Isolation and Functional Characterization of an Acidic Myotoxic Phospholipase A2 from Colombian Bothrops asper Venom
Abstract
:1. Introduction
2. Results
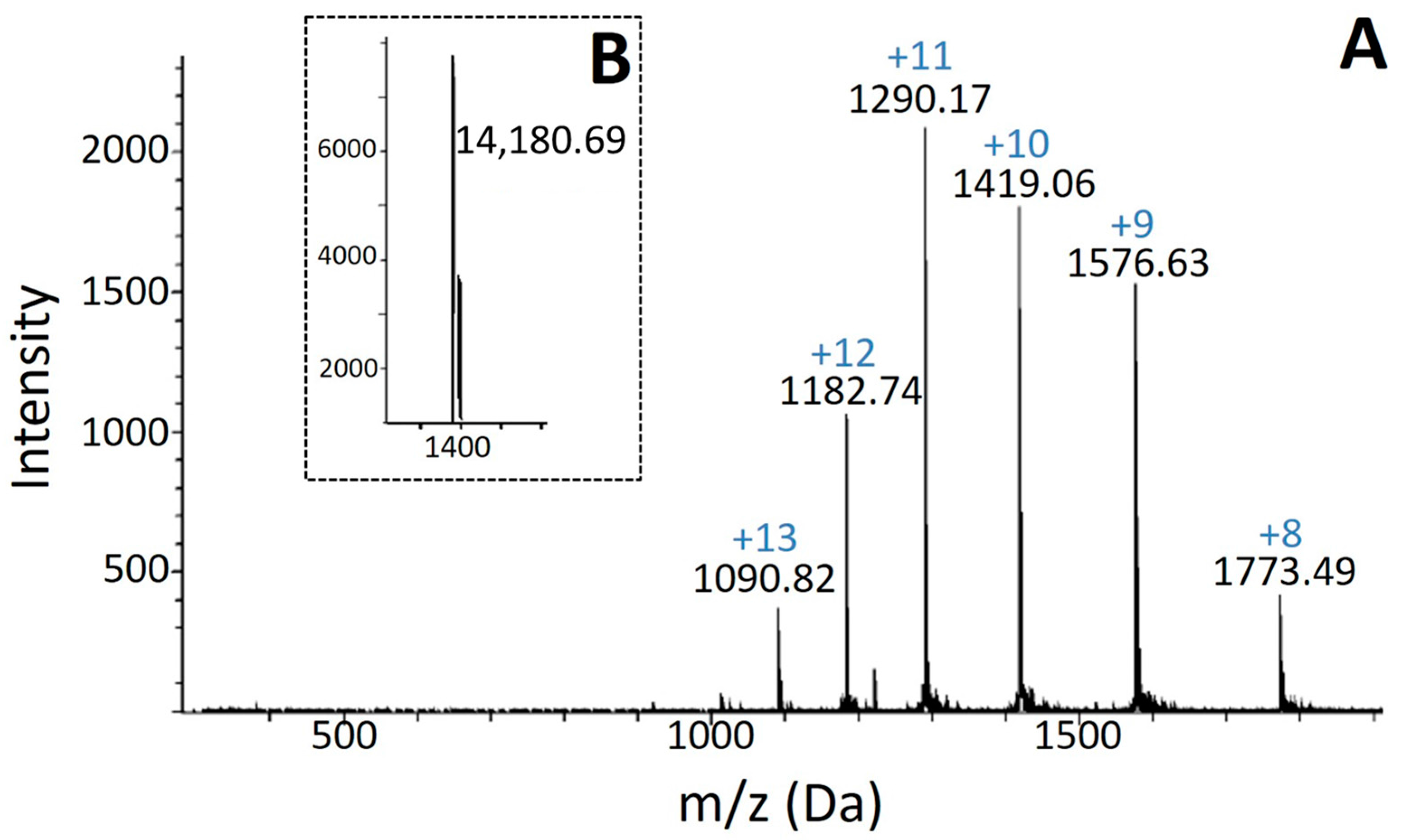
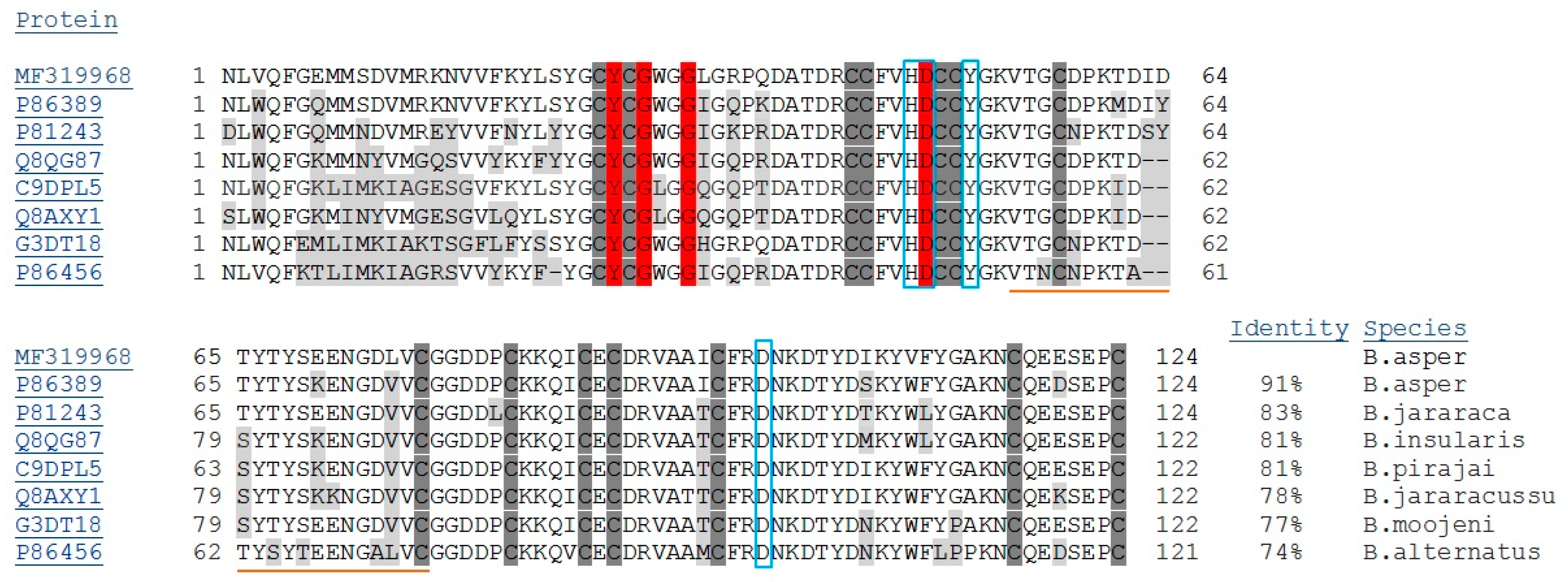
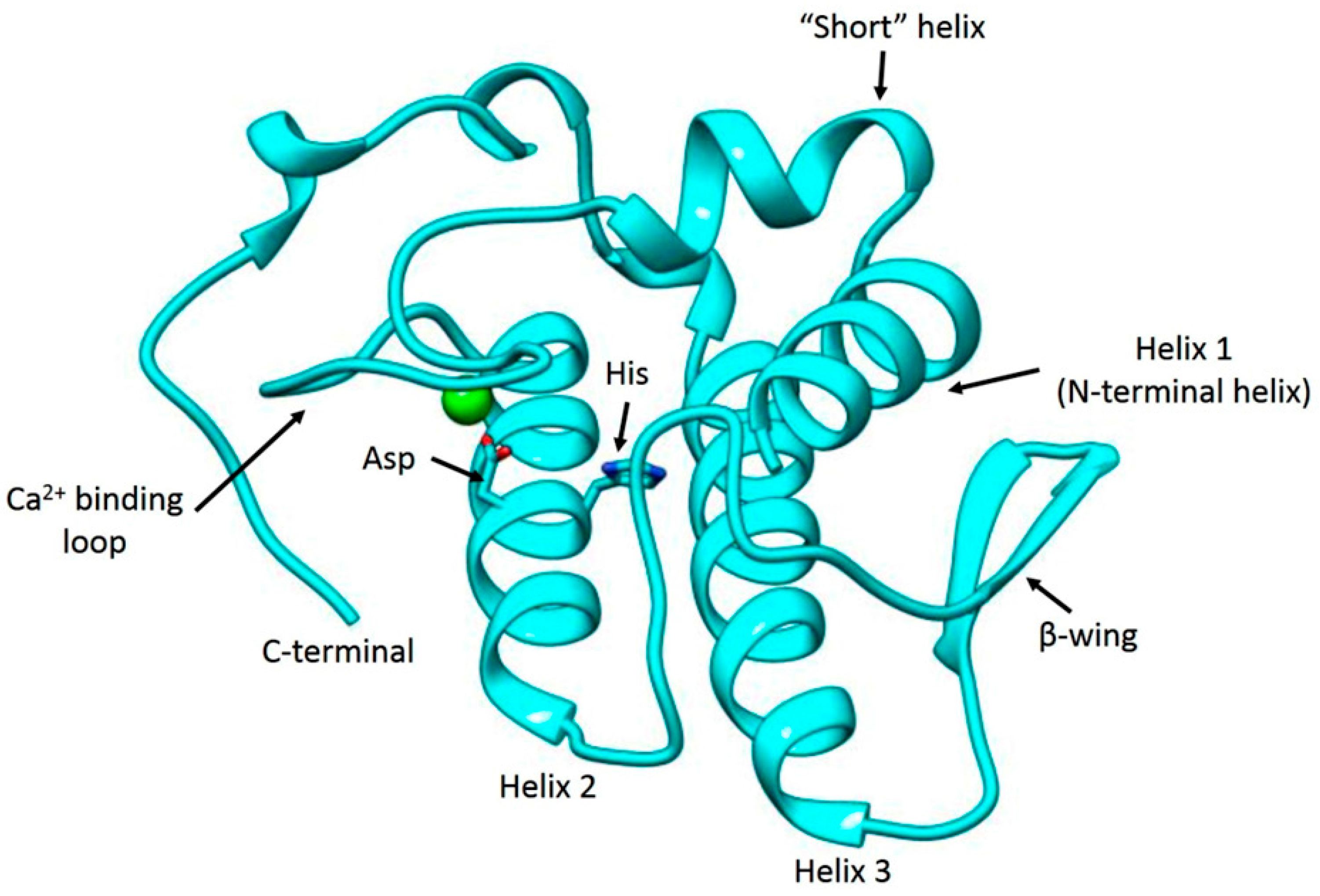
2.1. Isolation, Determination of Molecular Mass, Sequencing and Modeling of BaCol PLA2
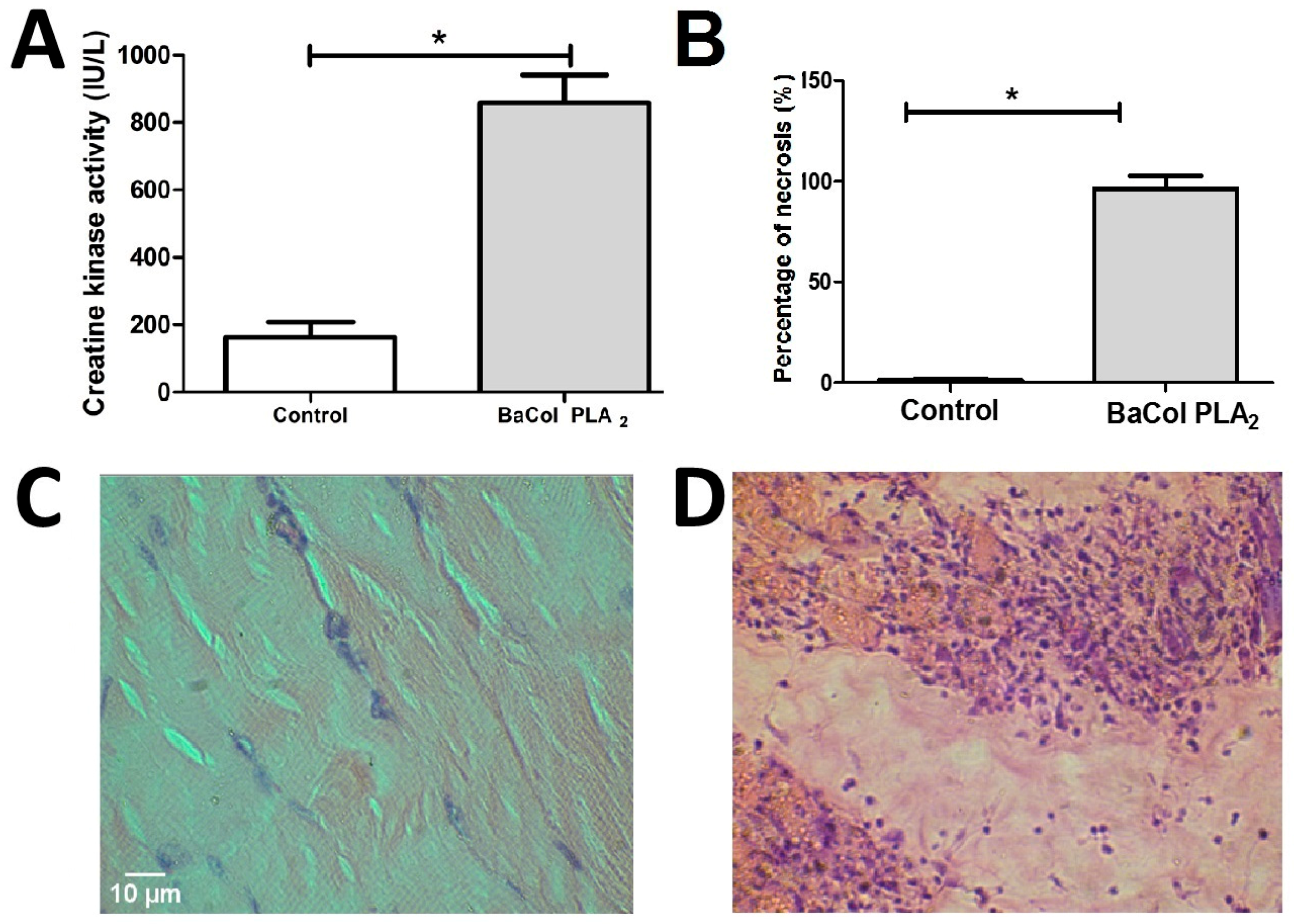
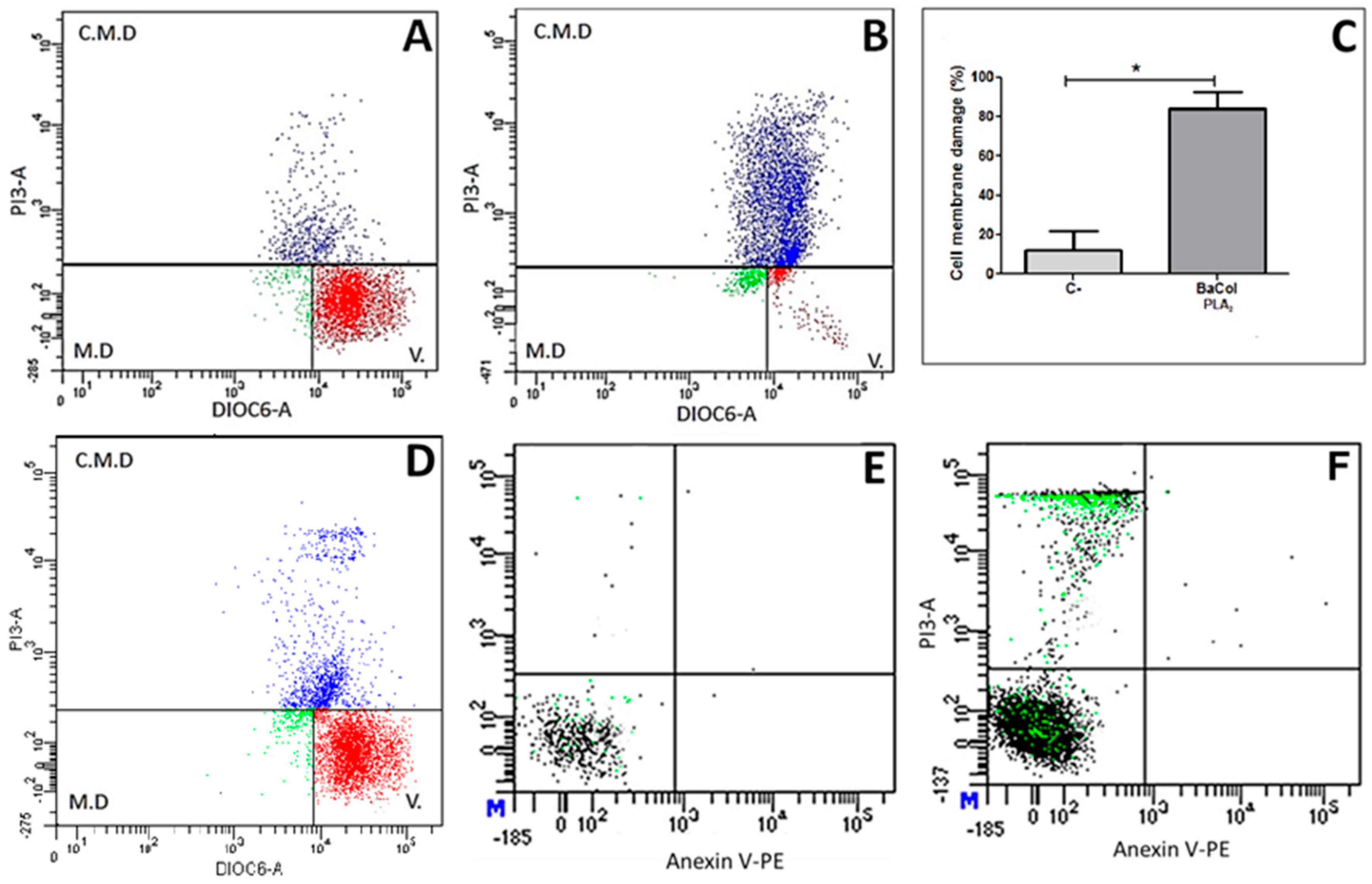
2.2. Biological Activities
3. Discussion
4. Materials and Methods
4.1. Venom
4.2. Animals
4.3. Isolation of BaCol PLA2
4.4. Molecular Mass and N-Terminal Determination
4.5. cDNA and Nucleotide Sequencing
4.6. Bidimensional Electrophoresis and Determination of Isoelectric Point
4.7. Molecular Modeling
4.8. PLA2 Activity
4.9. Edematogenic Activity
4.10. Myotoxicity
4.11. Anticoagulant Activity
4.12. Cell Viability and Cell Death
4.13. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sarmiento, K. Aspectos biomédicos del accidente ofídico. Univ. Med. 2012, 53, 68–82. [Google Scholar]
- León, L. Informe Final del Evento Accidente Ofídico; Instituto Nacional de Salud: Bogotá, Colombia, 2015.
- Otero, P. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009, 54, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon 1995, 33, 1405–1424. [Google Scholar] [CrossRef]
- Herrera, C.; Kele, J.; Feoli, M.A.; Esclante, T.; Rucavado, A.; Guitérrez, J.M. Muscle tissue damage induced by the venom of Bothrops asper: Identification of early and late pathological events through proteomic analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004599. [Google Scholar] [CrossRef] [PubMed]
