Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models
Abstract
:1. Introduction
2. Results
2.1. Envenomation Model and Cnidae Discharge
2.2. Testing of Potential Rinse Solutions Using the Tentacle Solution Assay
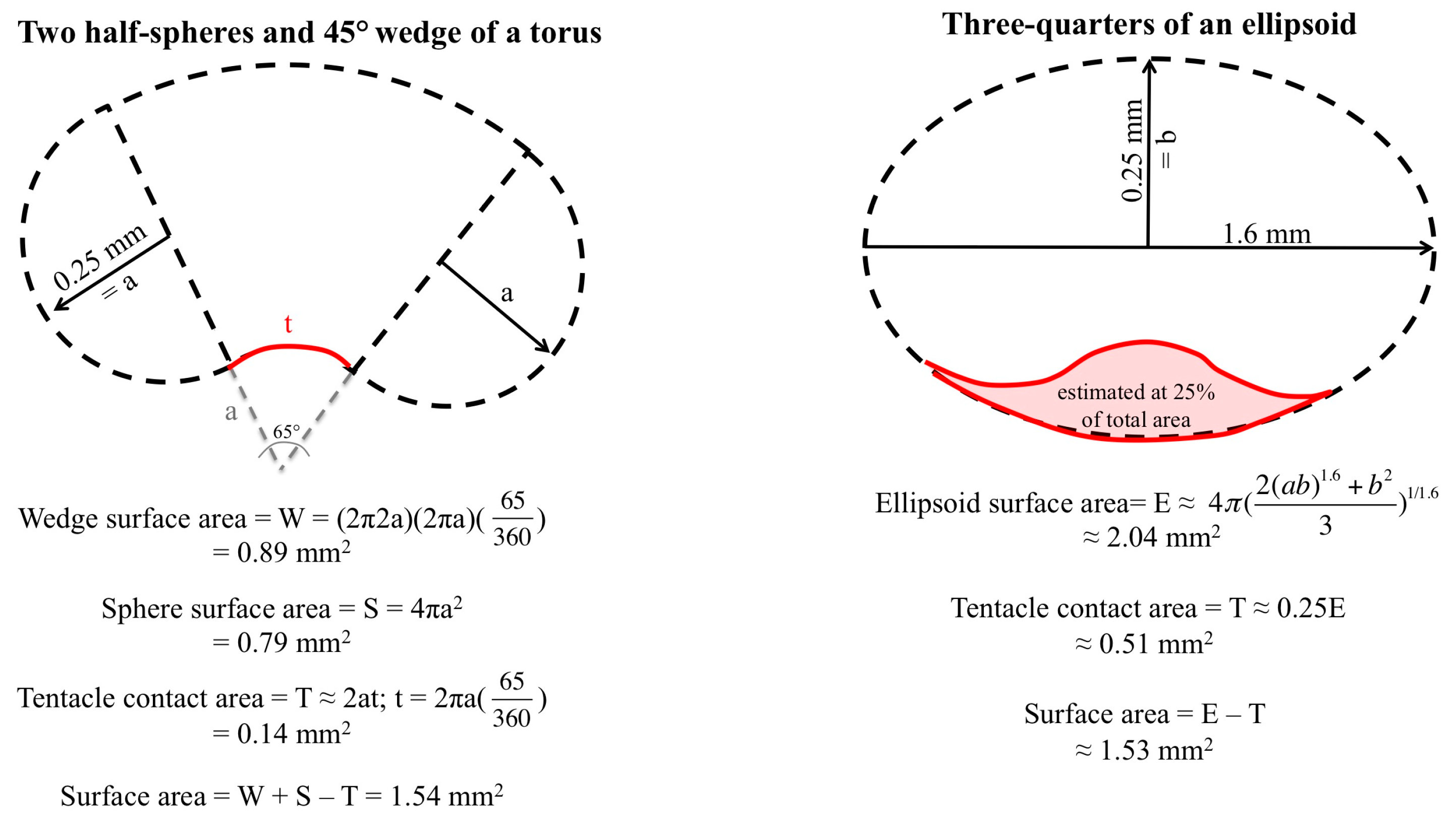
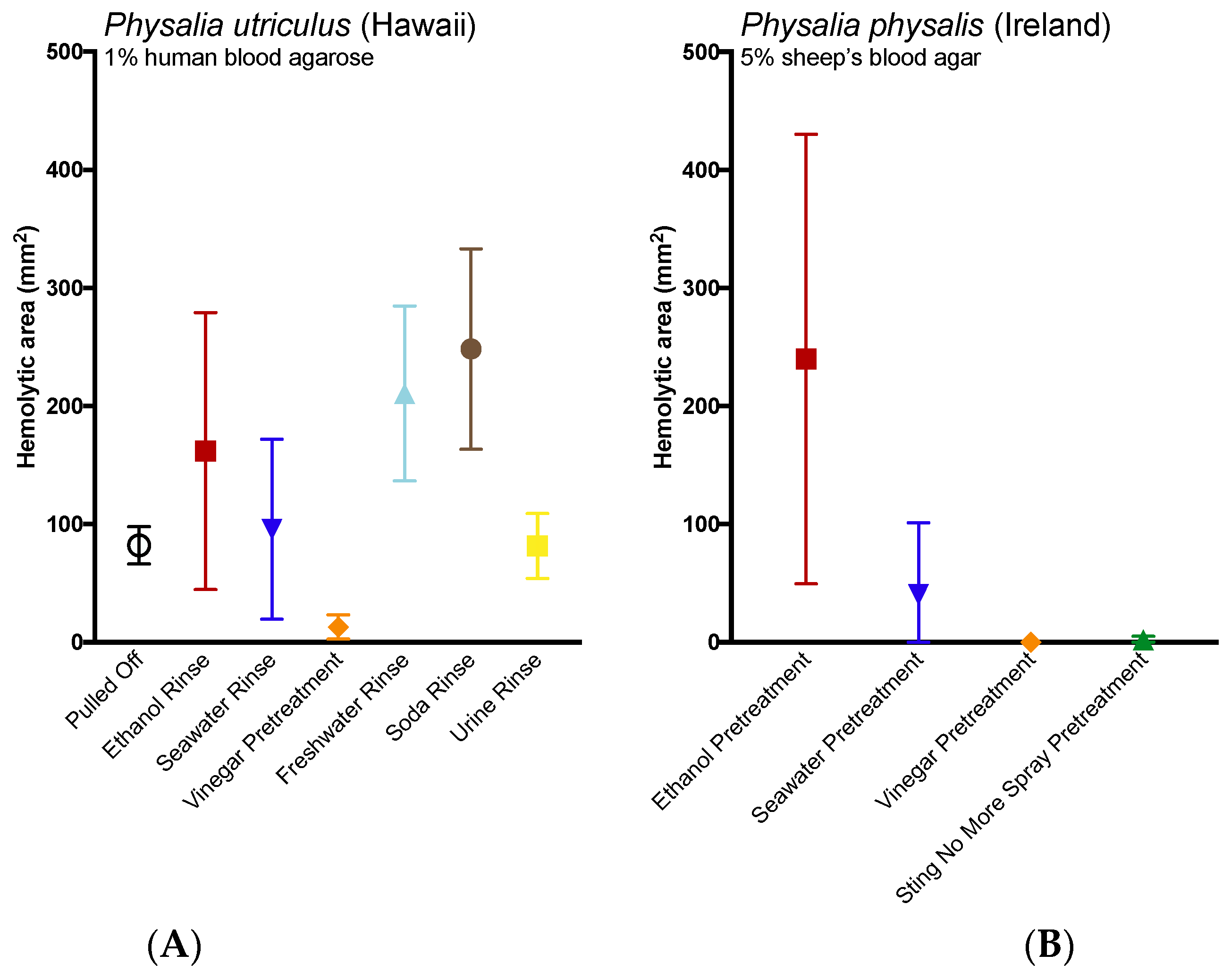
2.3. Evlauation of Potential Rinse Solutions Using the Tentacle Blood Agarose Assay
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Collection
5.2. Envenomation Modeling
5.3. Tentacle Solution Assay (TSA)
5.4. Tentacle Blood Agarose Assay
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TSBAA | Tentacle Skin Blood Agarose Assay |
| TBAA | Tentacle Blood Agarose Assay |
| TSA | Tentacle Solution Assay |
| Fisher’s LSD | Fisher’s Least Significant Difference |
Appendix A
| Test Solution | Cnidae Discharge (%) | Ethanol-Induced Discharge (%) | Pressure-Stimulated Discharge (%) |
|---|---|---|---|
| Seawater | 00.59 ± 00.26 | 43.40 ± 02.17 | 46.64 ± 02.97 |
| White Vinegar | 01.04 ± 00.04 | 00.09 ± 00.09 | 00.81 ± 00.37 |
| Cider Vinegar | 00.31 ± 00.13 | 00.21 ± 00.17 | 00.65 ± 00.23 |
| Malt Vinegar | 00.47 ± 00.38 | 00.77 ± 00.47 | not tested |
| Sting No More® Spray | 00.06 ± 00.06 | 00.03 ± 00.03 | 00.20 ± 00.20 |
| Test Solution | Cnidae Discharge (%) | Mean Difference (95% CI) | p Value | Pressure-Stimulated Discharge (%) | Mean Difference (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Seawater | 00.38 ± 0.13 | 44.06 ± 13.79 | ||||
| Tap Water | 02.01 ± 0.61 | −1.72 (−10.03–6.59) | 0.6616 | 15.50 ± 1.68 | 33.28 (11.99–54.58) | <0.0001 |
| Urine | 12.87 ± 2.60 | −12.58 (−20.8–4.27) | 0.002 | 14.73 ± 3.91 | 29.33 (8.037–50.63) | 0.0008 |
| Shaving Cream | 09.43 ± 6.45 | −6.76 (−13.79–0.26) | 0.045 | 22.45 ± 6.09 | 21.62 (0.32–42.91) | 0.0433 |
| Baking Soda (3:1 in Seawater) | 08.90 ± 2.67 | −8.61 (−16.92–0.3) | 0.0315 | 35.56 ± 7.24 | 8.507 (−12.79–29.8) | 0.9952 |
| Dish Soap | 01.68 ± 0.30 | −1.39 (−9.7–6.92) | 0.724 | 23.75 ± 3.34 | 20.31 (−0.99–41.6) | 0.0768 |
