Andrea, L.D.; Regan, L. TPR proteins: The versatile helix. Trends Biochem. Sci. 2003, 28, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Petosa, C.; O’Donoghue, S.I.; Müller, C.W.; Bork, P. Comparison of ARM and HEAT protein repeats. J. Mol. Biol. 2001, 309, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, F.; Huang, P.S.; Vorobiev, S.; Xiao, R.; Park, K.; Caprari, S.; Su, M.; Seetharaman, J.; Mao, L.; Janjua, H.; et al. A general computational approach for repeat protein design. J. Mol. Biol. 2015, 427, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Manna, S. An overview of pentatricopeptide repeat proteins and their applications. Biochimie 2015, 113, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Reichen, C.; Hansen, S.; Plückthun, A. Modular peptide binding: From a comparison of natural binders to designed armadillo repeat proteins. J. Struct. Biol. 2014, 185, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, K.; Blenner, M.; Banta, S. Design and application of stimulus-responsive peptide systems. Protein Eng. Des. Sel. 2007, 20, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Drepper, T.; Svensson, V.; Jaeger, K.-E.; Baumann, U. A calcium-gated lid and a large beta-roll sandwich are revealed by the crystal structure of extracellular lipase from Serratia marcescens. J. Biol. Chem. 2007, 282, 31477–31483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conway, J.F.; Thibodeau, P.H. Calcium-induced folding and stabilization of the Pseudomonas aeruginosa alkaline protease. J. Biol. Chem. 2012, 287, 4311–4343. [Google Scholar] [CrossRef] [PubMed]
- Linhartova, I.; Bumba, L.; Masin, J.; Basler, M.; Osicka, R.; Kamanova, J.; Prochazkova, K.; Adkins, I.; Hejnova-Holubova, J.; Sadilkova, L.; et al. RTX proteins: A highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 2010, 34, 1076–1112. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor-Pérez, A.C.; Ladant, D.; Chenal, A. Disorder-to-order transition in the CyaA toxin RTX domain: Implications for toxin secretion. Toxins 2015, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pojanapotha, P.; Thamwiriyasati, N.; Powthongchin, B.; Katzenmeier, G.; Angsuthanasombat, C. Bordetella pertussis CyaA-RTX subdomain requires calcium ions for structural stability against proteolytic degradation. Protein Expr. Purif. 2011, 75, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Functional roles of transiently and intrinsically disordered regions within proteins. FEBS J. 2015, 282, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Chenal, A.; Guijarro, J.I.; Raynal, B.; Delepierre, M.; Ladant, D. RTX calcium binding motifs are intrinsically disordered in the absence of calcium. J. Biol. Chem. 2009, 284, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Blenner, M.A.; Shur, O.; Szilvay, G.R.; Cropek, D.M.; Banta, S. Calcium-induced folding of a beta roll motif requires C-terminal entropic stabilization. J. Mol. Biol. 2010, 400, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor-Pérez, A.C.; Ladant, D.; Chenal, A. Calcium-induced folding of intrinsically disordered repeat-in-toxin (RTX) motifs via changes of protein charges and oligomerization states. J. Biol. Chem. 2011, 286, 16997–17004. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor-Pérez, A.C.; Karst, J.C.; Davi, M.; Guijarro, J.I.; Ladant, D.; Chenal, A. Characterization of the regions involved in the calcium-induced folding of the intrinsically disordered RTX motifs from the Bordetella pertussis adenylate cyclase toxin. J. Mol. Biol. 2010, 397, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Shur, O.; Banta, S. Rearranging and concatenating a native RTX domain to understand sequence modularity. Protein Eng. Des. Sel. 2013, 26, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Shur, O.; Dooley, K.; Blenner, M.; Baltimore, M.; Banta, S. A designed, phase changing RTX-based peptide for efficient bioseparations. Biotechniques 2013, 54, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Kim, Y.H.; Lu, H.D.; Tu, R.; Banta, S. Engineering of an environmentally responsive beta roll peptide for use as a calcium-dependent cross-linking domain for peptide hydrogel formation. Biomacromolecules 2012, 13, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Bulutoglu, B.; Banta, S. Doubling the cross-linking interface of a rationally designed beta roll peptide for calcium-dependent proteinaceous hydrogel formation. Biomacromolecules 2014, 15, 3617–3624. [Google Scholar] [CrossRef] [PubMed]
- Bulutoglu, B.; Dooley, K.; Szilvay, G.; Blenner, M.; Banta, S. Catch and release: Engineered allosterically-regulated β-roll peptides enable on/off biomolecular recognition. ACS Synth. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bulutoglu, B.; Yang, S.J.; Banta, S. Conditional network assembly and targeted protein retention via environmentally responsive, engineered β-roll peptides. Biomacromolecules 2017, 18, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Szilvay, G.R.; Blenner, M.A.; Shur, O.; Cropek, D.M.; Banta, S. A FRET-based method for probing the conformational behavior of an intrinsically disordered repeat domain from Bordetella pertussis adenylate cyclase. Biochemistry 2009, 48, 11273–11282. [Google Scholar] [CrossRef] [PubMed]