- Alape-Girón, A.; Flores-Díaz, M.; Sanz, L.; Madrigal, M.; Escolano, J.; Sasa, M.; Calvete, J.J. Studies on the venom proteome of Bothrops asper: Perspectives and applications. Toxicon 2009, 54, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.; Sinha, M.; Kumar, R.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.M.; Fontes, M.R.M.; Giglio, J.R. Phospholipase A2 myotoxins from Bothrops snake venoms: Structure-function relationship. Curr. Org. Chem. 2004, 8, 1–14. [Google Scholar] [CrossRef]
- Schaloske, R.; Dennis, E. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 2006, 1761, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Andrião-Escarso, S.; Soares, A.; Fontes, M.; Fuly, A.; Corrêa, F.; Rosa, J.; Greene, L.; Giglio, J. Structural and functional characterization of an acidic platelet aggregation inhibitor and hypotensive phospholipase A2 from Bothrops jararacussu snake venom. Biochem. Pharmacol. 2002, 64, 723–732. [Google Scholar] [CrossRef]
- Fernández, J.; Gutiérrez, J.M.; Angulo, Y.; Sanz, L.; Juárez, P.; Calvete, J.; Lomonte, B. Isolation of an acidic phospholipase A2 from the venom of the snake Bothrops asper of Costa Rica: biochemical and toxicological characterization. Biochimie 2010, 92, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Cogo, J.; Lilla, S.; Souza, G.; Hyslop, S.; De