| White Vinegar (3–5%) | 00.20 ± 0.20 | 0.09 (−8.22–8.4) | 0.9825 | 02.78 ± 0.88 | 41.29 (19.99–62.58) | <0.0001 |
| Cider Vinegar (3–5%) | 00.69 ± 0.39 | −0.4 (−8.71–7.91) | 0.9184 | 02.27 ± 0.29 | 41.79 (20.49–63.09) | <0.0001 |
| Malt Vinegar (3–5%) | 00.73 ± 0.45 | −0.45 (−8.76–7.86) | 0.9095 | 04.46 ± 2.22 | 39.6 (18.31–60.9) | <0.0001 |
| Balsamic Vinegar (3–5%) | 01.94 ± 1.13 | −1.65 (−9.96–6.66) | 0.675 | 03.47 ± 1.22 | 40.6 (19.3–61.89) | <0.0001 |
| 5% Acetic Acid in Distilled Water | 00.50 ± 0.44 | −0.21 (−8.52–8.1) | 0.9579 | 02.61 ± 1.45 | 41.45 (20.15–62.75) | <0.0001 |
| 75% White Vinegar in Seawater (~3.75%) | 01.82 ± 0.49 | −1.53 (−9.35–6.29) | 0.6975 | 20.40 ± 5.79 | 23.66 (2.363–44.96) | 0.0167 |
| 50% White Vinegar in Seawater (~2.5%) | 01.11 ± 0.36 | −0.83 (−8.65–6.99) | 0.8337 | 24.34 ± 7.30 | 19.73 (−1.57–41.02) | 0.0979 |
| 25% White Vinegar in Seawater (~1.88%) | 00.87 ± 0.51 | −0.59 (−8.41–7.23) | 0.8809 | 26.05 ± 5.29 | 18.01 (−3.287–39.31) | 0.1908 |
| 10% White Vinegar in Seawater (~0.5%) | 01.66 ± 1.22 | −1.37 (−9.19–6.45) | 0.7278 | 31.35 ± 8.31 | 12.71 (−8.587–34.01) | 0.7739 |
| 110 mM saline + HCl | 15.90 ± 7.70 | −15.61 (−23.92–7.3) | 0.0002 | 13.30 ± 1.48 | 30.77 (9.47–52.06) | 0.0004 |
| Lemon Juice | 12.40 ± 2.32 | −12.12 (−20.42–3.81) | 0.0029 | 12.27 ± 3.09 | 31.8 (10.5–53.1) | 0.0002 |
| Regular Cola | 02.80 ± 1.91 | −2.52 (−10.82–5.79) | 0.523 | 18.12 ± 11.07 | 25.94 (4.647–47.24) | 0.0053 |
| Ethanol (>95%) | 37.74 ± 2.78 | −33.68 (−40.97–26.38) | <0.0001 | 41.06 ± 7.57 | 3.006 (−16.92–22.93) | >0.9999 |
| Isopropanol (>95%) | 21.48 ± 6.76 | −21.2 (−29.5–12.89) | <0.0001 | 24.25 ± 8.91 | 19.82 (−1.48–41.11) | 0.0943 |
| Sting No More® Spray | 00.00 ± 0.00 | 0.29 (−8.02–8.6) | 0.9417 | 03.53 ± 1.2 † | 41.39 (22.95–59.84) | <0.0001 |
| 30 mM Copper Gluconate | 00.06 ± 0.06 | 0.23 (−8.08–8.54) | 0.9532 | 11.25 ± 1.49 | 32.81 (11.52–54.11) | 0.0001 |
