The Molecular Basis of Toxins’ Interactions with Intracellular Signaling via Discrete Portals
Abstract
:1. Introduction
2. Toxins and Their Target of Action
2.1. Toxins Acting via Plasma Membrane Lipid Domains
2.1.1. Toxins Acting via Pores Formation
2.1.1.1. Pardaxin
2.1.1.2. Staphylococcus aureus α-Toxin
2.1.2. Toxins Mimicking/or Activating Housekeeping Phospholipase A2 (PLA2)
2.1.2.1. Bee PLA2 Toxin
2.1.2.2. Crotoxin
2.1.2.3. Melittin
2.2. Toxins Acting via Ion Channels
2.2.1. Voltage-Gated Potassium Ion Channels (Kv)
Dendrotoxin
2.2.2. Voltage-Gated Sodium Channels (Nav)
α-Scorpion Toxin
2.2.3. Voltage-Gated Calcium Channels (VGCC)
2.2.3.1. Ω -Conotoxin
2.2.3.2. Ω-Agatoxin
2.2.4. Chloride Channels (CLCs)
Chlorotoxin (CTX)
2.3. Toxins Acting via Ion Pumps
2.3.1. Na+/K+ ATPase
Cardiac Glycoside Toxins
2.4. Toxins Acting via Ligand-Gated Ionotropic Channel Receptors
2.4.1. α-Bungarotoxin (α-Bgtx)
2.4.2. Domoic Acid (DA) Toxin
2.4.3. Kainic Acid (KA) Toxin
2.5. Toxins Acting via G-Protein Coupled Receptors (GPCRs)
2.5.1. G-Protein Coupled Cholinergic Muscarinic Receptors
Dendroaspis Toxin
2.5.2. GTP Binding Proteins (G-Protein)
2.5.2.1. Cholera Toxin (CT)
2.5.2.2. Pertussis Toxin (PTX)
2.5.3. House-Keeping Enzymes
2.5.3.1. Adenylate cyclase
Anthrax Toxin
2.5.3.2. PI-specific phospholipase C (PLC)
Staphylococcus aureus (S. aureus) PI-PLC Toxin
2.6. Toxins Acting on Small GTP-Binding Proteins
Botulinum C3 exoenzyme Toxin
2.7. Toxins Affecting Tyrosine Kinase Receptors (RTKs)
Staphylococcus aureus α-Toxin
2.8. Toxins Acting on Integrins Receptors Affecting Cell Adhesion
2.8.1. Disintegrins
2.8.2. C-Type Lectin-Like (CTL) Toxins
2.9. Toxins Acting on the Nucleic Acids
2.9.1. DNA-Targeted Mycotoxins
2.9.2. Histone Deacetylases Toxins
2.10. Toxins Acting on the Mitochondrial Respiratory System
Rotenone Toxin
2.11. Toxins Acting on the Ribosome
Diphtheria Toxin (DT)
2.12. Toxins Acting on Exocytotic Vesicles
2.12.1. Botulinum Neurotoxin (BoNT)
2.12.2. Tetanus Neurotoxin (TeNT)
2.13. Toxins Acting on Protein Kinases and Phosphatases
2.13.1. Staurosporine (St)
2.13.2. Okadaic acid (OA)
3. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gutman, Y.; Lazarovici, P. Toxins and Signal Transduction; The Netherland by Harwood Academic Publishers, Harwood Academic: Amsterdam, The Netherlands, 1997; Volume 1, pp. 1–14. [Google Scholar]
- Cecchini, M.; Changeux, J.P. The nicotinic acetylcholine receptor and its prokaryotic homologues: Structure, conformational transitions & allosteric modulation. Neuropharmacology 2015, 96, 137–149. [Google Scholar] [PubMed]
- Luiken, J.J.; Glatz, J.F.; Neumann, D. Cardiac contraction-induced GLUT4 translocation requires dual signaling input. Trends Endocrinol. Metable 2015, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Bee venom phospholipase A2: Yesterday’s enemy becomes today’s friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Burack, W.R.; Biltonen, R.L. Lipid bilayer heterogeneities and modulation of phospholipase A2 activity. Chem. Phys. Lipids 1994, 73, 209–222. [Google Scholar] [CrossRef]
- Dal Peraro, M.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.J.; Dalla Serra, M.; Froelich, C.J.; Wallace, M.I.; Anderluh, G. Membrane pore formation at protein-lipid interfaces. Trends Biochem. Sci. 2014, 39, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Gutman, Y.; Lazarovici, P. Toxins and Signal Transduction; The Netherland by Harwood Academic Publishers, Harwood Academic: Amsterdam, The Netherlands, 1997; Volume 1, pp. 211–232. [Google Scholar]
- Berube, B.J.; Bubeck Wardenburg, J. Staphylococcus aureus alpha-toxin: Nearly a century of intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Tranum-Jensen, J. Damage to mammalian cells by proteins that form transmembrane pores. Rev. Physiol. Biochem. Pharmacol. 1987, 107, 147–223. [Google Scholar] [PubMed]
- Lazarovici, P.; Primor, N.; Loew, L.M. Purification and pore-forming activity of two hydrophobic polypeptides from the secretion of the red sea moses sole (pardachirus marmoratus). J. Biol. Chem. 1986, 261, 16704–16713. [Google Scholar] [PubMed]
- Renner, P.; Caratsch, C.G.; Waser, P.G.; Lazarovici, P.; Primor, N. Presynaptic effects of the pardaxins, polypeptides isolated from the gland secretion of the flatfish pardachirus marmoratus. Neuroscience 1987, 23, 319–325. [Google Scholar] [CrossRef]
- Arribas, M.; Blasi, J.; Lazarovici, P.; Marsal, J. Calcium-dependent and -independent acetylcholine release from electric organ synaptosomes by pardaxin: Evidence of a biphasic action of an excitatory neurotoxin. J. Neurochem. 1993, 60, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Friedman, E. Increased 5-hydroxytryptamine and norepinephrine release from rat brain slices by the red sea flatfish toxin pardaxin. J. Neurochem. 1986, 47, 656–658. [Google Scholar] [PubMed]
- Lazarovici, P.; Lelkes, P.I. Pardaxin induces exocytosis in bovine adrenal medullary chromaffin cells independent of calcium. J. Pharmacol. Exp. Ther. 1992, 263, 1317–1326. [Google Scholar] [PubMed]
- Abu-Raya, S.; Bloch-Shilderman, E.; Lelkes, P.I.; Trembovler, V.; Shohami, E.; Gutman, Y.; Lazarovici, P. Characterization of pardaxin-induced dopamine release from pheochromocytoma cells: Role of calcium and eicosanoids. J. Pharmacol. Exp. Ther. 1999, 288, 399–406. [Google Scholar] [PubMed]
- Shai, Y.; Fox, J.; Caratsch, C.; Shih, Y.L.; Edwards, C.; Lazarovici, P. Sequencing and synthesis of pardaxin, a polypeptide from the red sea moses sole with ionophore activity. FEBS Lett. 1988, 242, 161–166. [Google Scholar] [CrossRef]
- Adermann, K.; Raida, M.; Paul, Y.; Abu-Raya, S.; Bloch-Shilderman, E.; Lazarovici, P.; Hochman, J.; Wellhoner, H. Isolation, characterization and synthesis of a novel paradaxin isoform. FEBS Lett. 1998, 435, 173–177. [Google Scholar] [CrossRef]
- Shi, Y.L.; Edwards, C.; Lazarovici, P. Ion selectivity of the channels formed by pardaxin, an ionophore, in bilayer membranes. Nat. Toxins 1995, 3, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, P.; Edwards, C.; Raghunathan, G.; Guy, H.R. Secondary structure, permeability and molecular modeling of pardaxin pores. J. Nat. Toxins 1992, 1, 1–15. [Google Scholar]
- Primor, N.; Lazarovici, P. Pardachirus marmoratus (red sea flatfish) secretion and its isolated toxic fraction pardaxin: The relationship between hemolysis and atpase inhibition. Toxicon 1981, 19, 573–578. [Google Scholar] [CrossRef]
- Bloch-Shilderman, E.; Abu-Raya, S.; Trembovler, V.; Boschwitz, H.; Gruzman, A.; Linial, M.; Lazarovici, P. Pardaxin stimulation of phospholipases A2 and their involvement in exocytosis in PC-12 cells. J. Pharmacol. Exp. Ther. 2002, 301, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Pardaxin: Channel formation by a shark repellant peptide from fish. Toxicology 1994, 87, 109–129. [Google Scholar] [CrossRef]
- Nikodijevic, B.; Nikodijevic, D.; Lazarovici, P. Pardaxin-stimulated calcium uptake in PC12 cells is blocked by cadmium and is not mediated by L-type calcium channels. J. Basic Clin. Physiol. Pharmacol. 1992, 3, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Fussle, R.; Bhakdi, S.; Sziegoleit, A.; Tranum-Jensen, J.; Kranz, T.; Wellensiek, H.J. On the mechanism of membrane damage by staphylococcus aureus alpha-toxin. J. Cell Biol. 1981, 91, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751. [Google Scholar] [PubMed]
- Bayley, H. Triggers and switches in a self-assembling pore-forming protein. J. Cell. Biochem. 1994, 56, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [PubMed]
- Acker, J.P.; Lu, X.M.; Young, V.; Cheley, S.; Bayley, H.; Fowler, A.; Toner, M. Measurement of trehalose loading of mammalian cells porated with a metal-actuated switchable pore. Biotechnol. Bioeng. 2003, 82, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Balsinde, J.; Winstead, M.V.; Dennis, E.A. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett. 2002, 531, 2–6. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Miki, Y.; Yamamoto, K.; Taketomi, Y. A new era of secreted phospholipase A(2). J. Lipid Res. 2015, 56, 1248–1261. [Google Scholar] [PubMed]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Ramanadham, S.; Ali, T.; Ashley, J.W.; Bone, R.N.; Hancock, W.D.; Lei, X. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J. Lipid Res. 2015, 56, 1643–1668. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.; Vindrieux, D. PLA2R1: Expression and function in cancer. Biochim. Biophys. Acta 2014, 1846, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Fabbro, D.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The concise guide to pharmacology 2015/16: Enzymes. Br. J. Pharmacol. 2015, 172, 6024–6109. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.P.; Lin, Y.; Lambeau, G.; Ghomashchi, F.; Lazdunski, M.; Gelb, M.H. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J. Biol. Chem. 1997, 272, 7173–7181. [Google Scholar] [CrossRef] [PubMed]
- Annand, R.R.; Kontoyianni, M.; Penzotti, J.E.; Dudler, T.; Lybrand, T.P.; Gelb, M.H. Active site of bee venom phospholipase A2: The role of histidine-34, aspartate-64 and tyrosine-87. Biochemistry 1996, 35, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Vadas, P.; Pruzanski, W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab. Investig. 1986, 55, 391–404. [Google Scholar]
- Schumacher, M.J.; Egen, N.B.; Tanner, D. Neutralization of bee venom lethality by immune serum antibodies. Am. J. Trop. Med. Hyg. 1996, 55, 197–201. [Google Scholar] [PubMed]
- Prado, M.; Solano-Trejos, G.; Lomonte, B. Acute physiopathological effects of honeybee (apis mellifera) envenoming by subcutaneous route in a mouse model. Toxicon 2010, 56, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Saul, F. Crystallographic characterization of functional sites of crotoxin and ammodytoxin, potent beta-neurotoxins from viperidae venom. Toxicon 2012, 60, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Campelo, I.S.; Pereira, A.F.; Alcantara-Neto, A.S.; Canel, N.G.; Souza-Fabjan, J.M.; Teixeira, D.I.; Camargo, L.S.; Melo, L.M.; Radis-Baptista, G.; Salamone, D.F.; et al. Effect of crotamine, a cell-penetrating peptide, on blastocyst production and gene expression of in vitro fertilized bovine embryos. Zygote 2016, 24, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Hendon, R.A.; Fraenkel-Conrat, H. Biological roles of the two components of crotoxin. Proc. Natl. Acad. Sci. USA 1971, 68, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, M.C.; Yen, C.H.; Hseu, M.J.; Tseng, C.C.; Tsai, M.D.; Dupureur, C.M. Binding proteins on synaptic membranes for crotoxin and taipoxin, two phospholipases A2 with neurotoxicity. Toxicon 1995, 33, 451–457. [Google Scholar] [CrossRef]
- Bon, C.; Changeux, J.P.; Jeng, T.W.; Fraenkel-Conrat, H. Postsynaptic effects of crotoxin and of its isolated subunits. Eur. J. Biochem. 1979, 99, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Repo, H. Leukocyte migration agarose test for the assessment of human neutrophil chemotaxis. I. Effects of environmental factors on neutrophil migration under agarose. Scand. J. Immunol. 1977, 6, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Donato, N.J.; Martin, C.A.; Perez, M.; Newman, R.A.; Vidal, J.C.; Etcheverry, M. Regulation of epidermal growth factor receptor activity by crotoxin, a snake venom phospholipase A2 toxin. A novel growth inhibitory mechanism. Biochem. Pharmacol. 1996, 51, 1535–1543. [Google Scholar] [CrossRef]