Nucci, G. Purification, sequencing and structural analysis of two acidic phospholipases A2 from the venom of Bothrops insularis (jararaca ilhoa). Biochimie 2006, 88, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Renetseder, R.; Brunie, S.; Dijkstrat, B.W.; Drentht, J.; Sigler, P.B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox venom. J. Biol. Chem. 1985, 260, 11627–11634. [Google Scholar] [PubMed]
- Teixeira, S.; Silveira, L.; Da Silva, F.; Marchi-Salvador, D.; Silva, F., Jr.; Izidoro, L.; Fuly, A.; Juliano, M.; Dos Santos, C.; Murakami, M.; et al. Molecular characterization of an acidic phospholipase A2 from Bothrops pirajai snake venom: Synthetic C-terminal peptide identifies its antiplatelet region. Arch. Toxicol. 2011, 85, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Van der Laat, M.; Fernández, J.; Durban, J.; Villalobos, E.; Camacho, E.; Calvete, J.; Lomonte, B. Amino acid sequence and biological characterization of BlatPLA2, a non-toxic acidic phospholipase A2 from the venom of the arboreal snake Bothriechis lateralis from Costa Rica. Toxicon 2013, 73, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Izidoro, L.; Teixeira, S.; Silveira, L.; Hamaguchi, A.; Homsi-Brandeburgo, M.; Selistre-de-Araújo, H.; Giglio, J.; Fuly, A.; Soares, A.; et al. Isolation and functional characterization of a new myotoxic acidic phospholipase A2 from Bothrops pauloensis snake venom. Toxicon 2007, 50, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Santos-Filho, N.; Silveira, L.; Oliveira, C.; Bernardes, C.; Menaldo, D.; Fuly, A.; Arantes, E.; Sampaio, S.; Mameded, C.; Beletti, M.; et al. A new acidic myotoxic, anti-platelet and prostaglandin I2 inductor phospholipase A2 isolated from Bothrops moojeni snake venom. Toxicon 2008, 52, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.; Reichl, A.; Mentele, R.; Auerswald, E.; Santoro, M.; Sampaio, C.; Camargo, A.; Assakura, M. A novel Phospholipase A2, BJ-PLA2, from the venom of the snake Bothrops jararaca: purification, primary structure analysis, and its characterization as a platelet-aggregation-inhibiting factor. Arch. Biochem. Biophys. 1999, 367, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.; Marchi-Salvador, D.; Santos-Filho, N.; Silva, F., Jr.; Marcussie, S.; Fuly, A.; Nomizo, A.; Da Silva, S.; Stábeli, R.; Arantes, E.; et al. Isolation and expression of a hypotensive and anti-platelet acidic phospholipase A2 from Bothrops moojeni snake venom. J. Pharm. Biomed. Anal. 2013, 73, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Rodrigues, R.; Lucena, M.; Cologna, C.; Oliveira, A.; Hamaguchi, A.; Homsi-Brandeburgo, M.; Arantes, E.; Teixeira, D.; Ueira-Vieira, C.; et al. Isolation and functional characterization of proinflammatory acidic phospholipase A2 from Bothrops leucurus snake venom. Comp. Biochem. Physiol. C 2011, 154, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Mora-Obando, D.; Fernández, J.; Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Synergism between basic Asp49 and Lys49 phospholipase A2 myotoxins of viperid snake venom in vitro and in vivo. PLoS ONE 2014, 9, e109846. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Structure–function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon 2005, 45, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Edman, P.; Begg, G. A protein sequentor. Eur. J. Biochem. 1967, 1, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis, version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.R.; Lipman, D.J. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 1988, 85, 2444–2448. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative protein structure modeling using Modeller. Protein Struct. Predict. 2014, 47, 1–15. [Google Scholar]
- Laskowski, R.; MacArthur, M.; Moss, D.; Hornton, D. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Sippl, M. Recognition of errors in three-dimensional structures of proteins. Proteins 1993, 17, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.U.; Luthy, R.; Eisenberg, D. A method to identify protein sequences that fold into a known three-dimensional structure. Science 1991, 253, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B.; Chaves, F.; Moreno, E.; Cerdas, L. Pharmacological activities of a toxic phospholipase A2 isolated from the venom of the snake Bothrops asper. Comp. Biochem. Physiol. C 1986, 84, 159–164. [Google Scholar] [CrossRef]
- Cho, W.; Kézdy, F.J. Chromogenic phospholipase A2 substrates and assays. Methods Enzymol. 1991, 197, 75–79. [Google Scholar] [PubMed]
- Holzer, M.; Mackessy, S.P. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
- Lomonte, B.; Tarkowski, A.; Hanson, L.A. Host response to Bothrops asper snake venom: analysis of edema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation 1993, 17, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B.; Cerdas, L. Isolation and partial characterization of a myotoxin from the venom of the snake Bothrops nummifer. Toxicon 1986, 24, 885–894. [Google Scholar] [CrossRef]
- Herrera, C.; Rucavado, A.; Warrell, D.A.; Gutiérrez, J.M. Systemic effects induced by the venom of the snake Bothrops caribbaeus in a murine model. Toxicon 2013, 63, 19–31. [Google Scholar] [CrossRef] [PubMed]



| Reference | Phospholipase | Isoelectric Point | Myotoxic Activity | Edematogenic Activity | Platelet Aggregation | Anti-Clotting Activity |
|---|---|---|---|---|---|---|
| Posada et al, 2017 | BaCol PLA2 | 4.4 | Yes | Yes | - | Moderate |
| Cogo et al., 2006 | BinTx-I | 5.05 | Yes | Yes | - | - |
| Rodrigues et al., 2007 | Bp-PLA2 | 4.3 | Yes | Yes | Inhibits | - |
| Santos et al., 2008 | BmooTx-I | 4.2 | Yes | Yes | Inhibits | - |
| Fernandez et al., 2010 | Basp PLA2-II | 4.9 | No | Yes | Does not inhibit | No |
| Teixeira et al., 2011 | Bpir PLA2-I | 4.8 | No | Yes | Inhibits | - |
| Silveira et al., 2013 | Bmoo PLA2 | 5.2 | No | Yes | Inhibits | Yes |
| AndriaoEscarso et al., 2002 | BthA-I-PLA2 | 4.5 | No | Yes | Inhibits | Low |