| Magnesium Sulfate | 08.57 ± 3.35 | −8.28 (−16.1–0.46) | 0.0383 | — | — | — |
| Lidocaine (4%) | 00.50 ± 0.14 | −0.21 (−7.9–7.49) | 0.9545 | 51.22 ± 12.16 | −7.153 (−28.45–14.14) | 0.9996 |
References
- Halstead, B.W. Coelenterates. In Poisonous and Venomous Marine Animals of the World, 2nd ed.; Darwin Press: Princeton, NJ, USA, 1988; pp. 99–186. [Google Scholar]
- Tibballs, J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon 2006, 48, 830–859. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.A.; Fenner, P.J.; Burnett, J.W.; Rifkin, J.F. Venomous & Poisonous Marine Animals: A Medical and Biological Handbook; Surf Life Saving Australia and University of New South Wales Press: Sydney, Australia, 1996; pp. 120–311. [Google Scholar]
- Cegolon, L.; Heymann, W.; Lange, J.; Mastrangelo, G. Jellyfish stings and their management: A review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Gable, W.D. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon 1989, 27, 823–824. [Google Scholar] [CrossRef]
- Stein, M.R.; Marraccini, J.V.; Rothschild, N.E.; Burnett, JW. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann. Emerg. Med. 1989, 18, 312–315. [Google Scholar] [CrossRef]
- Cazorla-Perfetti, D.J.; Loyo, J.; Lugo, L.; Acosta, M.E.; Morales, P.; Haddad, V.; Rodriguez-Morales, A.J. Epidemiology of the cnidarian Physalia physalis stings attended at a health care center in beaches of Adicora, Venezuela. Travel Med. Infect. Dis. 2012, 10, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Fenner, P.J.; Kokelj, F.; Williamson, J.A. Serious Physalia (Portuguese man o’war) stings: implications for scuba divers. J. Wilderness Med. 1994, 5, 71–76. [Google Scholar] [CrossRef]
- Virga, R.; Bechara, A.; da Silveira, F.L.; Morandini, A.C. Portuguese man-of-war envenoming in Southeastern Brazil: Report of 331 cases with follow-up after two years and data on the treatment using cold water packs and vinegar. Rev. Soc. Bras. Med. Trop. 2013, 46. [Google Scholar]