- Cura, J.E.; Blanzaco, D.P.; Brisson, C.; Cura, M.A.; Cabrol, R.; Larrateguy, L.; Mendez, C.; Sechi, J.C.; Silveira, J.S.; Theiller, E.; et al. Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA(2), NSC-624244) in patients with advanced cancer. Clin. Cancer Res. 2002, 8, 1033–1041. [Google Scholar] [PubMed]
- Magalhaes, T.; Viotti, A.P.; Gomes, R.T.; de Freitas, T.V. Effect of membrane composition and of Co-encapsulation of immunostimulants in a liposome-entrapped crotoxin. Biotechnol. Appl. Biochem. 2001, 33, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Gluskin, A.H. Rethinking clinical endodontic diagnosis. J. Calif. Dent. Assoc. 1991, 19, 15–22. [Google Scholar] [PubMed]
- Palm, N.W.; Rosenstein, R.K.; Yu, S.; Schenten, D.D.; Florsheim, E.; Medzhitov, R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 2013, 39, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.R.; Bomalaski, J.S.; Clark, M.A. Responses of purified phospholipases A2 to phospholipase A2 activating protein (PLAP) and melittin. Biochim. Biophys. Acta 1993, 1166, 124–130. [Google Scholar] [CrossRef]
- Yue, H.Y.; Fujita, T.; Kumamoto, E. Phospholipase A2 activation by melittin enhances spontaneous glutamatergic excitatory transmission in rat substantia gelatinosa neurons. Neuroscience 2005, 135, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Vernon, L.P.; Bell, J.D. Membrane structure, toxins and phospholipase A2 activity. Pharmacol. Ther. 1992, 54, 269–295. [Google Scholar] [CrossRef]
- Jones, R.G.; Corteling, R.L.; Bhogal, G.; Landon, J. A novel fab-based antivenom for the treatment of mass bee attacks. Am. J. Trop. Med. Hyg. 1999, 61, 361–366. [Google Scholar] [PubMed]
- Liu, P.; Davis, P.; Liu, H.; Krishnan, T.R. Evaluation of cytotoxicity and absorption enhancing effects of melittin—A novel absorption enhancer. Eur. J. Pharm. Biopharm. 1999, 48, 85–87. [Google Scholar] [CrossRef]
- Aliwarga, Y.; Hume, E.B.; Lan, J.; Willcox, M.D. Antimicrobial peptides: A potential role in ocular therapy. Clin. Exp. Ophthalmol. 2001, 29, 157–160. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000, 20, 323–349. [Google Scholar] [CrossRef]
- Morales-Lazaro, S.L.; Hernandez-Garcia, E.; Serrano-Flores, B.; Rosenbaum, T. Organic toxins as tools to understand ion channel mechanisms and structure. Curr. Top. Med. Chem. 2015, 15, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Catterall, W.A.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The concise guide to pharmacology 2015/16: Voltage-gated ion channels. Br. J. Pharmacol. 2015, 172, 5904–5941. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. The concise guide to pharmacology 2015/16: Other ion channels. Br. J. Pharmacol. 2015, 172, 5942–5955. [Google Scholar] [CrossRef] [PubMed]
- Baden, D.G. Brevetoxins: Unique polyether dinoflagellate toxins. FASEB J. 1989, 3, 1807–1817. [Google Scholar] [PubMed]
- Wang, S.Y.; Wang, G.K. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal. 2003, 15, 151–159. [Google Scholar] [CrossRef]
- Lewis, R.J. Conotoxins as selective inhibitors of neuronal ion channels, receptors and transporters. IUBMB Life 2004, 56, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hernandez, V.; Jimenez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L.D. Scorpion venom components that affect ion-channels function. Toxicon 2013, 76, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Rowan, E.G.; Harvey, A.L. Snake toxins from mamba venoms: Unique tools for the physiologist. Acta Chim. Slovenica 2011, 58, 689–692. [Google Scholar]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef] [PubMed]
- Teisseyre, A.; Gasiorowska, J.; Michalak, K. Voltage-gated potassium channels kv1.3--potentially new molecular target in cancer diagnostics and therapy. Adv. Clin. Exp. Med. 2015, 24, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Robertson, B. Dendrotoxins: Structure-activity relationships and effects on potassium ion channels. Curr. Med. Chem. 2004, 11, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Karlsson, E. Dendrotoxin from the venom of the green mamba, dendroaspis angusticeps. A neurotoxin that enhances acetylcholine release at neuromuscular junction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1980, 312, 1–6. [Google Scholar] [CrossRef]
- Joubert, F.J.; Taljaard, N. Snake venoms. The amino acid sequences of two proteinase inhibitor homologues from dendroaspis angusticeps venom. Hoppe-Seyler’s Z. Physiol. Chem. 1980, 361, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Mehraban, F.; Haines, A.; Dolly, J.O. Monoclonal and polyclonal antibodies against dendrotoxin: Their effects on its convulsive activity and interaction with neuronal acceptors. Neurochem. Int. 1986, 9, 11–22. [Google Scholar] [CrossRef]
- Benishin, C.G.; Sorensen, R.G.; Brown, W.E.; Krueger, B.K.; Blaustein, M.P. Four polypeptide components of green mamba venom selectively block certain potassium channels in rat brain synaptosomes. Mol. Pharmacol. 1988, 34, 152–159. [Google Scholar] [PubMed]
- Harvey, A.L.; Karlsson, E. Protease inhibitor homologues from mamba venoms: Facilitation of acetylcholine release and interactions with prejunctional blocking toxins. Br. J. Pharmacol. 1982, 77, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Harvey, A.L. Effects of the potassium channel blocking dendrotoxins on acetylcholine release and motor nerve terminal activity. Br. J. Pharmacol. 1988, 93, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, F.; Penner, R. The actions of presynaptic snake toxins on membrane currents of mouse motor nerve terminals. J. Physiol. 1987, 386, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Grissmer, S.; Nguyen, A.N.; Aiyar, J.; Hanson, D.C.; Mather, R.J.; Gutman, G.A.; Karmilowicz, M.J.; Auperin, D.D.; Chandy, K.G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 1994, 45, 1227–1234. [Google Scholar] [PubMed]
- Pongs, O. Molecular biology of voltage-dependent potassium channels. Physiol. Rev. 1992, 72, S69–S88. [Google Scholar] [PubMed]
- Black, A.R.; Donegan, C.M.; Denny, B.J.; Dolly, J.O. Solubilization and physical characterization of acceptors for dendrotoxin and β-bungarotoxin from synaptic membranes of rat brain. Biochemistry 1988, 27, 6814–6820. [Google Scholar] [CrossRef] [PubMed]
- Rehm, H.; Lazdunski, M. Purification and subunit structure of a putative K+-channel protein identified by its binding properties for dendrotoxin I. Proc. Natl. Acad. Sci. USA 1988, 85, 4919–4923. [Google Scholar] [CrossRef] [PubMed]
- Bidard, J.N.; Mourre, C.; Gandolfo, G.; Schweitz, H.; Widmann, C.; Gottesmann, C.; Lazdunski, M. Analogies and differences in the mode of action and properties of binding sites (localization and mutual interactions) of two K+ channel toxins, mcd peptide and dendrotoxin I. Brain Res. 1989, 495, 45–57. [Google Scholar] [CrossRef]
- Pelchen-Matthews, A.; Dolly, J.O. Distribution in the rat central nervous system of acceptor sub-types for dendrotoxin, a K+ channel probe. Neuroscience 1989, 29, 347–361. [Google Scholar] [CrossRef]
- Marban, E.; Yamagishi, T.; Tomaselli, G.F. Structure and function of voltage-gated sodium channels. J. Physiol. 1998, 508, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Cestele, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-gated ion channels and gating modifier toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possani, L.D.; Becerril, B.; Delepierre, M.; Tytgat, J. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 1999, 264, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Denac, H.; Mevissen, M.; Scholtysik, G. Structure, function and pharmacology of voltage-gated sodium channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 453–479. [Google Scholar] [CrossRef]
- Cestele, S.; Catterall, W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar] [CrossRef]
- Gilles, N.; Krimm, I.; Bouet, F.; Froy, O.; Gurevitz, M.; Lancelin, J.M.; Gordon, D. Structural implications on the interaction of scorpion α-like toxins with the sodium channel receptor site inferred from toxin iodination and ph-dependent binding. J. Neurochem. 2000, 75, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.C.; Qu, Y.; Tanada, T.N.; Scheuer, T.; Catterall, W.A. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J. Biol. Chem. 1996, 271, 15950–15962. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yarov-Yarovoy, V.; Kahn, R.; Gordon, D.; Gurevitz, M.; Scheuer, T.; Catterall, W.A. Mapping the receptor site for alpha-scorpion toxins on a Na+ channel voltage sensor. Proc. Natl. Acad. Sci. USA 2011, 108, 15426–15431. [Google Scholar] [CrossRef] [PubMed]
- Kopeyan, C.; Mansuelle, P.; Martin-Eauclaire, M.F.; Rochat, H.; Miranda, F. Characterization of toxin III of the scorpion leiurus quinquestriatus quinquestriatus: A new type of alpha-toxin highly toxic both to mammals and insects. Nat. Toxins 1993, 1, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Wang, B.; Alam, M.; Beck, A.; Stoeva, S.; Voelter, W.; Abbasi, A.; Duszenko, M. Structure-activity relationship of an alpha-toxin Bs-Tx28 from scorpion (buthus sindicus) venom suggests a new alpha-toxin subfamily. Arch. Biochem. Biophys. 2006, 445, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J.; Berecki, G. Mechanisms of conotoxin inhibition of N-type (Ca(v)2.2) calcium channels. Biochim. Biophys. Acta 2013, 1828, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Nimmrich, V.; Gross, G. P/Q-type calcium channel modulators. Br. J. Pharmacol. 2012, 167, 741–759. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.J.; Adams, D.; Thomas, L.; Bond, T.; Alewood, P.F.; Craik, D.J.; Lewis, R.J. Structure-activity relationships of omega-conotoxins MVIIA, MVIIC and 14 loop splice hybrids at n and P/Q-type calcium channels. J. Mol. Biol. 1999, 289, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Bingham, J.P.; Mitsunaga, E.; Bergeron, Z.L. Drugs from slugs—Past, present and future perspectives of omega-conotoxin research. Chemico-Biol. Interact. 2010, 183, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.; Khalil, Z.; Satkunanthan, N.; Livett, B.G. Drugs from the sea: Conotoxins as drug leads for neuropathic pain and other neurological conditions. Mini Rev. Med. Chem. 2003, 3, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Wright, C.E.; Angus, J.A. Actions of intrathecal omega-conotoxins CVID, GVIA, MVIIA, and morphine in acute and neuropathic pain in the rat. Eur. J. Pharmacol. 2002, 451, 279–286. [Google Scholar] [CrossRef]
- Cruz, L.J.; Olivera, B.M. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J. Biol. Chem. 1986, 261, 6230–6233. [Google Scholar] [PubMed]
- Wang, Y.X.; Gao, D.; Pettus, M.; Phillips, C.; Bowersox, S.S. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal n-type voltage-sensitive calcium channels, with morphine on nociception in rats. Pain 2000, 84, 271–281. [Google Scholar] [CrossRef]
