Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom
Abstract
:1. Introduction
2. Results
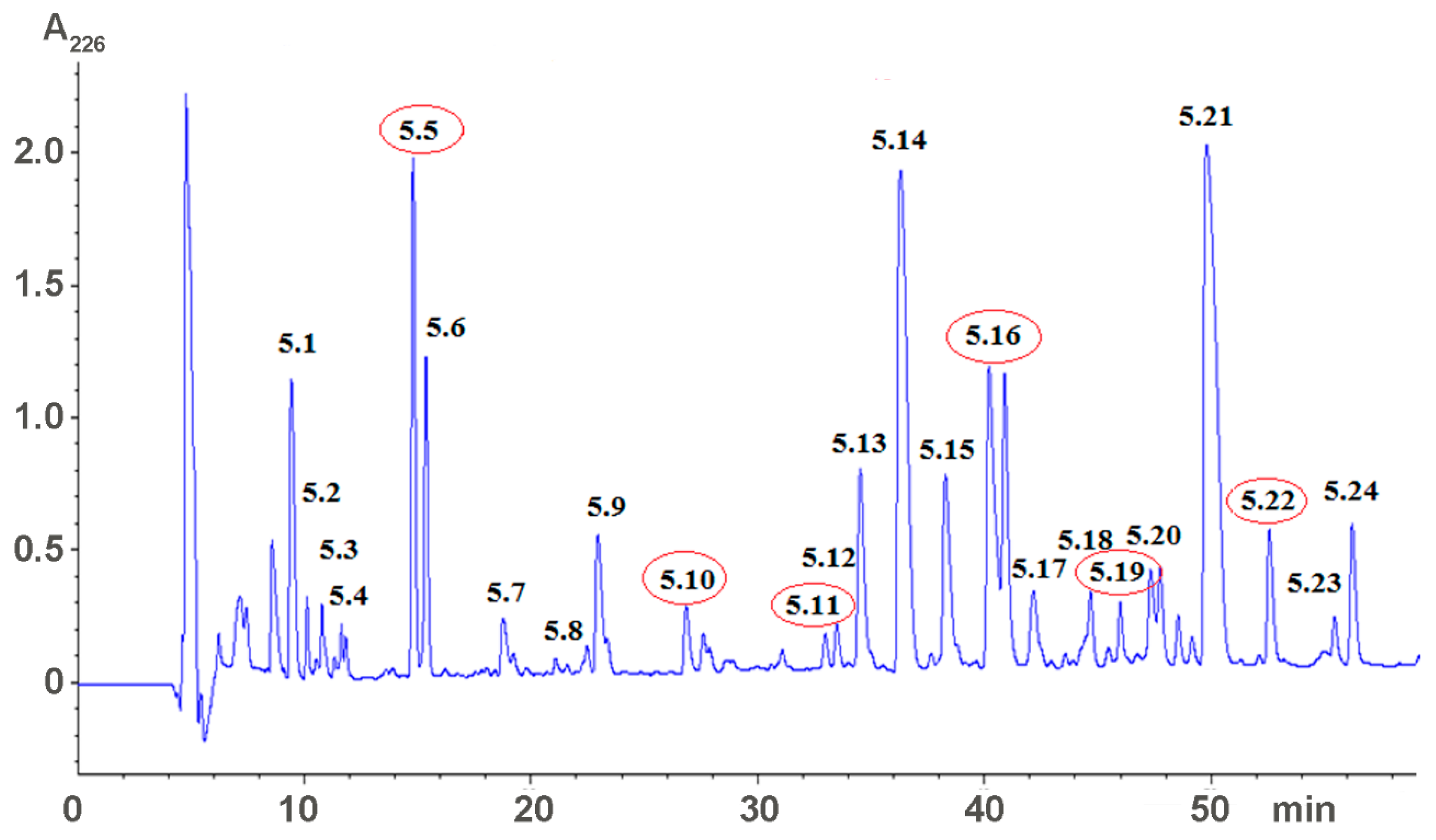
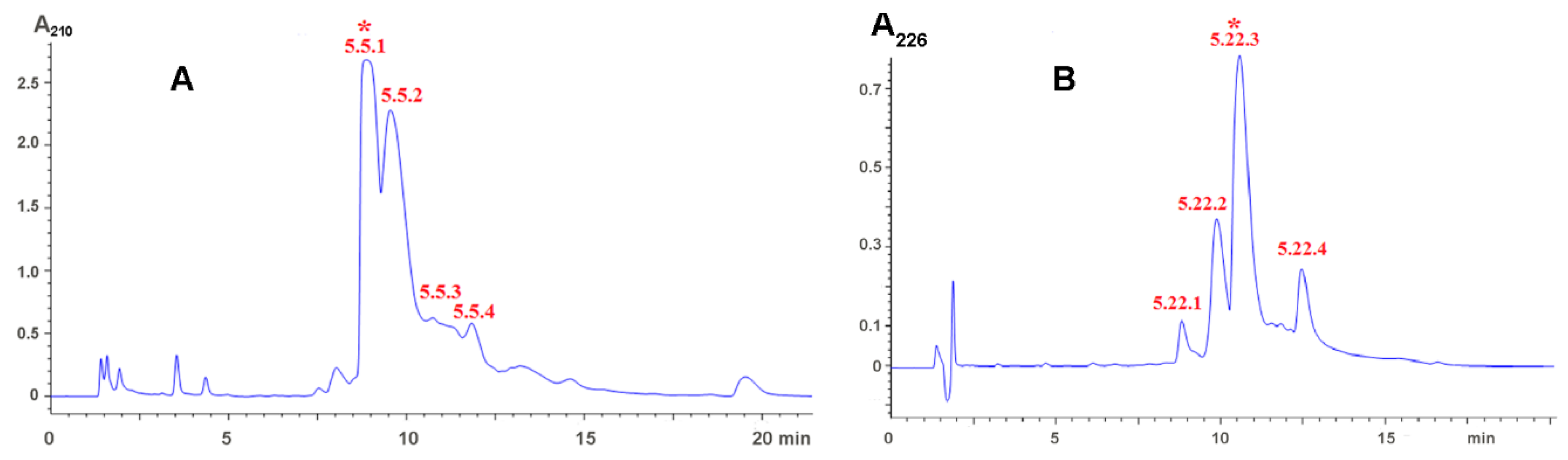
2.1. Isolation of Active Compounds
2.2. Studies of Anticoagulant Activity
2.2.1. Influence on Blood Coagulation In Vitro
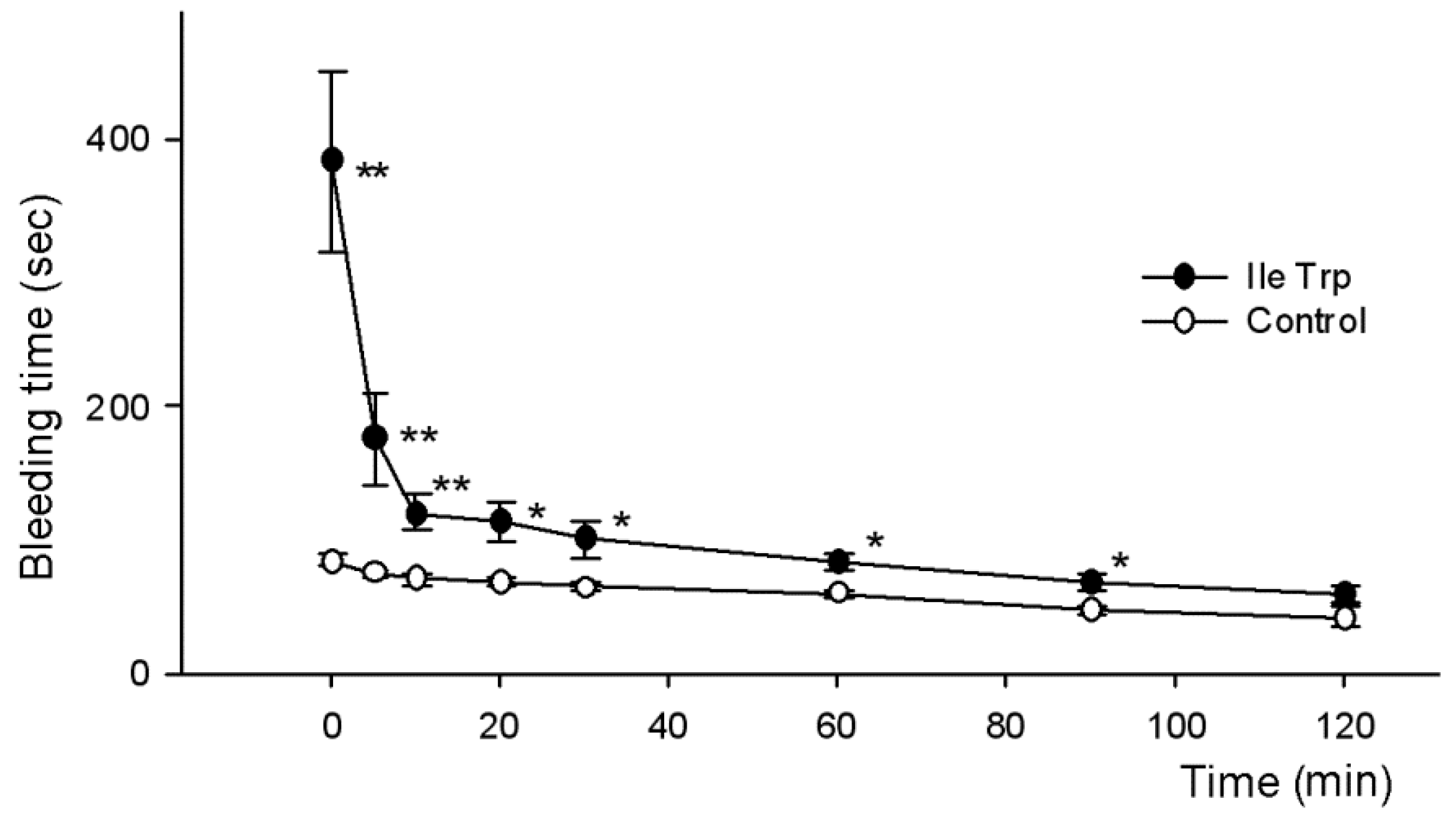
2.2.2. Influence on Bleeding Time In Vivo
2.2.3. Influence of Dipeptides on Platelet Aggregation In Vitro
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
Scorpions and Scorpion Venom
5.2. Venom Fractionation and Isolation of Low Molecular Weight Compounds
5.3. Characterization of Low Molecular Weight Compounds
5.4. Mice
5.5. Determination of Anticoagulant Activity
5.5.1. Determination of Blood Coagulation Time
5.5.2. Determination of In Vivo Bleeding Time
5.5.3. Platelet Aggregation Measurements
5.5.4. Plasma Coagulation Tests
5.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Couzijn, H.W.C. Revision of the genus Heterometrus Hemprich & Ehrenberg (Scorpionidae, Arachnoidea). Zoologische Verhandelingen 1981, 184, 1–196. [Google Scholar]
- Uawonggul, N.; Chaveerach, A.; Thammasirirak, S.; Arkaravichien, T.; Chuachan, C.; Daduang, S. Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J. Ethnopharmacol. 2006, 103, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.N.; Vo, H.D.; Vo, N.P.; Kudryashova, K.S.; Nekrasova, O.V.; Feofanov, A.V.; Kirpichnikov, M.P.; Andreeva, T.V.; Serebryakova, M.V.; Tsetlin, V.I.; et al. Vietnamese Heterometrus laoticus scorpion venom: evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 2014, 77, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Uawonggul, N.; Thammasirirak, S.; Chaveerach, A.; Arkaravichien, T.; Bunyatratchata, W.; Ruangjirachuporn, W.; Jearranaiprepame, P.; Nakamura, T.; Matsuda, M.; Kobayashi, M.; et al. Purification and characterization of Heteroscorpine-1 (HS-1) toxin from Heterometrus laoticus scorpion venom. Toxicon 2007, 49, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, T.; Kopljar, I.; Jenkins, D.P.; Diego-Garcia, E.; Abdel-Mottaleb, Y.; Vermassen, E.; Clynen, E.; Schoofs, L.; Wulff, H.; Snyders, D.; et al. Purification, molecular cloning and functional characterization of HelaTx1 (Heterometrus laoticus): the first member of a new κ-KTX subfamily. Biochem. Pharmacol. 2012, 83, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Ponnudurai, G. Comparative study of the enzymatic, hemorrhagic, procoagulant and anticoagulant activities of some animal venoms. Comp. Biochem. Physiol. C 1992, 103, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Gajalakshmi, B.S. Coagulation studies following scorpion venom injection in animals. Indian J. Med. Res. 1982, 76, 337. [Google Scholar] [PubMed]
- Hamilton, P.J.; Ogston, D.; Douglas, A.S. Coagulant activity of the scorpion venoms Palamneus gravimanus and Leiurus quinquestriatus. Toxicon 1974, 12, 291–296. [Google Scholar] [CrossRef]
- D’Suze, G.; Moncada, S.; Gonzalez, C.; Sevcik, C.; Aguilar, V.; Alagon, A. Relationship between plasmatic levels of various cytokines, tumour necrosis factor, enzymes, glucose and venom concentration following Tityus scorpion sting. Toxicon 2003, 41, 367–375. [Google Scholar] [CrossRef]
- Brazón, J.; Guerrero, B.; Arocha-Piñango, C.L.; Sevcik, C.; D’Suze, G. Effect of Tityus discrepans scorpion venom on global coagulation test. Preliminary studies. Investig. Clin. 2008, 49, 49–58. [Google Scholar]
- Brazón, J.; Guerrero, B.; D’Suze, G.; Sevcik, C.; Arocha-Piñango, C.L. Fibrin(ogen)olytic enzymes in scorpion (Tityus discrepans) venom. Comp. Biochem. Physiol. B 2014, 168, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, F.Z.; Conde, R.; Arévalo, C.; Becerril, B.; Martin, B.M.; Valdivia, H.H.; Possani, L.D. The mechanism of inhibition of ryanodine receptor channels by imperatoxin I, a heterodimeric protein from the scorpion Pandinus imperator. J. Biol. Chem. 1997, 272, 11886–11894. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Cruz, N.A.; Batista, C.V.; Possani, L.D. Phaiodactylipin, a glycosylated heterodimeric phospholipase A from the venom of the scorpion Anuroctonus phaiodactylus. Eur. J. Biochem. 2004, 271, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Tang, X.X.; Chen, X.G.; Gao, B.B.; Gao, E.; Bai, L.; Lv, X.R. Effects of scorpion venom bioactive polypolypeptides on platelet aggregation and thrombosis and plasma 6-keto-PG F1α and TXB2 in rabbits and rats. Toxicon 2005, 46, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Brazón, J.; D’Suze, G.; D’Errico, M.L.; Arocha-Piñango, C.L.; Guerrero, B. Discreplasminin, a plasmin inhibitor isolated from Tityus discrepans scorpion venom. Arch. Toxicol. 2009, 83, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.A.; Vo, D.M.H.; Nikitin, I.; Utkin, Y. Isolation and preliminary study of short toxins from scorpion Heterometrus laoticus. Tap Chi Hoa Hoc (J. Chem.) 2011, 49, 118–122. [Google Scholar]
- Tran, T.V.; Hoang, A.N.; Nguyen, T.T.T.; Phung, T.V.; Nguyen, K.C.; Osipov, A.V.; Dubovskii, P.V.; Ivanov, I.A.; Arseniev, A.S.; Tsetlin, V.I.; et al. Low-molecular compounds with anticoagulant activity from scorpion Heterometrus laoticus venom. Dokl. Biochem. Biophys. 2017, 476. in press. [Google Scholar] [CrossRef]
- Hoang, A.N.; Hoang, T.M.Q.; Nguyen, T.T.T.; Nguyen, T.T.T.; Pham, Y.N.D.; Vo, H.D.M. Isolation and characterization of anticoagulant components from scorpion venom Heterometrus laoticus. Tap Chi Hoa Hoc (J. Chem.) 2013, 51, 520–524. [Google Scholar]
- Barker, L.F. The Clinical Diagnosis of Internal Diseases. In Monographic Medicine, D; Appleton and Company: New York, NY, USA; London, UK, 1917; Volume III, p. 131. [Google Scholar]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Johnston-Cox, H.A.; Ravid, K. Adenosine and blood platelets. Purinergic Signal. 