- Yanagihara, A.A.; Kuroiwa, J.M.; Oliver, L.M.; Kunkel, D.D. The ultrastructure of nematocysts from the fishing tentacle of the Hawaiian bluebottle, Physalia utriculus (Cnidaria, Hydrozoa, Siphonophora). Hydrobiologia 2002, 489, 139–150. [Google Scholar] [CrossRef]
- Labadie, M.; Aldabe, B.; Ong, N.; Joncquiert-Latarjet, A.; Groult, V.; Poulard, A.; Coudreuse, M.; Cordier, L.; Rolland, P.; Chanseau, P.; et al. Portuguese man-of-war (Physalia physalis) envenomation on the Aquitaine Coast of France: An emerging health risk. Clin. Toxicol. 2012, 50, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Bracker, M.D. Signs, symptoms, and management of jellyfish envenomation. Phys. Sportsmed. 1988, 16, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Haddad, V., Jr.; Silveira, F.L.D.; Migotto, Á.E. Skin lesions in envenoming by cnidarians (Portuguese man-of-war and jellyfish): Etiology and severity of accidents on the Brazilian coast. Rev. Inst. Med. Trop. São Paulo 2010, 52, 47–50. [Google Scholar] [CrossRef]
- Yanagihara, A.A.; Wilcox, C.; King, R.; Hurwitz, K.; Castelfranco, A.M. Experimental assays to assess the efficacy of vinegar and other topical first-aid approaches on cubozoan (Alatina alata) tentacle firing and venom toxicity. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.E.; Doge, E. The toxicity of Physalia nematocysts. Biol. Bull. 1958, 115, 219–226. [Google Scholar] [CrossRef]
- Yanagihara, A.A.; Wilcox, C.L. Cubozoan sting-site seawater rinse, scraping, and ice can increase venom load: upending current first aid recommendations. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Rubinstein, H.; Calton, G.J. First aid for jellyfish envenomation. South. Med. J. 1983, 76, 870–872. [Google Scholar] [CrossRef] [PubMed]
- Mianzan, H.W.; Fenner, P.J.; Cornelius, P.F.S.; Ramírez, F.C. Vinegar as a disarming agent to prevent further discharge of the nematocysts of the stinging hydromedusa Olindias sambaquiensis. Cutis 2001, 68, 45–49. [Google Scholar] [PubMed]