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.S.; Rauck, R.; Fisher, R.; Charapata, S.G.; Ellis, D.; Dissanayake, S.; Ziconotide 98–022 Study Group. Intrathecal ziconotide for severe chronic pain: Safety and tolerability results of an open-label, long-term trial. Anesth. Analg. 2008, 106, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.P.; Hamid, J.; Doering, C.; Bosey, G.M.; Snutch, T.P.; Zamponi, G.W. Residue Gly1326 of the N-type calcium channel alpha 1b subunit controls reversibility of omega-conotoxin GVIA and MVIIA block. J. Biol. Chem. 2001, 276, 15728–15735. [Google Scholar] [CrossRef] [PubMed]
- Bindokas, V.P.; Venema, V.J.; Adams, M.E. Differential antagonism of transmitter release by subtypes of omega-agatoxins. J. Neurophysiol. 1991, 66, 590–601. [Google Scholar] [PubMed]
- Bindokas, V.P.; Adams, M.E. Omega-aga-I: A presynaptic calcium channel antagonist from venom of the funnel web spider, agelenopsis aperta. J. Neurobiol. 1989, 20, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Venema, V.J.; Swiderek, K.M.; Lee, T.D.; Hathaway, G.M.; Adams, M.E. Antagonism of synaptosomal calcium channels by subtypes of omega-agatoxins. J. Biol. Chem. 1992, 267, 2610–2615. [Google Scholar] [PubMed]
- Adams, M.E.; Mintz, I.M.; Reily, M.D.; Thanabal, V.; Bean, B.P. Structure and properties of omega-agatoxin IVB, a new antagonist of P-type calcium channels. Mol. Pharmacol. 1993, 44, 681–688. [Google Scholar] [PubMed]
- Ertel, E.A.; Smith, M.M.; Leibowitz, M.D.; Cohen, C.J. Isolation of myocardial l-type calcium channel gating currents with the spider toxin omega-aga-IIIA. J. Gen. Physiol. 1994, 103, 731–753. [Google Scholar] [CrossRef] [PubMed]
- Mintz, I.M. Block of ca channels in rat central neurons by the spider toxin omega-aga-IIIA. J. Neurosci. 1994, 14, 2844–2853. [Google Scholar] [PubMed]
- Mintz, I.M.; Venema, V.J.; Adams, M.E.; Bean, B.P. Inhibition of N- and L-type Ca2+ channels by the spider venom toxin omega-aga-IIIA. Proc. Natl. Acad. Sci. USA 1991, 88, 6628–6631. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Ertel, E.A.; Smith, M.M.; Venema, V.J.; Adams, M.E.; Leibowitz, M.D. High affinity block of myocardial L-type calcium channels by the spider toxin omega-aga-toxin IIIA: Advantages over 1,4-dihydropyridines. Mol. Pharmacol. 1992, 42, 947–951. [Google Scholar] [PubMed]
- Cohen, C.J.; Spires, S.; Van Skiver, D. Block of t-type ca channels in guinea pig atrial cells by antiarrhythmic agents and ca channel antagonists. J. Gen. Physiol. 1992, 100, 703–728. [Google Scholar] [CrossRef] [PubMed]
- Mintz, I.M.; Venema, V.J.; Swiderek, K.M.; Lee, T.D.; Bean, B.P.; Adams, M.E. P-type calcium channels blocked by the spider toxin omega-aga-IVA. Nature 1992, 355, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.B.; Randall, A.; Tsien, R.W. Roles of n-type and q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science 1994, 264, 107–111. [Google Scholar] [CrossRef] [PubMed]
- McDonough, S.I.; Mintz, I.M.; Bean, B.P. Alteration of P-type calcium channel gating by the spider toxin omega-aga-IVA. Biophys. J. 1997, 72, 2117–2128. [Google Scholar] [CrossRef]
- Turner, T.J.; Adams, M.E.; Dunlap, K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc. Natl. Acad. Sci. USA 1993, 90, 9518–9522. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.J.; Adams, M.E.; Dunlap, K. Calcium channels coupled to glutamate release identified by omega-aga-iva. Science 1992, 258, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.; Ginsburg, S.; Butkevich, A.; Kachalsky, S.G.; Kaiserman, I.; Ahdut, R.; Demirgoren, S.; Rahamimoff, R. Ion channels in presynaptic nerve terminals and control of transmitter release. Physiol. Rev. 1999, 79, 1019–1088. [Google Scholar] [PubMed]
- Mintz, I.M.; Sabatini, B.L.; Regehr, W.G. Calcium control of transmitter release at a cerebellar synapse. Neuron 1995, 15, 675–688. [Google Scholar] [PubMed]
- Takahashi, T.; Momiyama, A. Different types of calcium channels mediate central synaptic transmission. Nature 1993, 366, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.; Thompson, C.H.; Xiao, Q.; Hartzell, H.C. Chloride channels: Often enigmatic, rarely predictable. Annu. Rev. Physiol. 2010, 72, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, L.; Rani, D.; Aziz, T.A.; Bazin, I.; Sabatier, J.M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Lippens, G.; Najib, J.; Wodak, S.J.; Tartar, A. Nmr sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels. Biochemistry 1995, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol. 1993, 264, C361–C369. [Google Scholar] [PubMed]
- Fu, Y.J.; Yin, L.T.; Wang, W.; Chai, B.F.; Liang, A.H. Synthesis, expression and purification of a type of chlorotoxin-like peptide from the scorpion, buthus martensii karsch, and its acute toxicity analysis. Biotechnol. Lett. 2005, 27, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, N.; Bordey, A.; Gillespie, G.Y.; Sontheimer, H. Expression of voltage-activated chloride currents in acute slices of human gliomas. Neuroscience 1998, 83, 1161–1173. [Google Scholar] [CrossRef]
- Olsen, M.L.; Schade, S.; Lyons, S.A.; Amaral, M.D.; Sontheimer, H. Expression of voltage-gated chloride channels in human glioma cells. J. Neurosci. 2003, 23, 5572–5582. [Google Scholar] [PubMed]
- Lyons, S.A.; O′Neal, J.; Sontheimer, H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia 2002, 39, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Hockaday, D.C.; Shen, S.; Fiveash, J.; Raubitschek, A.; Colcher, D.; Liu, A.; Alvarez, V.; Mamelak, A.N. Imaging glioma extent with 131I-TM-601. J. Nucl. Med. 2005, 46, 580–586. [Google Scholar] [PubMed]
- Shen, S.; Khazaeli, M.B.; Gillespie, G.Y.; Alvarez, V.L. Radiation dosimetry of 131I-chlorotoxin for targeted radiotherapy in glioma-bearing mice. J. Neuro-Oncol. 2005, 71, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, A.N.; Rosenfeld, S.; Bucholz, R.; Raubitschek, A.; Nabors, L.B.; Fiveash, J.B.; Shen, S.; Khazaeli, M.B.; Colcher, D.; Liu, A.; et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J. Clin. Oncol. 2006, 24, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, J.; Zhao, L.; Xiong, Z.; Tang, Y.; Liu, C.; Guo, L.; Qiao, W.; Shi, X.; Zhao, J. (131)I-labeled multifunctional dendrimers modified with bmk ct for targeted spect imaging and radiotherapy of gliomas. Nanomedicine 2016, 11, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, D.C. Ion channels versus ion pumps: The principal difference, in principle. Nat. Rev. Mol. Cell Biol. 2009, 10, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. The concise guide to pharmacology 2015/16: Transporters. Br. J. Pharmacol. 2015, 172, 6110–6202. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Ambudkar, S.V. Overview: Abc transporters and human disease. J. Bioenerg. Biomembr. 2001, 33, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 2007, 293, C509–C536. [Google Scholar] [CrossRef] [PubMed]
- Nesher, M.; Shpolansky, U.; Rosen, H.; Lichtstein, D. The digitalis-like steroid hormones: New mechanisms of action and biological significance. Life Sci. 2007, 80, 2093–2107. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Contreras, R.G.; Wang, R.; Fernandez, S.V.; Shoshani, L.; Russo, I.H.; Cereijido, M.; Russo, J. Sodium/potassium atpase (Na+, K+-atpase) and ouabain/related cardiac glycosides: A new paradigm for development of anti- breast cancer drugs? Breast Cancer Res. Treat. 2006, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. Fxyd proteins: New regulators of na-k-atpase. Am. J. Physiol. Renal Physiol. 2006, 290, F241–F250. [Google Scholar] [CrossRef] [PubMed]
- Garty, H.; Karlish, S.J. Role of fxyd proteins in ion transport. Annu. Rev. Physiol. 2006, 68, 431–459. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cai, T. Na+-K+--atpase-mediated signal transduction: From protein interaction to cellular function. Mol. Interv. 2003, 3, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Lytton, J.; Westlin, M.; Hanley, M.R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-atpase family of calcium pumps. J. Biol. Chem. 1991, 266, 17067–17071. [Google Scholar] [PubMed]
- Hammond, C.E.; Beeson, C.; Suarez, G.; Peek, R.M., Jr.; Backert, S.; Smolka, A.J. Helicobacter pylori virulence factors affecting gastric proton pump expression and acid secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hamad, E.; Mather, P.J.; Srinivasan, S.; Rubin, S.; Whellan, D.J.; Feldman, A.M. Pharmacologic therapy of chronic heart failure. Am. J. Cardiovasc. Drugs 2007, 7, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; van Veldhuisen, D.J.; Colucci, W.S. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation 2006, 113, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z. Ouabain interaction with cardiac Na/K-atpase reveals that the enzyme can act as a pump and as a signal transducer. Cell. Mol. Biol. 2001, 47, 383–390. [Google Scholar] [PubMed]
- Liu, J.; Tian, J.; Haas, M.; Shapiro, J.I.; Askari, A.; Xie, Z. Ouabain interaction with cardiac Na+/K+-atpase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 2000, 275, 27838–27844. [Google Scholar] [PubMed]
- Newman, R.A.; Yang, P.; Pawlus, A.D.; Block, K.I. Cardiac glycosides as novel cancer therapeutic agents. Mol. Interv. 2008, 8, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Robey, T.E.; Tarabar, A.F.; Hodsdon, M.E. Rapid detection of convallatoxin using five digoxin immunoassays. Clin. Toxicol. 2014, 52, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Peters, J.A.; Kelly, E.; Marrion, N.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. The concise guide to pharmacology 2015/16: Ligand-gated ion channels. Br. J. Pharmacol. 2015, 172, 5870–5903. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom—Milestones of discovery. Toxicon 2013, 62, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Olsen, R.W.; Peters, J.; Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharmacology 2009, 56, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.R.; Manalo, J.L. The activation of glutamate receptors by kainic acid and domoic acid. Nat. Toxins 1998, 6, 153–158. [Google Scholar] [CrossRef]
- Gotti, C.; Omini, C.; Berti, F.; Clementi, F. Isolation of a polypeptide from the venom of bungarus multicinctus that binds to ganglia and blocks the ganglionic transmission in mammals. Neuroscience 1985, 15, 563–575. [Google Scholar] [CrossRef]
- Rosenthal, J.A.; Hsu, S.H.; Schneider, D.; Gentile, L.N.; Messier, N.J.; Vaslet, C.A.; Hawrot, E. Functional expression and site-directed mutagenesis of a synthetic gene for α-bungarotoxin. J. Biol. Chem. 1994, 269, 11178–11185. [Google Scholar] [PubMed]
- Menez, A. Functional architectures of animal toxins: A clue to drug design? Toxicon 1998, 36, 1557–1572. [Google Scholar] [CrossRef]
- Love, R.A.; Stroud, R.M. The crystal structure of α-bungarotoxin at 2.5 A resolution: Relation to solution structure and binding to acetylcholine receptor. Protein Eng. 1986, 1, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Tamiya, N. Current view on the structure-function relationship of postsynaptic neurotoxins from snake venoms. Pharmacol. Ther. 1987, 34, 403–451. [Google Scholar] [CrossRef]
- Stroud, R.M.; McCarthy, M.P.; Shuster, M. Nicotinic acetylcholine receptor superfamily of ligand-gated ion channels. Biochemistry 1990, 29, 11009–11023. [Google Scholar] [CrossRef] [PubMed]