2011, 7, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Söderbäck, U.; Sollevi, A.; Wallen, N.H.; Larsson, P.T.; Hjemdahl, P. Anti-aggregatory effects of physiological concentrations of adenosine in human whole blood as assessed by filtragometry. Clin. Sci. (Lond.) 1991, 81, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, R.E. Dipyridamole inhibition of adenosine metabolism in human blood. Eur. J. Pharmacol. 1983, 93, 21–26. [Google Scholar] [CrossRef]
- Lunow, D.; Kaiser, S.; Rückriemen, J.; Pohl, C.; Henle, T. Tryptophan-containing dipeptides are C-domain selective inhibitors of angiotensin converting enzyme. Food Chem. 2015, 166, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Skowasch, D.; Viktor, A.; Schneider-Schmitt, M.; Lüderitz, B.; Nickenig, G.; Bauriedel, G. Differential antiplatelet effects of angiotensin converting enzyme inhibitors: comparison of ex vivo platelet aggregation in cardiovascular patients with ramipril, captopril and enalapril. Clin. Res. Cardiol. 2006, 95, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzam, S.I.; Alzoubi, K.H.; Khabour, O.F.; Quttina, M.; Zayadeen, R. Evaluation of the effect of angiotensin converting enzyme inhibitors and angiotensin receptors blockers on aspirin antiplatelet effect. Int. J. Clin. Pharmacol. Ther. 2016, 54, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wojewódzka-Zelezniakowicz, M.; Chabielska, E.; Mogielnicki, A.; Kramkowski, K.; Karp, A.; Opadczuk, A.; Domaniewski, T.; Malinowska-Zaprzałka, M.; Buczko, W. Antithrombotic effect of tissue and plasma type angiotensin converting enzyme inhibitors in experimental thrombosis in rats. J. Physiol. Pharmacol. 2006, 57, 231–245. [Google Scholar] [PubMed]
- Phase II Study of BNC210 in PTSD. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02933606 (accessed on 13 October 2017).
- O’Connor, S.; Thebault, V.; Danjou, P.; Mikkelsen, J.D.; Doolin, E.; Simpson, J.; Tadie, E. A multiple ascending dose study with evidence for target engagement of BNC210; a negative allosteric modulator of alpha7 nAChR in development for anxiety. Eur. Neuropsychopharmacol. 2016, 26, S609. [Google Scholar] [CrossRef]