- Turner, B.; Sullivan, P.; Pennefather, J. Disarming the bluebottle: treatment of Physalia envenomation. Med. J. Aust. 1980, 2, 394–395. [Google Scholar] [PubMed]
- Exton, D.R. Treatment of Physalia physalis envenomation. Med. J. Aust. 1988, 149, 54. [Google Scholar] [PubMed]
- Birsa, L.; Verity, P.; Lee, R. Evaluation of the effects of various chemicals on discharge of and pain caused by jellyfish nematocysts. Comp. Biochem. Physiol. C 2010, 151, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Fenner, P.J.; Williamson, J.A.; Burnett, J.W.; Rifkin, J. First aid treatment of jellyfish stings in Australia: Response to a newly differentiated species. Med. J. Aust. 1993, 158, 498–501. [Google Scholar] [PubMed]
- Kajfasz, P. A case of severe stinging caused by venomous marine animal, “Portuguese man of war” (Physalia species) in all probability. Int. Marit. Health 2015, 66, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Australian Resuscitation Council Guideline 9.4.5. Envenomation—Jellyfish Stings. Available online: http://resus.org.au/?wpfb_dl = 41 (accessed on 30 June 2016).
- National Health Service, United Kingdom. Jellyfish and Other Sea Creature Stings—Treatment. Available online: http://www.nhs.uk/Conditions/Stings-marine-creatures/Pages/Treatment.aspx (accessed on 30 June 2016).
- Diver’s Alert Network. Portuguese Man-of-War. Available online: http://www.diversalertnetwork.org/health/hazardous-marine-life/portuguese-man-of-war (accessed on 30 June 2016).
- Ward, N.; Darracq, M.; Tomaszewski, C.; Clark, R. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann. Emerg. Med. 2012, 60, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Jellyfish Stings. Available online: http://www.emedicinehealth.com/jellyfish_stings/page4_em.htm (accessed on 30 June 2016).
- How to Treat Jellyfish Stings. Available online: http://www.wikihow.com/Treat-Jellyfish-Stings (accessed on 30 June 2016).