- Polz-Tejera, G.; Schmidt, J.; Karten, H.J. Autoradiographic localisation of α-bungarotoxin-binding sites in the central nervous system. Nature 1975, 258, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Crosland, R.D. Effect of chloroquine on toxicity in mice of the venom and neurotoxins from the snake bungarus multicinctus. J. Pharmacol. Exp. Ther. 1988, 246, 992–995. [Google Scholar] [PubMed]
- Elgoyhen, A.B.; Johnson, D.S.; Boulter, J.; Vetter, D.E.; Heinemann, S. Alpha 9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 1994, 79, 705–715. [Google Scholar] [CrossRef]
- Sargent, P.B. The diversity of neuronal nicotinic acetylcholine receptors. Annu. Rev. Neurosci. 1993, 16, 403–443. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Ogando, A.E.; Hanke, W.; Schlue, R.; Moretti, M.; Clementi, F. Purification and characterization of an alpha-bungarotoxin receptor that forms a functional nicotinic channel. Proc. Natl. Acad. Sci. USA 1991, 88, 3258–3262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Vijayaraghavan, S.; Berg, D.K. Neuronal acetylcholine receptors that bind alpha-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron 1994, 12, 167–177. [Google Scholar] [CrossRef]
- Chang, C.C.; Chiou, L.C.; Hwang, L.L. Selective antagonism to succinylcholine-induced depolarization by α-bungarotoxin with respect to the mode of action of depolarizing agents. Br. J. Pharmacol. 1989, 98, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Acosta, C.P.; Bermudez de la Puente, M.; Salgado, C. Depuration and anatomical distribution of the amnesic shellfish poisoning (ASP) toxin domoic acid in the king scallop pecten maximus. Aquat. Toxicol. 2002, 60, 111–121. [Google Scholar] [CrossRef]
- Hampson, D.R.; Huang, X.P.; Wells, J.W.; Walter, J.A.; Wright, J.L. Interaction of domoic acid and several derivatives with kainic acid and ampa binding sites in rat brain. Eur. J. Pharmacol. 1992, 218, 1–8. [Google Scholar] [CrossRef]
- Stewart, G.R.; Zorumski, C.F.; Price, M.T.; Olney, J.W. Domoic acid: A dementia-inducing excitotoxic food poison with kainic acid receptor specificity. Exp. Neurol. 1990, 110, 127–138. [Google Scholar] [CrossRef]
- Biscoe, T.J.; Evans, R.H.; Headley, P.M.; Martin, M.; Watkins, J.C. Domoic and quisqualic acids as potent amino acid excitants of frog and rat spinal neurones. Nature 1975, 255, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Biscoe, T.J.; Evans, R.H.; Headley, P.M.; Martin, M.R.; Watkins, J.C. Structure-activity relations of excitatory amino acids on frog and rat spinal neurones. Br. J. Pharmacol. 1976, 58, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Ramsdell, J.S. Glutamate receptors and calcium entry mechanisms for domoic acid in hippocampal neurons. Neuroreport 1996, 7, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Nijjar, M.S.; Nijjar, S.S. Domoic acid-induced neurodegeneration resulting in memory loss is mediated by Ca2+ overload and inhibition of Ca2+ + calmodulin-stimulated adenylate cyclase in rat brain (review). Int. J. Mol. Med. 2000, 6, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Grimmelt, B.; Nijjar, M.S.; Brown, J.; Macnair, N.; Wagner, S.; Johnson, G.R.; Amend, J.F. Relationship between domoic acid levels in the blue mussel (mytilus edulis) and toxicity in mice. Toxicon 1990, 28, 501–508. [Google Scholar] [CrossRef]
- Sutherland, R.J.; Hoesing, J.M.; Whishaw, I.Q. Domoic acid, an environmental toxin, produces hippocampal damage and severe memory impairment. Neurosci. Lett. 1990, 120, 221–223. [Google Scholar] [CrossRef]
- Tasker, R.A.; Connell, B.J.; Strain, S.M. Pharmacology of systemically administered domoic acid in mice. Can. J. Physiol. Pharmacol. 1991, 69, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.F.; Pinsky, C.; Standish, N.M.; Bose, R.; Glavin, G.B. Parenteral domoic acid impairs spatial learning in mice. Pharmacol. Biochem. Behav. 1992, 41, 211–214. [Google Scholar] [CrossRef]
- Shinozaki, H.; Konishi, S. Actions of several anthelmintics and insecticides on rat cortical neurones. Brain Res. 1970, 24, 368–371. [Google Scholar] [CrossRef]
- Coyle, J.T. Neurotoxic action of kainic acid. J. Neurochem. 1983, 41, 1–11. [Google Scholar] [PubMed]
- Monaghan, D.T.; Bridges, R.J.; Cotman, C.W. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 1989, 29, 365–402. [Google Scholar] [CrossRef] [PubMed]
- Lerma, J.; Paternain, A.V.; Naranjo, J.R.; Mellstrom, B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 1993, 90, 11688–11692. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Cossart, R. Kainate, a double agent that generates seizures: Two decades of progress. Trends Neurosci. 2000, 23, 580–587. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: Mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 1985, 14, 375–403. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Anderson, D.K.; Horrocks, L.A. Effect of glutamate and its analogs on diacylglycerol and monoacylglycerol lipase activities of neuron-enriched cultures. Brain Res. 1993, 604, 180–184. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Yi Ong, W.; Lu, X.R.; Halliwell, B.; Horrocks, L.A. Neurochemical consequences of kainate-induced toxicity in brain: Involvement of arachidonic acid release and prevention of toxicity by phospholipase A2 inhibitors. Brain Res. Brain Res. Rev. 2001, 38, 61–78. [Google Scholar] [CrossRef]
- Kaasinen, K.; Koistinaho, J.; Alhonen, L.; Janne, J. Overexpression of spermidine/spermine n-acetyltransferase in transgenic mice protects the animals from kainate-induced toxicity. Eur. J. Neurosci. 2000, 12, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Rhee, V.; Ho, O.L. Kainic acid: A powerful neurotoxic analogue of glutamate. Brain Res. 1974, 77, 507–512. [Google Scholar] [CrossRef]
- Alexander, S.P.; Davenport, A.P.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The concise guide to pharmacology 2015/16: G protein-coupled receptors. Br. J. Pharmacol. 2015, 172, 5744–5869. [Google Scholar] [PubMed]
- Nareoja, K.; Nasman, J. Selective targeting of G-protein-coupled receptor subtypes with venom peptides. Acta Physiol. 2012, 204, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Servent, D.; Blanchet, G.; Mourier, G.; Marquer, C.; Marcon, E.; Fruchart-Gaillard, C. Muscarinic toxins. Toxicon 2011, 58, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bonner, T.I. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989, 12, 148–151. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Birdsall, N.J. International union of pharmacology. Xvii. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar] [PubMed]
- Wess, J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annual Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.P. Muscarinic receptors—Characterization, coupling and function. Pharmacol. Ther. 1993, 58, 319–379. [Google Scholar] [CrossRef]
- Eglen, R.M.; Hegde, S.S.; Watson, N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996, 48, 531–565. [Google Scholar] [PubMed]
- Nathanson, N.M. A multiplicity of muscarinic mechanisms: Enough signaling pathways to take your breath away. Proc. Natl. Acad. Sci. USA 2000, 97, 6245–6247. [Google Scholar] [CrossRef] [PubMed]
- Van Koppen, C.J.; Kaiser, B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol. Ther. 2003, 98, 197–220. [Google Scholar] [CrossRef]
- Bradley, K.N. Muscarinic toxins from the green mamba. Pharmacol. Ther. 2000, 85, 87–109. [Google Scholar] [CrossRef]
- Harvey, A.L.; Kornisiuk, E.; Bradley, K.N.; Cervenansky, C.; Duran, R.; Adrover, M.; Sanchez, G.; Jerusalinsky, D. Effects of muscarinic toxins MT1 and MT2 from green mamba on different muscarinic cholinoceptors. Neurochem. Res. 2002, 27, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.J. The amino acid sequence of protein cm-3 from dendroaspis polylepis polylepis (black mamba) venom. Int. J. Biochem. 1985, 17, 695–699. [Google Scholar] [CrossRef]
- Jerusalinsky, D.; Harvey, A.L. Toxins from mamba venoms: Small proteins with selectivities for different subtypes of muscarinic acetylcholine receptors. Trends Pharmacol. Sci. 1994, 15, 424–430. [Google Scholar] [CrossRef]
- Gilman, A.G. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987, 56, 615–649. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Offermanns, S. Mammalian g proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.G.; Gotow, L.F.; Yang, Z.; Holmes, R.K. A mutational analysis of residues in cholera toxin a1 necessary for interaction with its substrate, the stimulatory g protein gsalpha. Toxins 2015, 7, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Katada, T. The inhibitory g protein G(i) identified as pertussis toxin-catalyzed ADP-ribosylation. Biol. Pharm. Bull. 2012, 35, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Bharati, K.; Ganguly, N.K. Cholera toxin: A paradigm of a multifunctional protein. Indian J. Med. Res. 2011, 133, 179–187. [Google Scholar] [PubMed]
- Zhang, R.G.; Scott, D.L.; Westbrook, M.L.; Nance, S.; Spangler, B.D.; Shipley, G.G.; Westbrook, E.M. The three-dimensional crystal structure of cholera toxin. J. Mol. Biol. 1995, 251, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.W. Action of cholera toxin on fluid and electrolyte movement in the small intestine. Annu. Rev. Med. 1973, 24, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Montecucco, C. Guidebook to Protein Toxins and Their Use in Cell Biology; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Sanchez, J.; Holmgren, J. Cholera toxin—A foe & a friend. Indian J. Med. Res. 2011, 133, 153–163. [Google Scholar] [PubMed]
- De Haan, L.; Hirst, T.R. Cholera toxin: A paradigm for multi-functional engagement of cellular mechanisms (review). Mol. Membr. Biol. 2004, 21, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Vanden Broeck, D.; Horvath, C.; De Wolf, M.J. Vibrio cholerae: Cholera toxin. Int. J. Biochem. Cell Biol. 2007, 39, 1771–1775. [Google Scholar] [CrossRef]
- Aktories, K. Adp-Ribosylating Toxins; Springer-Verlag: Berlin, Germany, 1992. [Google Scholar]
- Moss, J.; Vaughan, M. Activation of cholera toxin and escherichia coli heat-labile enterotoxins by adp-ribosylation factors, a family of 20 kda guanine nucleotide-binding proteins. Mol. Microbiol. 1991, 5, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Dragunsky, E.M.; Rivera, E.; Aaronson, W.; Dolgaya, T.M.; Hochstein, H.D.; Habig, W.H.; Levenbook, I.S. Experimental evaluation of antitoxic protective effect of new cholera vaccines in mice. Vaccine 1992, 10, 735–736. [Google Scholar] [CrossRef]
- Patton, W.A. Cholera toxin and adp-ribosylation factors. In Toxins and Signal Transduction; Lazarovici, F., Gutman, Y., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; Volume 1, pp. 234–271. [Google Scholar]
- Stein, P.E.; Boodhoo, A.; Armstrong, G.D.; Cockle, S.A.; Klein, M.H.; Read, R.J. The crystal structure of pertussis toxin. Structure 1994, 2, 45–57. [Google Scholar] [CrossRef]
- Locht, C.; Antoine, R. A proposed mechanism of adp-ribosylation catalyzed by the pertussis toxin s1 subunit. Biochimie 1995, 77, 333–340. [Google Scholar] [CrossRef]
- Merritt, E.A.; Hol, W.G. AB5 toxins. Curr. Opin. Struct. Biol. 1995, 5, 165–171. [Google Scholar] [CrossRef]