- Schedel, A.; Thornton, S.; Schloss, P.; Klüter, H.; Bugert, P. Human platelets express functional alpha7-nicotinic acetylcholine receptors. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Fogari, R.; Zoppi, A. Antihypertensive drugs and fibrinolytic function. Am. J. Hypertens. 2006, 19, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Jurk, K.; Hobohm, L.; Jäckel, S.; Saffarzadeh, M.; Schwierczek, K.; Wenzel, P.; Langer, F.; Reinhardt, C.; Ruf, W. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood 2017, 129, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.; Gachet, C.; Cazenave, J.-P.; Packham, M.A. Adenosine diphosphate (ADP) does not induce thromboxane A2 generation in human platelets. Blood 2002, 99, 3868–3869. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.D.; Barnard, M.R.; Frelinger, A.L.; Michelson, A.D.; Przyklenk, K. Effect of adenosine A2 receptor stimulation on platelet activation-aggregation: Differences between canine and human models. Thromb. Res. 2008, 121, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N.; Weise, C.; Kasheverov, I.E.; Andreeva, T.V.; Kryukova, E.V.; Zhmak, M.N.; Starkov, V.G.; Hoang, N.A.; Bertrand, D.; Ramerstorfer, J.; et al. Azemiopsin from Azemiops feae viper venom, a novel polypeptide ligand of nicotinic acetylcholine receptor. J. Biol. Chem. 2012, 287, 27079–27086. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jennings, N.L.; Dart, A.M.; Du, X.J. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J. Exp. Med. 2012, 2, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Osipov, A.V.; Filkin, S.Y.; Makarova, Y.V.; Tsetlin, V.I.; Utkin, Y.N. A new type of thrombin inhibitor, noncytotoxic phospholipase A2, from the Naja haje cobra venom. Toxicon 2010, 55, 186–194. [Google Scholar] [CrossRef] [PubMed]