| Nunes et al., 2011 | Bl PLA2 | 5.4 | Low | Low | - | - |
| Serrano et al., 1999 | BJ PLA2 | - | - | No | Inhibits | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posada Arias, S.; Rey-Suárez, P.; Pereáñez J, A.; Acosta, C.; Rojas, M.; Delazari dos Santos, L.; Ferreira Jr, R.S.; Núñez, V. Isolation and Functional Characterization of an Acidic Myotoxic Phospholipase A2 from Colombian Bothrops asper Venom. Toxins 2017, 9, 342. https://doi.org/10.3390/toxins9110342
Posada Arias S, Rey-Suárez P, Pereáñez J A, Acosta C, Rojas M, Delazari dos Santos L, Ferreira Jr RS, Núñez V. Isolation and Functional Characterization of an Acidic Myotoxic Phospholipase A2 from Colombian Bothrops asper Venom. Toxins. 2017; 9(11):342. https://doi.org/10.3390/toxins9110342
Chicago/Turabian StylePosada Arias, Silvia, Paola Rey-Suárez, Andrés Pereáñez J, Cristian Acosta, Mauricio Rojas, Lucilene Delazari dos Santos, Rui Seabra Ferreira Jr, and Vitelbina Núñez. 2017. "Isolation and Functional Characterization of an Acidic Myotoxic Phospholipase A2 from Colombian Bothrops asper Venom" Toxins 9, no. 11: 342. https://doi.org/10.3390/toxins9110342
APA StylePosada Arias, S., Rey-Suárez, P., Pereáñez J, A., Acosta, C., Rojas, M., Delazari dos Santos, L., Ferreira Jr, R. S., & Núñez, V. (2017). Isolation and Functional Characterization of an Acidic Myotoxic Phospholipase A2 from Colombian Bothrops asper Venom. Toxins, 9(11), 342. https://doi.org/10.3390/toxins9110342