- Wong, W.S.; Rosoff, P.M. Pharmacology of pertussis toxin B-oligomer. Can. J. Physiol. Pharmacol. 1996, 74, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.L.; Hausman, S.Z.; Lindner, W.; Robey, F.A.; Manclark, C.R. Structural characterization of pertussis toxin a subunit. J. Biol. Chem. 1987, 262, 17677–17682. [Google Scholar] [PubMed]
- Del Giudice, G.; Rappuoli, R. Genetically derived toxoids for use as vaccines and adjuvants. Vaccine 1999, 17, S44–S52. [Google Scholar] [CrossRef]
- Rappuoli, R.; Montecucco, C. Guidebook to Protein Toxins and Their Use in Cell Biology; Sambrook and Tooze Publication at Oxford Universuty Press; Oxford University Press Inc.: New York, NY, USA, 1997; pp. 34–35. [Google Scholar]
- Reisine, T. Pertussis toxin in the analysis of receptor mechanisms. Biochem. Pharmacol. 1990, 39, 1499–1504. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]
- Sunahara, R.K.; Taussig, R. Isoforms of mammalian adenylyl cyclase: Multiplicities of signaling. Mol. Interv. 2002, 2, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.S.; Zhang, P.; Steichen, J.M.; Keshwani, M.M.; Kornev, A.P. Pka: Lessons learned after twenty years. Biochim. Biophys. Acta 2013, 1834, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Guo, Q. The adenylyl cyclase activity of anthrax edema factor. Mol. Asp. Med. 2009, 30, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Paccani, S.R.; Tonello, F.; Ghittoni, R.; Natale, M.; Muraro, L.; D'Elios, M.M.; Tang, W.J.; Montecucco, C.; Baldari, C.T. Anthrax toxins suppress t lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 2005, 201, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Baldari, C.T.; Tonello, F.; Paccani, S.R.; Montecucco, C. Anthrax toxins: A paradigm of bacterial immune suppression. Trends Immunol. 2006, 27, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A.; Collier, R.J. Anthrax toxin: Receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 2007, 76, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Alisaraie, L.; Rouiller, I. Molecular assembly of lethal factor enzyme and pre-pore heptameric protective antigen in early stage of translocation. J. Mol. Model. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Mourez, M.; Lacy, D.B.; Cunningham, K.; Legmann, R.; Sellman, B.R.; Mogridge, J.; Collier, R.J. 2001: A year of major advances in anthrax toxin research. Trends Microbiol. 2002, 10, 287–293. [Google Scholar] [CrossRef]
- Molloy, S.S.; Bresnahan, P.A.; Leppla, S.H.; Klimpel, K.R.; Thomas, G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 1992, 267, 16396–16402. [Google Scholar] [PubMed]
- Panchal, R.G.; Halverson, K.M.; Ribot, W.; Lane, D.; Kenny, T.; Abshire, T.G.; Ezzell, J.W.; Hoover, T.A.; Powell, B.; Little, S.; et al. Purified bacillus anthracis lethal toxin complex formed in vitro and during infection exhibits functional and biological activity. J. Biol. Chem. 2005, 280, 10834–10839. [Google Scholar] [CrossRef] [PubMed]
- Krantz, B.A.; Melnyk, R.A.; Zhang, S.; Juris, S.J.; Lacy, D.B.; Wu, Z.; Finkelstein, A.; Collier, R.J. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science 2005, 309, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Bodart, J.F.; Chopra, A.; Liang, X.; Duesbery, N. Anthrax, mek and cancer. Cell Cycle 2002, 1, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.P.; Boone, S.A.; Liang, X.; Duesbery, N.S. Anthrax lethal factor proteolysis and inactivation of mapk kinase. J. Biol. Chem. 2003, 278, 9402–9406. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhukovskaya, N.L.; Guo, Q.; Florian, J.; Tang, W.J. Calcium-independent calmodulin binding and two-metal-ion catalytic mechanism of anthrax edema factor. EMBO J. 2005, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, F.; Tonello, F.; Ladant, D.; Zornetta, I.; Zamparo, I.; Di Benedetto, G.; Zaccolo, M.; Montecucco, C. Cell entry and camp imaging of anthrax edema toxin. EMBO J. 2006, 25, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Bernardi, L.; Napolitani, G.; Mock, M.; Montecucco, C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 2000, 352, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Ezzell, J.W.; Ivins, B.E.; Leppla, S.H. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of bacillus anthracis toxin. Infect. Immunity 1984, 45, 761–767. [Google Scholar]
- Nagendra, S.; Vanlalhmuaka; Verma, S.; Tuteja, U.; Thavachelvam, K. Recombinant expression of bacillus anthracis lethal toxin components of indian isolate in escherichia coli and determination of its acute toxicity level in mouse model. Toxicon 2015, 108, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Leysath, C.E.; Phillips, D.D.; Crown, D.; Fattah, R.J.; Moayeri, M.; Leppla, S.H. Anthrax edema factor toxicity is strongly mediated by the n-end rule. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Abi-Habib, R.J.; Singh, R.; Leppla, S.H.; Greene, J.J.; Ding, Y.; Berghuis, B.; Duesbery, N.S.; Frankel, A.E. Systemic anthrax lethal toxin therapy produces regressions of subcutaneous human melanoma tumors in athymic nude mice. Clin. Cancer Res. 2006, 12, 7437–7443. [Google Scholar] [CrossRef] [PubMed]
- Duesbery, N.S.; Resau, J.; Webb, C.P.; Koochekpour, S.; Koo, H.M.; Leppla, S.H.; Vande Woude, G.F. Suppression of ras-mediated transformation and inhibition of tumor growth and angiogenesis by anthrax lethal factor, a proteolytic inhibitor of multiple mek pathways. Proc. Natl. Acad. Sci. USA 2001, 98, 4089–4094. [Google Scholar] [CrossRef] [PubMed]
- McEvers, K.; Elrefaei, M.; Norris, P.; Deeks, S.; Martin, J.; Lu, Y.; Cao, H. Modified anthrax fusion proteins deliver hiv antigens through mhc class I and II pathways. Vaccine 2005, 23, 4128–4135. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Rabideau, A.E.; Pentelute, B.L. Delivery of antibody mimics into mammalian cells via anthrax toxin protective antigen. Chembiochem 2014, 15, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Cella, L.N.; Sanchez, P.; Zhong, W.; Myung, N.V.; Chen, W.; Mulchandani, A. Nano aptasensor for protective antigen toxin of anthrax. Anal. Chem. 2010, 82, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Duverger, A.; Carre, J.M.; Jee, J.; Leppla, S.H.; Cormet-Boyaka, E.; Tang, W.J.; Tome, D.; Boyaka, P.N. Contributions of edema factor and protective antigen to the induction of protective immunity by bacillus anthracis edema toxin as an intranasal adjuvant. J. Immunol. 2010, 185, 5943–5952. [Google Scholar] [CrossRef] [PubMed]
- Vrentas, C.E.; Moayeri, M.; Keefer, A.B.; Greaney, A.J.; Tremblay, J.; O’Mard, D.; Leppla, S.H.; Shoemaker, C.B. A diverse set of single-domain antibodies (VHHs) against the anthrax toxin lethal and edema factors provides a basis for construction of a bispecific agent that protects against anthrax infection. J. Biol. Chem. 2016, 291, 21596–21606. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Follo, M.Y.; Cocco, L.; Suh, P.G. The physiological roles of primary phospholipase C. Adv. Biol. Regul. 2013, 53, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Katan, M. Families of phosphoinositide-specific phospholipase c: Structure and function. Biochim. Biophys. Acta 1998, 1436, 5–17. [Google Scholar] [CrossRef]
- Patterson, R.L.; van Rossum, D.B.; Nikolaidis, N.; Gill, D.L.; Snyder, S.H. Phospholipase c-gamma: Diverse roles in receptor-mediated calcium signaling. Trends Biochem. Sci. 2005, 30, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.H.; Ryan, M. Bacterial phosphatidylinositol-specific phospholipase C: Structure, function, and interaction with lipids. Biochim. Biophys. Acta 1999, 1441, 237–254. [Google Scholar] [CrossRef]
- Goldstein, R.; Cheng, J.; Stec, B.; Roberts, M.F. Structure of the S. Aureus PI-specific phospholipase c reveals modulation of active site access by a titratable PI-cation latched loop. Biochemistry 2012, 51, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.; Low, M.G. Cloning, expression, and mutagenesis of phosphatidylinositol-specific phospholipase c from staphylococcus aureus: A potential staphylococcal virulence factor. Infect. Immunity 1993, 61, 5078–5089. [Google Scholar]
- Hondal, R.J.; Zhao, Z.; Kravchuk, A.V.; Liao, H.; Riddle, S.R.; Yue, X.; Bruzik, K.S.; Tsai, M.D. Mechanism of phosphatidylinositol-specific phospholipase C: A unified view of the mechanism of catalysis. Biochemistry 1998, 37, 4568–4580. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.X.; Sanchez-Magraner, L.; Alonso, A.; Goni, F.M. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 2010, 584, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.B.; Weller, P.F.; Parsonnet, J.; Ransil, B.J.; Nicholson-Weller, A. Phosphatidylinositol-specific phospholipase C, a possible virulence factor of staphylococcus aureus. J. Clin. Microbiol. 1989, 27, 2451–2454. [Google Scholar] [PubMed]
- Zhou, C.; Qian, X.; Roberts, M.F. Allosteric activation of phosphatidylinositol-specific phospholipase C: Specific phospholipid binding anchors the enzyme to the interface. Biochemistry 1997, 36, 10089–10097. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Lambright, D.G. Invited review: Small gtpases and their gaps. Biopolymers 2016, 105, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Gysin, S.; Salt, M.; Young, A.; McCormick, F. Therapeutic strategies for targeting ras proteins. Genes Cancer 2011, 2, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Just, I.; Rohrbeck, A.; Huelsenbeck, S.C.; Hoeltje, M. Therapeutic effects of clostridium botulinum C3 exoenzyme. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K. Bacterial protein toxins that modify host regulatory gtpases. Nat. Rev. Microbiol. 2011, 9, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R. Bacterial factors exploit eukaryotic rho gtpase signaling cascades to promote invasion and proliferation within their host. Small Gtpases 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Visvikis, O.; Maddugoda, M.P.; Lemichez, E. Direct modifications of rho proteins: Deconstructing gtpase regulation. Biol. Cell 2010, 102, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.L.; Rucks, E.A.; Vincent, D.M.; Olson, J.C. Examination of the coordinate effects of pseudomonas aeruginosa exos on rac1. Infect. Immunity 2005, 73, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Rosener, S.; Blaschke, U.; Chhatwal, G.S. Botulinum adp-ribosyltransferase C3. Purification of the enzyme and characterization of the adp-ribosylation reaction in platelet membranes. Eur. J. Biochem. 1988, 172, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Arvai, A.S.; Clancy, S.B.; Tainer, J.A. Crystal structure and novel recognition motif of rho adp-ribosylating C3 exoenzyme from clostridium botulinum: Structural insights for recognition specificity and catalysis. J. Mol. Biol. 2001, 305, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Vogelsgesang, M.; Pautsch, A.; Aktories, K. C3 exoenzymes, novel insights into structure and action of rho-adp-ribosylating toxins. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 374, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Sehr, P.; Joseph, G.; Genth, H.; Just, I.; Pick, E.; Aktories, K. Glucosylation and adp ribosylation of rho proteins: Effects on nucleotide binding, gtpase activity, and effector coupling. Biochemistry 1998, 37, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Rohrbeck, A.; von Elsner, L.; Hagemann, S.; Just, I. Binding of clostridium botulinum C3 exoenzyme to intact cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, W.; Just, I.; Muller, H.; Hellwig, A.; Traub, P.; Aktories, K. Alteration of the cytoskeleton of mammalian cells cultured in vitro by clostridium botulinum C2 toxin and C3 adp-ribosyltransferase. Eur. J. Cell Biol. 1991, 54, 237–245. [Google Scholar] [PubMed]