| Compound | Time after Injection, min | ||||
|---|---|---|---|---|---|
| 20 | 30 | 60 | 90 | 120 | |
| Clotting Time, s | |||||
| Control | 307.7 ± 17.4 | 290.67 ± 9.58 | 286.00 ± 6.31 | 256.3 ± 20.4 | 229.7 ± 13.2 |
| Fraction 5 | 422.3 ± 8.4 1 | 391.7 ± 48.1 1 | 387.5 ± 35.0 | 360.2 ± 6.5 | 358.8 ± 26.6 1 |
| Adenosin | 442.5 ± 20.6 2 | 426.2 ± 25.6 2 | 366.2 ± 25.0 2 | 428.3 ± 51.1 | 296.3 ± 37.4 |
| LeuTrp | 401.5 ± 31.2 | 340.8 ± 29.1 | 300.3 ± 2.1 | 460.5 ± 41.7 | 313.3 ± 24.9 1 |
| IleTrp | 556.5 ± 87.2 2 | 426.2 ± 3.7 1 | 388.0 ± 46.3 1 | 367.0 ± 25.5 1 | 261.8 ± 16.4 |
| Compound | Time after Injection, min | ||||
|---|---|---|---|---|---|
| 20 | 30 | 60 | 90 | 120 | |
| Bleeding Time, s | |||||
| Control | 79.5 ± 13.7 | 43.33 ± 1.94 | 45.83 ± 3.95 | 40.67 ± 5.02 | 49.67 ± 7.85 |
| Fraction 5 | 386.2 ± 88.7 1 | 187.0 ± 64.6 1 | 86 ± 2.38 | 119.3 ± 29.2 1 | 183 ± 80.7 |
| Adenosin | 248.2 ± 66.7 1 | 314 ± 58.6 1 | 146.7 ± 46.0 1 | 65 ± 14.5 | 40.2 ± 10.3 |
| LeuTrp | 314.5 ± 85.2 1 | 84.8 ± 16.7 | 81.2 ± 15.4 | 61.8 ± 14.8 | 68.8 ± 16.4 |
| IleTrp | 233.0 ± 30.6 2 | 179.0 ± 41.4 1 | 218.7 ± 78.5 2 | 151.5 ± 57.4 | 83.8 ± 13.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.V.; Hoang, A.N.; Nguyen, T.T.T.; Phung, T.V.; Nguyen, K.C.; Osipov, A.V.; Ivanov, I.A.; Tsetlin, V.I.; Utkin, Y.N. Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom. Toxins 2017, 9, 343. https://doi.org/10.3390/toxins9110343
Tran TV, Hoang AN, Nguyen TTT, Phung TV, Nguyen KC, Osipov AV, Ivanov IA, Tsetlin VI, Utkin YN. Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom. Toxins. 2017; 9(11):343. https://doi.org/10.3390/toxins9110343
Chicago/Turabian StyleTran, Thien Vu, Anh Ngoc Hoang, Trang Thuy Thi Nguyen, Trung Van Phung, Khoa Cuu Nguyen, Alexey V. Osipov, Igor A. Ivanov, Victor I. Tsetlin, and Yuri N. Utkin. 2017. "Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom" Toxins 9, no. 11: 343. https://doi.org/10.3390/toxins9110343