- Shur, O.; Wu, J.; Cropek, D.M.; Banta, S. Monitoring the conformational changes of an intrinsically disordered peptide using a quartz crystal microbalance. Protein Sci. 2011, 20, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Ringler, P.; Schulz, G.E. Self-assembly of proteins into designed networks. Science 2003, 302, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Machielsen, R.; Uria, A.R.; Kengen, S.W.; van der Oost, J. Production and characterization of a thermostable alcohol dehydrogenase that belongs to the aldo-keto reductase superfamily. Appl. Environ. Microbiol. 2006, 72, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Wheeldon, I.R.; Banta, S. Broadening the cofactor specificity of a thermostable alcohol dehydrogenase using rational protein design introduces novel kinetic transient behavior. Biotechnol. Bioeng. 2010, 107, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Chuang, S.; Banta, S. Modular exchange of substrate-binding loops alters both substrate and cofactor specificity in a member of the aldo-keto reductase superfamily. Protein Eng. Des. Sel. 2013, 26, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, W.; Solanki, K.; Banta, S. Insertion of a calcium-responsive beta roll domain into a thermostable alcohol dehydrogenase enables tunable control over cofactor selectivity. 2017; submitted. [Google Scholar]
- Lilie, H.; Haehnel, W.; Rudolph, R.; Baumann, U. Folding of a synthetic parallel β-roll protein. FEBS Lett. 2000, 470, 173–177. [Google Scholar] [CrossRef]
- Scotter, A.J.; Guo, M.; Tomczak, M.M.; Daley, M.E.; Campbell, R.L.; Oko, R.J.; Bateman, D.A.; Chakrabartty, A.; Sykes, B.D.; Davies, P.L. Metal ion-dependent, reversible, protein filament formation by designed beta-roll polypeptides. BMC Struct. Biol. 2007, 7, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, Q.; Xing, L.; Zhou, B.; Wang, X. Aggregating tags for column-free protein purification. Biotechnol. J. 2015, 10, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.; Read, T.; Lalonde, J.F.; Jensen, P.K.; Heymann, W.; Lovelace, E.; Zimmermann, S.A.; Brasino, M.; Rokicki, J.; Dowell, R.D. Engineered calcium-precipitable restriction enzyme. ACS Synth. Biol. 2014, 3, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Alber, T. Structure of the leucine zipper. Curr. Opin. Genet. Dev. 1992, 2, 205–210. [Google Scholar] [CrossRef]
- Banta, S.; Wheeldon, I.R.; Blenner, M. Protein engineering in the development of functional hydrogels. Annu. Rev. Biomed. Eng. 2010, 12, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Petka, W.A.; Harden, J.L.; McGrath, K.P.; Wirtz, D.; Tirrell, D.A. Reversible hydrogels from self-assembling artificial proteins. Science 1998, 281, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Kornfield, J.A.; Tirrell, D.A. Structure and mechanical properties of artificial protein hydrogels assembled through aggregation of leucine zipper peptide domains. Soft Matter 2007, 3, 99–107. [Google Scholar] [CrossRef]
- Wheeldon, I.R.; Gallaway, J.W.; Barton, S.C.; Banta, S. Bioelectrocatalytic hydrogels from electron-conducting metallopolypeptides coassembled with bifunctional enzymatic building blocks. Proc. Nat. Acad. Sci. USA 2008, 105, 15275–15280. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, I.; Campbell, E.; Banta, S. A chimeric fusion protein engineered with disparate functionalities—Enzymatic activity and self-assembly. J. Mol. Biol. 2009, 392, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Wheeldon, I.R.; Banta, S. Catalytic biomaterials: Engineering organophosphate hydrolase to form self-assembling enzymatic hydrogels. Protein Eng. Des. Sel. 2010, 23, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.H.; Hu, B.-H.; Ruberti, J.W.; Zhang, J.; Shum, P.; Thompson, D.H.; Messersmith, P.B. Thermally and photochemically triggered self-assembly of peptide hydrogels. J. Am. Chem. Soc. 2001, 123, 9463–9464. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Hartgerink, J.D. Short homodimeric and heterodimeric coiled coils. Biomacromolecules 2006, 7, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Binz, H.K.; Amstutz, P.; Kohl, A.; Stumpp, M.T.; Briand, C.; Forrer, P.; Grütter, M.G.; Plückthun, A. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat. Biotechnol. 2004, 22, 575–582. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulutoglu, B.; Banta, S. Block V RTX Domain of Adenylate Cyclase from Bordetella pertussis: A Conformationally Dynamic Scaffold for Protein Engineering Applications. Toxins 2017, 9, 289. https://doi.org/10.3390/toxins9090289
Bulutoglu B, Banta S. Block V RTX Domain of Adenylate Cyclase from Bordetella pertussis: A Conformationally Dynamic Scaffold for Protein Engineering Applications. Toxins. 2017; 9(9):289. https://doi.org/10.3390/toxins9090289
Chicago/Turabian StyleBulutoglu, Beyza, and Scott Banta. 2017. "Block V RTX Domain of Adenylate Cyclase from Bordetella pertussis: A Conformationally Dynamic Scaffold for Protein Engineering Applications" Toxins 9, no. 9: 289. https://doi.org/10.3390/toxins9090289