- Aktories, K.; Schmidt, G.; Just, I. Rho gtpases as targets of bacterial protein toxins. Biol. Chem. 2000, 381, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Chardin, P.; Boquet, P.; Madaule, P.; Popoff, M.R.; Rubin, E.J.; Gill, D.M. The mammalian g protein rhoc is adp-ribosylated by clostridium botulinum exoenzyme C3 and affects actin microfilaments in vero cells. EMBO J. 1989, 8, 1087–1092. [Google Scholar] [PubMed]
- Dillon, S.T.; Feig, L.A. Purification and assay of recombinant C3 transferase. Methods Enzymol. 1995, 256, 174–184. [Google Scholar] [PubMed]
- Aktories, K.; Just, I. Clostridial rho-inhibiting protein toxins. Curr. Top. Microbiol. Immunol. 2005, 291, 113–145. [Google Scholar] [PubMed]
- Paterson, H.F.; Self, A.J.; Garrett, M.D.; Just, I.; Aktories, K.; Hall, A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J. Cell Biol. 1990, 111, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Aullo, P.; Giry, M.; Olsnes, S.; Popoff, M.R.; Kocks, C.; Boquet, P. A chimeric toxin to study the role of the 21 kda gtp binding protein rho in the control of actin microfilament assembly. EMBO J. 1993, 12, 921–931. [Google Scholar] [PubMed]
- Huelsenbeck, J.; Dreger, S.C.; Gerhard, R.; Fritz, G.; Just, I.; Genth, H. Upregulation of the immediate early gene product rhob by exoenzyme C3 from clostridium limosum and toxin B from clostridium difficile. Biochemistry 2007, 46, 4923–4931. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Schlessinger, J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef]
- Lazarovici, P.; Chan, K.F. Staphylococcus aureus α-toxin. 2. Reduction of epidermal growth factor receptor affinity in PC12 cells. Toxicon 1987, 25, 637–647. [Google Scholar] [CrossRef]
- Vandana, S.; Navneet, S.; Surinder, K.; Krishnasastry, M.V. Modulation of EGF receptor autophosphorylation by α-hemolysin of staphylococcus aureus via protein tyrosine phosphatase. FEBS Lett. 2003, 535, 71–76. [Google Scholar] [CrossRef]
- Fink, D.; Contreras, M.L.; Lelkes, P.I.; Lazarovici, P. Staphylococcus aureus alpha-toxin activates phospholipases and induces a Ca2+ influx in PC12 cells. Cell. Signal. 1989, 1, 387–393. [Google Scholar] [CrossRef]
- Raya, S.A.; Trembovler, V.; Shohami, E.; Lazarovici, P. Cytolysins increase intracellular calcium and induce eicosanoids release by pheochromocytoma PC12 cell cultures. Nat. Toxins 1993, 1, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Haugwitz, U.; Bobkiewicz, W.; Han, S.R.; Beckmann, E.; Veerachato, G.; Shaid, S.; Biehl, S.; Dersch, K.; Bhakdi, S.; Husmann, M. Pore-forming staphylococcus aureus alpha-toxin triggers epidermal growth factor receptor-dependent proliferation. Cell. Microbiol. 2006, 8, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C. Functional characteristic of snake venom disintegrins: Potential therapeutic implication. Curr. Pharm. Des. 2005, 11, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Marcinkiewicz, C.; Monleon, D.; Esteve, V.; Celda, B.; Juarez, P.; Sanz, L. Snake venom disintegrins: Evolution of structure and function. Toxicon 2005, 45, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C. Applications of snake venom components to modulate integrin activities in cell-matrix interactions. Int. J. Biochem. Cell Biol. 2013, 45, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Moreno-Murciano, M.P.; Theakston, R.D.; Kisiel, D.G.; Marcinkiewicz, C. Snake venom disintegrins: Novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem. J. 2003, 372, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Moreno-Murciano, M.P.; Sanz, L.; Jurgens, M.; Schrader, M.; Raida, M.; Benjamin, D.C.; Fox, J.W. The disulfide bond pattern of catrocollastatin C, a disintegrin-like/cysteine-rich protein isolated from crotalus atrox venom. Protein Sci. 2000, 9, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Momic, T.; Arlinghaus, F.T.; Arien-Zakay, H.; Katzhendler, J.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Pharmacological aspects of vipera xantina palestinae venom. Toxins 2011, 3, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C.; Calvete, J.J.; Marcinkiewicz, M.M.; Raida, M.; Vijay-Kumar, S.; Huang, Z.; Lobb, R.R.; Niewiarowski, S. EC3, a novel heterodimeric disintegrin from echis carinatus venom, inhibits α4 and α5 integrins in an rgd-independent manner. J. Biol. Chem. 1999, 274, 12468–12473. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, C.; Calvete, J.J.; Vijay-Kumar, S.; Marcinkiewicz, M.M.; Raida, M.; Schick, P.; Lobb, R.R.; Niewiarowski, S. Structural and functional characterization of EMF10, a heterodimeric disintegrin from eristocophis macmahoni venom that selectively inhibits α5β1 integrin. Biochemistry 1999, 38, 13302–13309. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Fox, J.W.; Agelan, A.; Niewiarowski, S.; Marcinkiewicz, C. The presence of the WGD motif in CC8 heterodimeric disintegrin increases its inhibitory effect on αIIbβ3, αvβ3, and α5β1 integrins. Biochemistry 2002, 41, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. Structures and functions of snake venom clps (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon 2005, 45, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Lu, Q.; Clemetson, J.M. Snake C-type lectin-like proteins and platelet receptors. Pathophysiol. Haemost. Thromb. 2005, 34, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon 2010, 56, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Navdaev, A.; Clemetson, J.M.; Clemetson, K.J. Snake venom c-type lectins interacting with platelet receptors. Structure-function relationships and effects on haemostasis. Toxicon 2005, 45, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Arruda Macedo, J.K.; Fox, J.W.; de Souza Castro, M. Disintegrins from snake venoms and their applications in cancer research and therapy. Curr. Protein Peptide Sci. 2015, 16, 532–548. [Google Scholar] [CrossRef]
- Venkatesh, S.; Workman, J.L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harbor Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Essigmann, J.M.; Croy, R.G.; Nadzan, A.M.; Busby, W.F., Jr.; Reinhold, V.N.; Buchi, G.; Wogan, G.N. Structural identification of the major DNA adduct formed by aflatoxin b1 in vitro. Proc. Natl. Acad. Sci. USA 1977, 74, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [Google Scholar] [PubMed]
- Thelestam, M.; Frisan, T. Cytolethal distending toxins. Rev. Physiol. Biochem. Pharmacol. 2004, 152, 111–133. [Google Scholar] [PubMed]
- De Rycke, J.; Oswald, E. Cytolethal distending toxin (CDT): A bacterial weapon to control host cell proliferation? FEMS Microbiol. Lett. 2001, 203, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.S. Mycotoxins, general view, chemistry and structure. Toxicol. Lett. 1995, 82–83, 843–851. [Google Scholar] [CrossRef]
- Johnson, W.W.; Guengerich, F.P. Reaction of aflatoxin b1 exo-8,9-epoxide with DNA: Kinetic analysis of covalent binding and DNA-induced hydrolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 6121–6125. [Google Scholar] [CrossRef]
- Bedard, L.L.; Massey, T.E. Aflatoxin b1-induced DNA damage and its repair. Cancer Lett. 2006, 241, 174–183. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.D.; Haseltine, W.A. Modification of DNA by aflatoxin B1 creates alkali-labile lesions in DNA at positions of guanine and adenine. Proc. Natl. Acad. Sci. USA 1978, 75, 4120–4124. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.L.; Huang, J.X.; Bender, W.; Wu, Z.R.; Chang, J.C. Evidence for the covalent binding of aflatoxin b1-dichloride to cytosine in DNA. Carcinogenesis 1991, 12, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Groopman, J.D. DNA damage by mycotoxins. Mutat. Res. 1999, 424, 167–181. [Google Scholar] [CrossRef]
- Hsu, I.C.; Metcalf, R.A.; Sun, T.; Welsh, J.A.; Wang, N.J.; Harris, C.C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 1991, 350, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, Y.; He, L.; Wang, N.J.; Gu, J.R. Aberrations of p53 gene in human hepatocellular carcinoma from china. Carcinogenesis 1993, 14, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M. P53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet 1991, 338, 1356–1359. [Google Scholar] [PubMed]
- Coulombe, R.A., Jr. Biological action of mycotoxins. J. Dairy Sci. 1993, 76, 880–891. [Google Scholar] [CrossRef]
- McKean, C.; Tang, L.; Billam, M.; Tang, M.; Theodorakis, C.W.; Kendall, R.J.; Wang, J.S. Comparative acute and combinative toxicity of aflatoxin b1 and t-2 toxin in animals and immortalized human cell lines. J. Appl. Toxicol. 2006, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Netke, S.P.; Roomi, M.W.; Tsao, C.; Niedzwiecki, A. Ascorbic acid protects guinea pigs from acute aflatoxin toxicity. Toxicol. Appl. Pharmacol. 1997, 143, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Kobayashi, M.; Nagashima, K.; Wakisaka, Y.; Koizumi, K. A new antifungal antibiotic, trichostatin. J. Antibiot. 1976, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Schreiber, S.L. Deacetylase enzymes: Biological functions and the use of small-molecule inhibitors. Chem. Biol. 2002, 9, 3–16. [Google Scholar] [CrossRef]
- Marks, P.A.; Richon, V.M.; Rifkind, R.A. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 2000, 92, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Finnin, M.S.; Donigian, J.R.; Cohen, A.; Richon, V.M.; Rifkind, R.A.; Marks, P.A.; Breslow, R.; Pavletich, N.P. Structures of a histone deacetylase homologue bound to the tsa and saha inhibitors. Nature 1999, 401, 188–193. [Google Scholar] [PubMed]
- Furumai, R.; Komatsu, Y.; Nishino, N.; Khochbin, S.; Yoshida, M.; Horinouchi, S. Potent histone deacetylase inhibitors built from trichostatin a and cyclic tetrapeptide antibiotics including trapoxin. Proc. Natl. Acad. Sci. USA 2001, 98, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Rifkind, R.A.; Richon, V.M.; Breslow, R.; Miller, T.; Kelly, W.K. Histone deacetylases and cancer: Causes and therapies. Nat. Rev. Cancer 2001, 1, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002, 1, 287–299. [Google Scholar] [CrossRef] [PubMed]
- West, A.E.; Griffith, E.C.; Greenberg, M.E. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 2002, 3, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef] [PubMed]
- Boutillier, A.L.; Trinh, E.; Loeffler, J.P. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J. Neurochem. 2003, 84, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Nervi, C.; Borello, U.; Fazi, F.; Buffa, V.; Pelicci, P.G.; Cossu, G. Inhibition of histone deacetylase activity by trichostatin a modulates gene expression during mouse embryogenesis without apparent toxicity. Cancer Res. 2001, 61, 1247–1249. [Google Scholar] [PubMed]