- Rifkin, J.; Fenner, P.J.; Williamson, J.A. First aid treatment from the sting from the hydroid Lytocarpus philippinus: The structure of, and in vitro discharge experiments with its nematocysts. J. Wilderness Med. 1993, 4, 252–260. [Google Scholar] [CrossRef]
- Baker, J.R. Principles of Biological Microtechnique; Methuen and Co. Ltd: London, UK, 1958; pp. 24–150. [Google Scholar]
- Calhoun, W. Jensen, S. (Director) The One with the Jellyfish [Friends]. Bright/Kauffman; Crane Productions, Warner Bros: Hollywood, CA, USA, 1997. [Google Scholar]
- Anderson, P.A.; Bouchard, C. The regulation of cnidocyte discharge. Toxicon 2009, 54, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Loten, C.; Stokes, B.; Worsley, D.; Seymour, J.E.; Jiang, S.; Isbister, G.K. A randomised controlled trial of hot water (45 °C) immersion versus ice packs for pain relief in bluebottle stings. Med. J. Aust. 2006, 184, 329–333. [Google Scholar] [PubMed]
- Li, L.; McGee, R.G.; Isbister, G.; Webster, A.C. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst. Rev. 2013, 12. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Xing, R.; Liu, S.; Li, K.; Wang, X.; Chen, X.; Li, P. Functional elucidation of Nemopilema nomurai and Cyanea nozakii nematocyst venoms’ lytic activity using mass spectrometry and zymography. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.L.; Yanagihara, A.A. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G. Wanderings In New South Wales, Batavia, Pedir Coast, Singapore and China; Richard Bentley: London, UK, 1834; p. 9. [Google Scholar]
- Barnes, J.H. Personal Notes, Quoted In Injuries to Man from Marine Invertebrates in the Australian Region; Cleland, J.B., Southcott, R.V., Eds.; Commonwealth of Australia: Canberra, Australia, 1965; pp. 31–32.
- Williamson, J.A.; Fenner, P.J.; Burnett, J.W. Principles of patient care in marine envenomations and poisonings. In Venomous and Poisonous Marine Animals: A Medical and Biological Handbook; Williamson, J.A., Fenner, P.J., Burnett, J.W., Rifkin, J.F., Eds.; Surf Life Saving Australia and University of New South Wales Press: Sydney, Australia, 1996; pp. 98–117. [Google Scholar]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef]



| First-Aid Solution | Cnidae Discharge (%) | |
|---|---|---|
| P. physalis (Atlantic) | P. utriculus (Pacific) | |
| Seawater | 00.59 ± 00.26 | 00.38 ± 00.13 |
| Freshwater | 40.94 ± 02.88 * | 02.01 ± 00.61 |
| Urine | 42.54 ± 06.88 * | 12.87 ± 02.60 * |
| Sting No More® Spray | 00.00 ± 00.00 | 00.06 ± 00.06 |
| Pressure | 46.64 ± 02.97 * | 44.06 ± 13.79 * |
| Alcohols | ||
| 10% Ethanol | not tested | 03.23 ± 01.32 |
| 30% Ethanol | not tested | 03.90 ± 01.62 |
| 70% Ethanol | 15.71 ± 03.68 * | 13.58 ± 05.63 * |
| >95% Ethanol | 33.96 ± 04.35 * | 37.74 ± 02.78 * |
| Vinegars | ||
| White Vinegar | 01.04 ± 00.04 | 00.20 ± 00.20 |
| Cider Vinegar | 00.31 ± 00.13 | 00.69 ± 00.39 |
| Malt Vinegar | 00.47 ± 00.38 | 00.73 ± 00.45 |
| Test Solution | pH | Cnidae Discharge (%) | Ethanol-Induced Discharge (%) | Pressure-Stimulated Discharge (%) | Discharge Increase from Pressure (%) |