- Hassig, C.A.; Schreiber, S.L. Nuclear histone acetylases and deacetylases and transcriptional regulation: Hats off to hdacs. Curr. Opin. Chem. Biol. 1997, 1, 300–308. [Google Scholar] [CrossRef]
- Taunton, J.; Hassig, C.A.; Schreiber, S.L. A mammalian histone deacetylase related to the yeast transcriptional regulator RPD3P. Science 1996, 272, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A.; et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Doherty, J.J.; Dingledine, R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J. Neurosci. 2002, 22, 8422–8428. [Google Scholar] [PubMed]
- Hockly, E.; Richon, V.M.; Woodman, B.; Smith, D.L.; Zhou, X.; Rosa, E.; Sathasivam, K.; Ghazi-Noori, S.; Mahal, A.; Lowden, P.A.; et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.P. Mitochondrial electron-transport inhibitors. Methods Enzymol. 1979, 55, 454–462. [Google Scholar] [PubMed]
- Koopman, W.J.; Nijtmans, L.G.; Dieteren, C.E.; Roestenberg, P.; Valsecchi, F.; Smeitink, J.A.; Willems, P.H. Mammalian mitochondrial complex I: Biogenesis, regulation, and reactive oxygen species generation. Antioxid. Redox Signal. 2010, 12, 1431–1470. [Google Scholar] [CrossRef] [PubMed]
- Ling, N. Rotenone—A Review of Its Toxicity and Use for Fisheries Management; Department of Conservation: Wellington, New Zealand, 2003. [Google Scholar]
- Bove, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRx 2005, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Klinge, S.; Voigts-Hoffmann, F.; Leibundgut, M.; Ban, N. Atomic structures of the eukaryotic ribosome. Trends Biochem. Sci. 2012, 37, 189–198. [Google Scholar] [CrossRef]
- Kaul, G.; Pattan, G.; Rafeequi, T. Eukaryotic elongation factor-2 (eEF2): Its regulation and peptide chain elongation. Cell Biochem. Funct. 2011, 29, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Barbieri, J.T. Molecular mechanisms of the cytotoxicity of adp-ribosylating toxins. Annu. Rev. Microbiol. 2008, 62, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.J.; Dodd, J.E.; Hautbergue, G.M. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Menestrina, G.; Schiavo, G.; Montecucco, C. Molecular mechanisms of action of bacterial protein toxins. Mol. Asp. Med. 1994, 15, 79–193. [Google Scholar] [CrossRef]
- Choe, S.; Bennett, M.J.; Fujii, G.; Curmi, P.M.; Kantardjieff, K.A.; Collier, R.J.; Eisenberg, D. The crystal structure of diphtheria toxin. Nature 1992, 357, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Shapira, A.; Benhar, I. Toxin-based therapeutic approaches. Toxins 2010, 2, 2519–2583. [Google Scholar] [CrossRef] [PubMed]
- Falnes, P.O.; Sandvig, K. Penetration of protein toxins into cells. Curr. Opin. Cell Biol. 2000, 12, 407–413. [Google Scholar] [CrossRef]
- Chiron, M.F.; Fryling, C.M.; FitzGerald, D.J. Cleavage of pseudomonas exotoxin and diphtheria toxin by a furin-like enzyme prepared from beef liver. J. Biol. Chem. 1994, 269, 18167–18176. [Google Scholar] [PubMed]
- Tsuneoka, M.; Nakayama, K.; Hatsuzawa, K.; Komada, M.; Kitamura, N.; Mekada, E. Evidence for involvement of furin in cleavage and activation of diphtheria toxin. J. Biol. Chem. 1993, 268, 26461–26465. [Google Scholar] [PubMed]
- Papini, E.; Rappuoli, R.; Murgia, M.; Montecucco, C. Cell penetration of diphtheria toxin. Reduction of the interchain disulfide bridge is the rate-limiting step of translocation in the cytosol. J. Biol. Chem. 1993, 268, 1567–1574. [Google Scholar] [PubMed]
- Donovan, J.J.; Simon, M.I.; Draper, R.K.; Montal, M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc. Natl. Acad. Sci. USA 1981, 78, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Bomsel, M.; Devilliers, G.; vanderSpek, J.; Murphy, J.R.; Lukianov, E.V.; Olsnes, S.; Boquet, P. Membrane translocation of diphtheria toxin fragment a exploits early to late endosome trafficking machinery. Mol. Microbiol. 1997, 23, 445–457. [Google Scholar] [CrossRef]
- Ratts, R.; Zeng, H.; Berg, E.A.; Blue, C.; McComb, M.E.; Costello, C.E.; vanderSpek, J.C.; Murphy, J.R. The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J. Cell Biol. 2003, 160, 1139–1150. [Google Scholar] [CrossRef]
- Aniento, F.; Gu, F.; Parton, R.G.; Gruenberg, J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J. Cell Biol. 1996, 133, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, H.; Bonavida, B. Diphtheria toxin- and pseudomonas a toxin-mediated apoptosis. Adp ribosylation of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-alpha. J. Immunol. 1992, 149, 2089–2094. [Google Scholar] [PubMed]
- Thorburn, A.; Thorburn, J.; Frankel, A.E. Induction of apoptosis by tumor cell-targeted toxins. Apoptosis 2004, 9, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yamaizumi, M.; Mekada, E.; Uchida, T.; Okada, Y. One molecule of diphtheria toxin fragment a introduced into a cell can kill the cell. Cell 1978, 15, 245–250. [Google Scholar] [CrossRef]
- Pappenheimer, A.M., Jr. The diphtheria bacillus and its toxin: A model system. J. Hyg. 1984, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.H.; Fayad, L.; McLaughlin, P.; Romaguara, J.E.; Hagemeister, F.; Goy, A.; Neelapu, S.; Samaniego, F.; Walker, P.L.; Wang, M.; et al. Phase ii trial of the combination of denileukin diftitox and rituximab for relapsed/refractory B-cell non-hodgkin lymphoma. Br. J. Haematol. 2007, 138, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Gutierrez, E.; Muglia, J.; McDonald, C.J.; Guzzo, C.; Gottlieb, A.; Pappert, A.; Garland, W.T.; Bagel, J.; Bacha, P. A multicenter dose-escalation trial with denileukin diftitox (ONTAK, DAB(389)IL-2) in patients with severe psoriasis. J. Am. Acad. Dermatol. 2001, 45, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, P.J.; Bachier, C.; Grimley, M.; Freytes, C.O.; Callander, N.S.; Essell, J.H.; Flomenberg, N.; Selby, G.; Lemaistre, C.F. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2005, 11, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Urieto, J.O.; Liu, T.; Black, J.H.; Cohen, K.A.; Hall, P.D.; Willingham, M.C.; Pennell, L.K.; Hogge, D.E.; Kreitman, R.J.; Frankel, A.E. Expression and purification of the recombinant diphtheria fusion toxin dt388il3 for phase i clinical trials. Protein Expr. Purif. 2004, 33, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Machado, J.D.; Betancor, G.; Camacho, M. Pharmacological regulation of the late steps of exocytosis. Ann. N. Y. Acad. Sci. 2002, 971, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. The molecular machinery of neurotransmitter release (nobel lecture). Angew. Chem. 2014, 53, 12696–12717. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Martin-Urdiroz, M.; Deeks, M.J.; Horton, C.G.; Dawe, H.R.; Jourdain, I. The exocyst complex in health and disease. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankratov, Y.; Lalo, U.; Verkhratsky, A.; North, R.A. Vesicular release of atp at central synapses. Pflugers Arch. 2006, 452, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; de Bernard, M.; Pellizzari, R.; Vitale, G.; Caccin, P.; Schiavo, G.; Montecucco, C. Bacterial toxins with intracellular protease activity. Clin. Chim. Acta Int. J. Clin. Chem. 2000, 291, 189–199. [Google Scholar] [CrossRef]
- Dolly, J.O.; Aoki, K.R. The structure and mode of action of different botulinum toxins. Eur. J. Neurol. 2006, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.L.; Latham, C.F.; Wen, P.J.; Cavaignac, S.; Fanning, J.; Foran, P.G.; Meunier, F.A. The janus faces of botulinum neurotoxin: Sensational medicine and deadly biological weapon. J. Neurosci. Res. 2007, 85, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, R.; Rossetto, O.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: Mechanism of action and therapeutic uses. Philos. Trans. R. Soc.Lond. Ser. B Biol. Sci. 1999, 354, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Turton, K.; Chaddock, J.A.; Acharya, K.R. Botulinum and tetanus neurotoxins: Structure, function and therapeutic utility. Trends Biochem. Sci. 2002, 27, 552–558. [Google Scholar] [CrossRef]
- Rummel, A. The long journey of botulinum neurotoxins into the synapse. Toxicon 2015, 107, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.R.; Guyer, B. Botulinum toxin type a and other botulinum toxin serotypes: A comparative review of biochemical and pharmacological actions. Eur. J. Neurol. 2001, 8, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Adrion, C.B.; Keller, J.E. Comparison of extracellular and intracellular potency of botulinum neurotoxins. Infect. Immunity 2006, 74, 5617–5624. [Google Scholar] [CrossRef] [PubMed]
- Grumelli, C.; Verderio, C.; Pozzi, D.; Rossetto, O.; Montecucco, C.; Matteoli, M. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology 2005, 26, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Zanetti, G.; Megighian, A.; Scorzeto, M.; Fillo, S.; Shone, C.C.; Binz, T.; Rossetto, O.; Lista, F.; et al. Thioredoxin and its reductase are present on synaptic vesicles, and their inhibition prevents the paralysis induced by botulinum neurotoxins. Cell Rep. 2014, 8, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Tonello, F.; Schiavo, G.; Montecucco, C. Metal substitution of tetanus neurotoxin. Biochem. J. 1997, 322, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Whitemarsh, R.C.; Tepp, W.H.; Bradshaw, M.; Lin, G.; Pier, C.L.; Scherf, J.M.; Johnson, E.A.; Pellett, S. Characterization of botulinum neurotoxin a subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect. Immunity 2013, 81, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J. Dermatol. 2010, 55, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Luvisetto, S.; Rossetto, O.; Montecucco, C.; Pavone, F. Toxicity of botulinum neurotoxins in central nervous system of mice. Toxicon 2003, 41, 475–481. [Google Scholar] [CrossRef]
- Walker, T.J.; Dayan, S.H. Comparison and overview of currently available neurotoxins. J. Clin. Aesthet. Dermatol. 2014, 7, 31–39. [Google Scholar] [PubMed]
- Fujita, K.; Guroff, G.; Yavin, E.; Goping, G.; Orenberg, R.; Lazarovici, P. Preparation of affinity-purified, biotinylated tetanus toxin, and characterization and localization of cell surface binding sites on nerve growth factor-treated PC12 cells. Neurochem. Res. 1990, 15, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yavin, E.; Yavin, Z.; Habig, W.H.; Hardegree, M.C.; Kohn, L.D. Tetanus toxin association with developing neuronal cell cultures. Kinetic parameters and evidence for ganglioside-mediated internalization. J. Biol. Chem. 1981, 256, 7014–7022. [Google Scholar] [PubMed]
- Yavin, Z.; Yavin, E.; Kohn, L.D. Sequestration of tetanus toxin in developing neuronal cell cultures. J. Neurosci. Res. 1982, 7, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Leka, O.; Zanetti, G.; Rossetto, O.; Montecucco, C. On the translocation of botulinum and tetanus neurotoxins across the membrane of acidic intracellular compartments. Biochim. Biophys. Acta 2016, 1858, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Lazarovici, P.; Fujita, K.; Contreras, M.L.; DiOrio, J.P.; Lelkes, P.I. Affinity purified tetanus toxin binds to isolated chromaffin granules and inhibits catecholamine release in digitonin-permeabilized chromaffin cells. FEBS Lett. 1989, 253, 121–128. [Google Scholar] [CrossRef]
- Fischer, A.; Montal, M. Molecular dissection of botulinum neurotoxin reveals interdomain chaperone function. Toxicon 2013, 75, 101–107. [Google Scholar] [CrossRef]
- Bercsenyi, K.; Schmieg, N.; Bryson, J.B.; Wallace, M.; Caccin, P.; Golding, M.; Zanotti, G.; Greensmith, L.; Nischt, R.; Schiavo, G. Tetanus toxin entry. Nidogens are therapeutic targets for the prevention of tetanus. Science 2014, 346, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Ulrich, W.P.; Wetterich, F.; Weller, U.; Galla, H.J. Gangliosides in phospholipid bilayer membranes: Interaction with tetanus toxin. Chem. Phys. Lipids 1996, 81, 21–34. [Google Scholar] [CrossRef]
- Yavin, E.; Nathan, A. Tetanus toxin receptors on nerve cells contain a trypsin-sensitive component. Eur. J. Biochem. 1986, 154, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Binz, T.; Rummel, A. Cell entry strategy of clostridial neurotoxins. J. Neurochem. 2009, 109, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Caleo, M.; Schiavo, G. Central effects of tetanus and botulinum neurotoxins. Toxicon 2009, 54, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Bercsenyi, K.; Giribaldi, F.; Schiavo, G. The elusive compass of clostridial neurotoxins: Deciding when and where to go? Curr. Top. Microbiol. Immunol. 2013, 364, 91–113. [Google Scholar] [PubMed]
- Yeh, F.L.; Dong, M.; Yao, J.; Tepp, W.H.; Lin, G.; Johnson, E.A.; Chapman, E.R. Sv2 mediates entry of tetanus neurotoxin into central neurons. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef]
- Yavin, E.; Yavin, Z.; Kohn, L.D. Temperature-mediated interaction of tetanus toxin with cerebral neuron cultures: Characterization of a neuraminidase-insensitive toxin-receptor complex. J. Neurochem. 1983, 40, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Bertasio, C.; Bordin, F.; Shone, C.C.; Binz, T.; Montecucco, C. Time course and temperature dependence of the membrane translocation of tetanus and botulinum neurotoxins c and d in neurons. Biochem. Biophys. Res. Commun. 2013, 430, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.R.; Baldwin, M.R. Tetanus neurotoxin utilizes two sequential membrane interactions for channel formation. J. Biol. Chem. 2014, 289, 22450–22458. [Google Scholar] [CrossRef]
- Pirazzini, M.; Bordin, F.; Rossetto, O.; Shone, C.C.; Binz, T.; Montecucco, C. The thioredoxin reductase-thioredoxin system is involved in the entry of tetanus and botulinum neurotoxins in the cytosol of nerve terminals. FEBS Lett. 2013, 587, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Benfenati, F.; Poulain, B.; Rossetto, O.; Polverino de Laureto, P.; DasGupta, B.R.; Montecucco, C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 1992, 359, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.L.; O’Connor, V.; Lottspeich, F.; Betz, H. Clostridial neurotoxins compromise the stability of a low energy snare complex mediating nsf activation of synaptic vesicle fusion. EMBO J. 1995, 14, 4705–4713. [Google Scholar] [PubMed]
- Gil, C.; Ruiz-Meana, M.; Alava, M.; Yavin, E.; Aguilera, J. Tetanus toxin enhances protein kinase c activity translocation and increases polyphosphoinositide hydrolysis in rat cerebral cortex preparations. J. Neurochem. 1998, 70, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Okabe, T.; Sugimoto, N.; Senda, T.; Fujita, H. Tetanus toxin and clostridium perfringens enterotoxin as tools for the study of exocytosis. Ann. N. Y. Acad. Sci. 1994, 710, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, J.; Hawkins, C.; Mellanby, H.; Rawlins, J.N.; Impey, M.E. Tetanus toxin as a tool for studying epilepsy. J. Physiol. 1984, 79, 207–215. [Google Scholar]
- Toivonen, J.M.; Olivan, S.; Osta, R. Tetanus toxin c-fragment: The courier and the cure? Toxins 2010, 2, 2622–2644. [Google Scholar] [CrossRef]
- Johnston, L.; Mawas, F.; Tierney, R.; Qazi, O.; Fairweather, N.; Sesardic, D. Transcutaneous delivery of tetanus toxin hc fragment induces superior tetanus toxin neutralizing antibody response compared to tetanus toxoid. Hum. Vaccines 2009, 5, 230–236. [Google Scholar] [CrossRef]
- Ruegg, U.T.; Burgess, G.M. Staurosporine, K-252 and UCN-01: Potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 1989, 10, 218–220. [Google Scholar] [CrossRef]
- Rasouley, D.; Matsuda, Y.; Lazarovici, P. Biochemical and pharmacological properties of K252 microbial alkaloids. In The Toxic Action of Marine and Terrestrial Alkaloids; Blom, M.S., Ed.; Alken Inc.: Fort Collins, CO, USA, 1995; pp. 161–190. [Google Scholar]
- Lazarovici, P.; Rasouly, D.; Friedman, L.; Tabekman, R.; Ovadia, H.; Matsuda, Y. K252a and staurosporine microbial alkaloid toxins as prototype of neurotropic drugs. Adv. Exp. Med. Biol. 1996, 391, 367–377. [Google Scholar] [PubMed]
- Vale, C.; Botana, L.M. Marine toxins and the cytoskeleton: Okadaic acid and dinophysistoxins. FEBS J. 2008, 275, 6060–6066. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 of streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Sanokawa, R.; Fujita, Y.; Zhou, D.; Kawasaki, K.; Tanaka, H.; Komatsu, T.; Nagasawa, T.; Oka, S. Cytoplasmic elongation and rupture in megakaryoblastic leukemia cells via activation of adhesion and motility by staurosporine on fibronectin-bound substratum. J. Cell. Physiol. 1999, 179, 179–192. [Google Scholar] [CrossRef]
- Prade, L.; Engh, R.A.; Girod, A.; Kinzel, V.; Huber, R.; Bossemeyer, D. Staurosporine-induced conformational changes of camp-dependent protein kinase catalytic subunit explain inhibitory potential. Structure 1997, 5, 1627–1637. [Google Scholar] [CrossRef]
- Rasouly, D.; Lazarovici, P. Staurosporine induces tyrosine phosphorylation of a 145 kda protein but does not activate gp140trk in PC12 cells. Eur. J. Pharmacol. 1994, 269, 255–264. [Google Scholar] [CrossRef]
- Rasouly, D.; Rahamim, E.; Ringel, I.; Ginzburg, I.; Muarakata, C.; Matsuda, Y.; Lazarovici, P. Neurites induced by staurosporine in PC12 cells are resistant to colchicine and express high levels of tau proteins. Mol. Pharmacol. 1994, 45, 29–35. [Google Scholar] [PubMed]
- Way, K.J.; Chou, E.; King, G.L. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol. Sci. 2000, 21, 181–187. [Google Scholar] [CrossRef]
- Tainton, K.M.; Ruefli, A.A.; Smyth, M.J.; Johnstone, R.W. Equivalent death of P-glycoprotein expressing and nonexpressing cells induced by the protein kinase C inhibitor staurosporine. Biochem. Biophys. Res. Commun. 2000, 276, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kohn, E.A.; Yoo, C.J.; Eastman, A. The protein kinase C inhibitor Go6976 is a potent inhibitor of DNA damage-induced S and G2 cell cycle checkpoints. Cancer Res. 2003, 63, 31–35. [Google Scholar] [PubMed]
- Takai, A.; Bialojan, C.; Troschka, M.; Ruegg, J.C. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987, 217, 81–84. [Google Scholar] [CrossRef]
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Holmes, C.F.; Tsukitani, Y. Okadaic acid: A new probe for the study of cellular regulation. Trends Biochem. Sci. 1990, 15, 98–102. [Google Scholar] [CrossRef]
- Suganuma, M.; Fujiki, H.; Suguri, H.; Yoshizawa, S.; Hirota, M.; Nakayasu, M.; Ojika, M.; Wakamatsu, K.; Yamada, K.; Sugimura, T. Okadaic acid: An additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc. Natl. Acad. Sci. USA 1988, 85, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.F.; Luu, H.A.; Carrier, F.; Schmitz, F.J. Inhibition of protein phosphatases-1 and -2a with acanthifolicin. Comparison with diarrhetic shellfish toxins and identification of a region on okadaic acid important for phosphatase inhibition. FEBS Lett. 1990, 270, 216–218. [Google Scholar] [CrossRef]
- Haystead, T.A.; Sim, A.T.; Carling, D.; Honnor, R.C.; Tsukitani, Y.; Cohen, P.; Hardie, D.G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature 1989, 337, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Ishida, Y.; Kitano, H.; Ohizumi, Y.; Habon, J.; Tsukitani, Y.; Kikuchi, H. Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscles under the condition of ca deficiency. J. Pharmacol. Exp.Ther. 1982, 223, 135–143. [Google Scholar] [PubMed]
- Wang, L.Y.; Salter, M.W.; MacDonald, J.F. Regulation of kainate receptors by camp-dependent protein kinase and phosphatases. Science 1991, 253, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Yan, X.; Qin, K.; Shi, M.; Lin, T.; Zhu, Y.; Kang, T.; Zhao, G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011, 138, 135–141. [Google Scholar] [CrossRef]
- Berven, G.; Saetre, F.; Halvorsen, K.; Seglen, P.O. Effects of the diarrhetic shellfish toxin, okadaic acid, on cytoskeletal elements, viability and functionality of rat liver and intestinal cells. Toxicon 2001, 39, 349–362. [Google Scholar] [CrossRef]
- McCluskey, A.; Sim, A.T.; Sakoff, J.A. Serine-threonine protein phosphatase inhibitors: Development of potential therapeutic strategies. J. Med. Chem. 2002, 45, 1151–1175. [Google Scholar] [CrossRef]
- Alape-Giron, A.; Persson, B.; Cederlund, E.; Flores-Diaz, M.; Gutierrez, J.M.; Thelestam, M.; Bergman, T.; Jornvall, H. Elapid venom toxins: Multiple recruitments of ancient scaffolds. Eur. J. Biochem. 1999, 259, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Mackessy, S.P.; Kini, R.M. Role of accelerated segment switch in exons to alter targeting (asset) in the molecular evolution of snake venom proteins. BMC Evolut. Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-venom peptides as therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.D. Botulinum neurotoxin serotype a: A clinical update on non-cosmetic uses. Am. J. Health-Syst. Pharm. 2004, 61, S11–S23. [Google Scholar]
- Pope, J.E.; Deer, T.R. Ziconotide: A clinical update and pharmacologic review. Expert Opin. Pharmacother. 2013, 14, 957–966. [Google Scholar] [CrossRef]
- Hagemeyer, C.E.; Peter, K. Targeting the platelet integrin GPIIb/IIIa. Curr. Pharm. Des. 2010, 16, 4119–4133. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Morgenstern, D.; Reitzel, A.M.; Moran, Y. Ecological venomics: How genomics, transcriptomics and proteomics can shed new light on the ecology and evolution of venom. J. Proteom. 2016, 135, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Dutertre, S.; Vetter, I.; Christie, M.J. Conus venom peptide pharmacology. Pharmacol. Rev. 2012, 64, 259–298. [Google Scholar] [CrossRef] [PubMed]
- Verdes, A.; Anand, P.; Gorson, J.; Jannetti, S.; Kelly, P.; Leffler, A.; Simpson, D.; Ramrattan, G.; Holford, M. From mollusks to medicine: A venomics approach for the discovery and characterization of therapeutics from terebridae peptide toxins. Toxins 2016, 8. [Google Scholar] [CrossRef]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
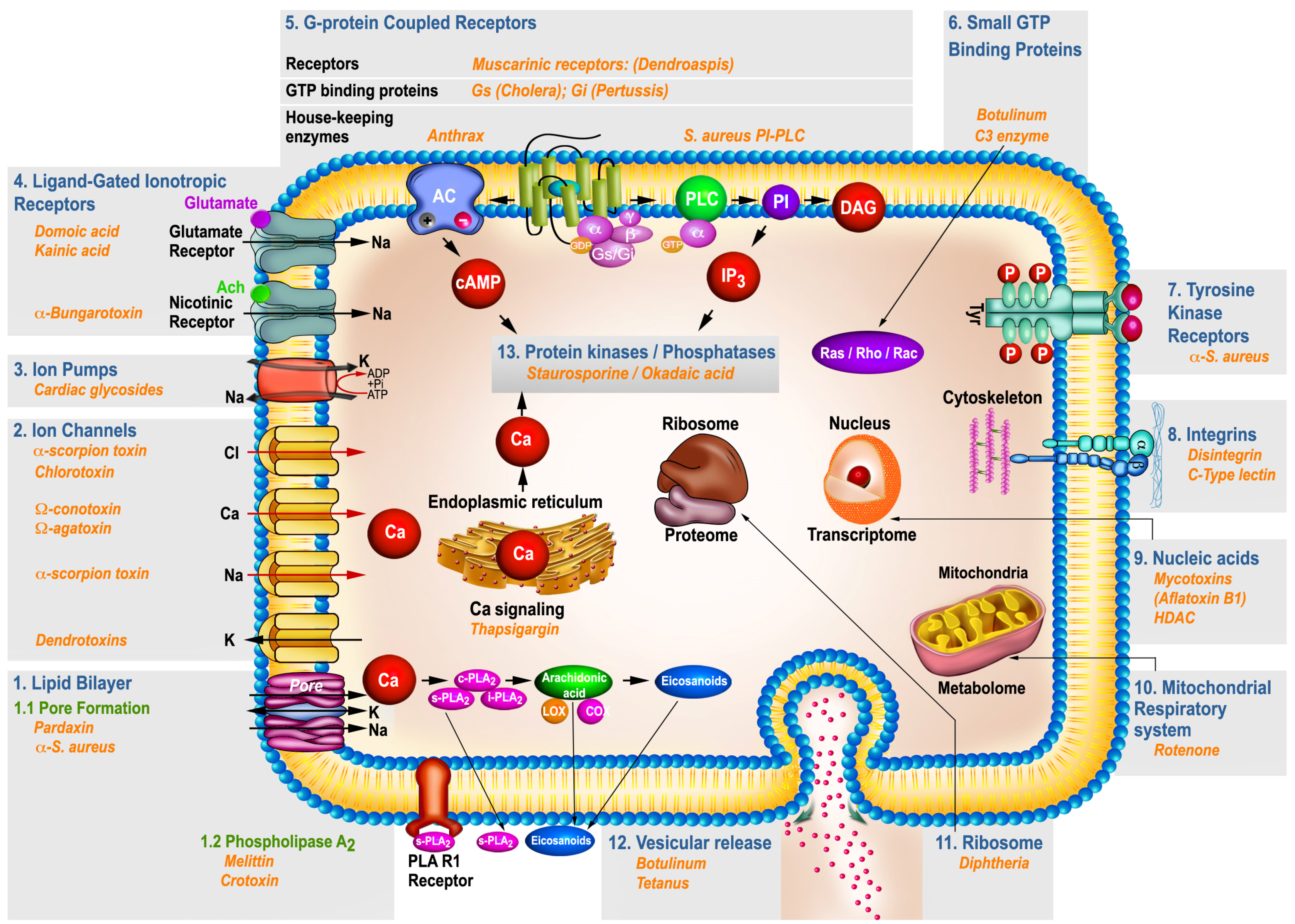
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahiani, A.; Yavin, E.; Lazarovici, P. The Molecular Basis of Toxins’ Interactions with Intracellular Signaling via Discrete Portals. Toxins 2017, 9, 107. https://doi.org/10.3390/toxins9030107
Lahiani A, Yavin E, Lazarovici P. The Molecular Basis of Toxins’ Interactions with Intracellular Signaling via Discrete Portals. Toxins. 2017; 9(3):107. https://doi.org/10.3390/toxins9030107
Chicago/Turabian StyleLahiani, Adi, Ephraim Yavin, and Philip Lazarovici. 2017. "The Molecular Basis of Toxins’ Interactions with Intracellular Signaling via Discrete Portals" Toxins 9, no. 3: 107. https://doi.org/10.3390/toxins9030107