|---|---|---|---|---|---|
| Readily-Available | |||||
| Seawater | 7.9 | 00.38 ± 0.13 | 37.74 ± 2.78 | 44.06 ± 13.79 | 43.7 |
| Tap Water | 6.0 | 02.01 ± 0.61 | 10.78 ± 6.85 | 15.50 ± 1.68 † | 13.5 |
| Urine | 6.2 | 12.87 ± 2.60 * | — | 14.73 ± 3.91 † | 1.9 |
| Shaving Cream | 6.5 | 09.43 ± 6.45 * | — | 22.45 ± 6.09 † | 13.0 |
| Baking Soda (3:1 in Seawater) | 9.0 | 08.90 ± 2.67 * | — | 35.56 ± 7.24 | 26.7 |
| Dish Soap | n/a | 01.68 ± 0.30 | 09.40 ± 4.81 | 23.75 ± 3.34 | 22.1 |
| Acetic Acid Solutions | |||||
| White Vinegar (3–5%) | 2.5 | 00.20 ± 0.20 | 00.00 ± 0.00 | 02.78 ± 0.88 † | 2.5 |
| Cider Vinegar (3–5%) | 3.2 | 00.69 ± 0.39 | 00.00 ± 0.00 | 02.27 ± 0.29 † | 1.6 |
| Malt Vinegar (3–5%) | 2.9 | 00.73 ± 0.45 | 00.24 ± 0.20 | 04.46 ± 2.22 † | 3.7 |
| Balsamic Vinegar (3–5%) | 3.0 | 01.94 ± 1.13 | 00.80 ± 0.28 | 03.47 ± 1.22 † | 1.5 |
| 5% Acetic Acid in Distilled Water | 2.4 | 00.50 ± 0.44 | 00.96 ± 0.42 | 02.61 ± 1.45 † | 2.1 |
| 75% White Vinegar in Seawater (~3.75%) | 2.6 | 01.82 ± 0.49 | 01.50 ± 0.79 | 20.40 ± 5.79 † | 18.6 |
| 50% White Vinegar in Seawater (~2.5%) | 2.6 | 01.11 ± 0.36 | 01.48 ± 0.50 | 24.34 ± 7.30 | 23.2 |
| 25% White Vinegar in Seawater (~1.88%) | 2.6 | 00.87 ± 0.51 | 06.79 ± 3.50 | 26.05 ± 5.29 | 25.2 |
| 10% White Vinegar in Seawater (~0.5%) | 2.7 | 01.66 ± 1.22 | 03.17 ± 1.70 | 31.35 ± 8.31 | 29.7 |
| Other Acidic Solutions | |||||
| 110 mM saline + HCl | 2.0 | 15.90 ± 7.70 * | — | 13.30 ± 1.48 † | −2.6 |
| Lemon Juice | 4.0 | 12.40 ± 2.32 * | — | 12.27 ± 3.09 † | −0.1 |
| Regular Cola | 4.0 | 02.80 ± 1.91 | 07.72 ± 5.37 | 18.12 ± 11.07 † | 15.3 |
| Alcohols | |||||
| Ethanol (>95%) | n/a | 37.74 ± 2.78 * | — | 41.06 ± 7.57 | 3.3 |
| Isopropanol (>95%) | n/a | 21.48 ± 6.76 * | — | 24.25 ± 8.91 | 2.8 |
| Commercial Products and Active Ingredients | |||||
| Sting No More® Spray | 3.0 | 00.00 ± 0.00 | 00.00 ± 0.00 | 03.53 ± 1.27 † | 3.5 |
| Copper Gluconate (30 mM in 110 mM saline) | 4.0 | 00.06 ± 0.06 | 05.11 ± 3.08 | 11.25 ± 1.49 † | 11.2 |
| Magnesium Sulfate | 4.9 | 08.57 ± 3.35 * | — | — | — |
| Lidocaine (4%) | 4.6 | 00.50 ± 0.14 | 00.46 ± 0.31 | 51.22 ± 12.16 | 50.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilcox, C.L.; Headlam, J.L.; Doyle, T.K.; Yanagihara, A.A. Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models. Toxins 2017, 9, 149. https://doi.org/10.3390/toxins9050149
Wilcox CL, Headlam JL, Doyle TK, Yanagihara AA. Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models. Toxins. 2017; 9(5):149. https://doi.org/10.3390/toxins9050149
Chicago/Turabian StyleWilcox, Christie L., Jasmine L. Headlam, Thomas K. Doyle, and Angel A. Yanagihara. 2017. "Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models" Toxins 9, no. 5: 149. https://doi.org/10.3390/toxins9050149
APA StyleWilcox, C. L., Headlam, J. L., Doyle, T. K., & Yanagihara, A. A. (2017). Assessing the Efficacy of First-Aid Measures in Physalia sp. Envenomation, Using Solution- and Blood Agarose-Based Models. Toxins, 9(5), 149. https://doi.org/10.3390/toxins9050149






