Anti-Salmonella Activity Modulation of Mastoparan V1—A Wasp Venom Toxin—Using Protease Inhibitors, and Its Efficient Production via an Escherichia coli Secretion System
Abstract
:1. Introduction
2. Results
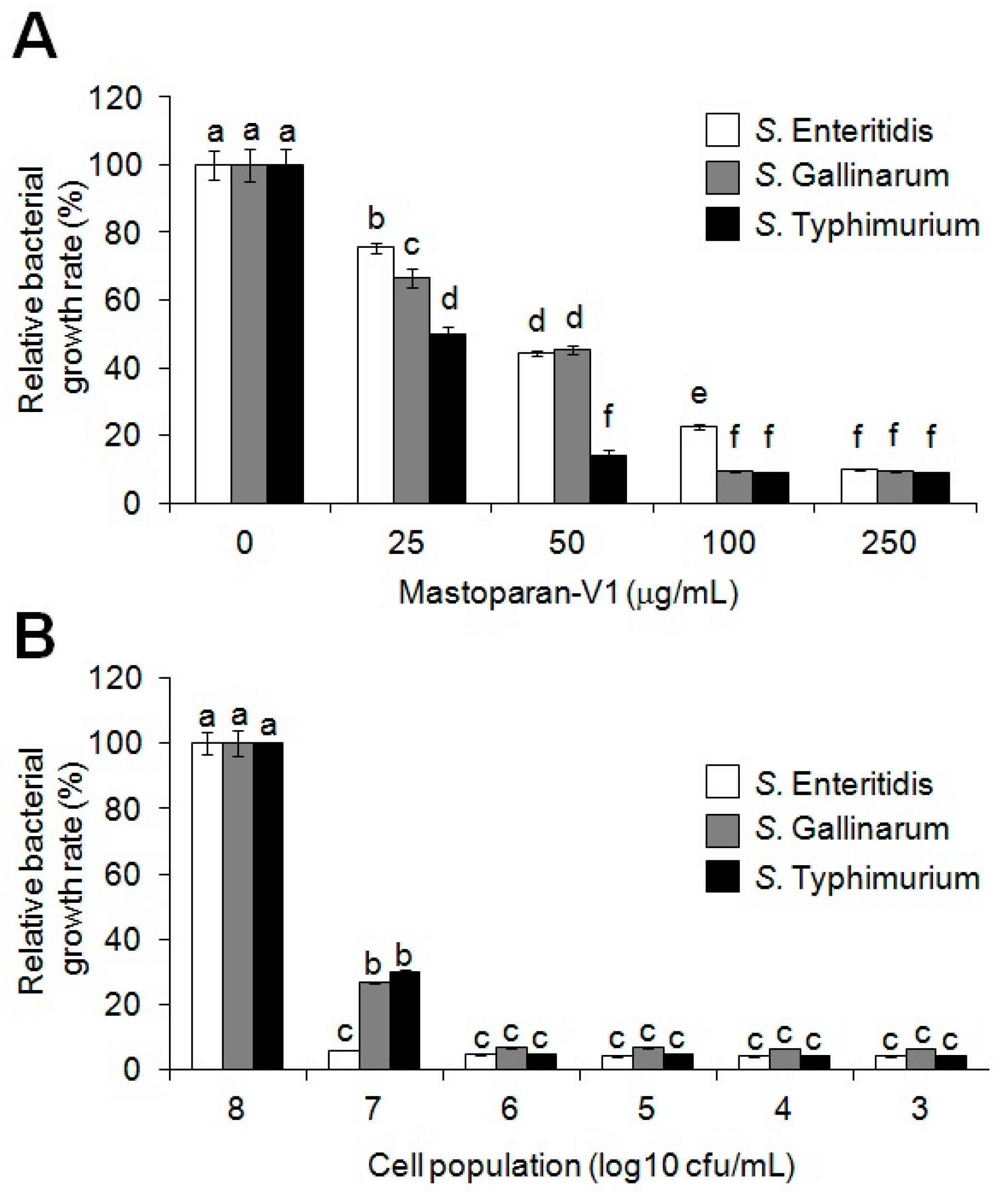
2.1. Antimicrobial Activity of Synthetic MP-V1 against the Three Salmonella Serotypes
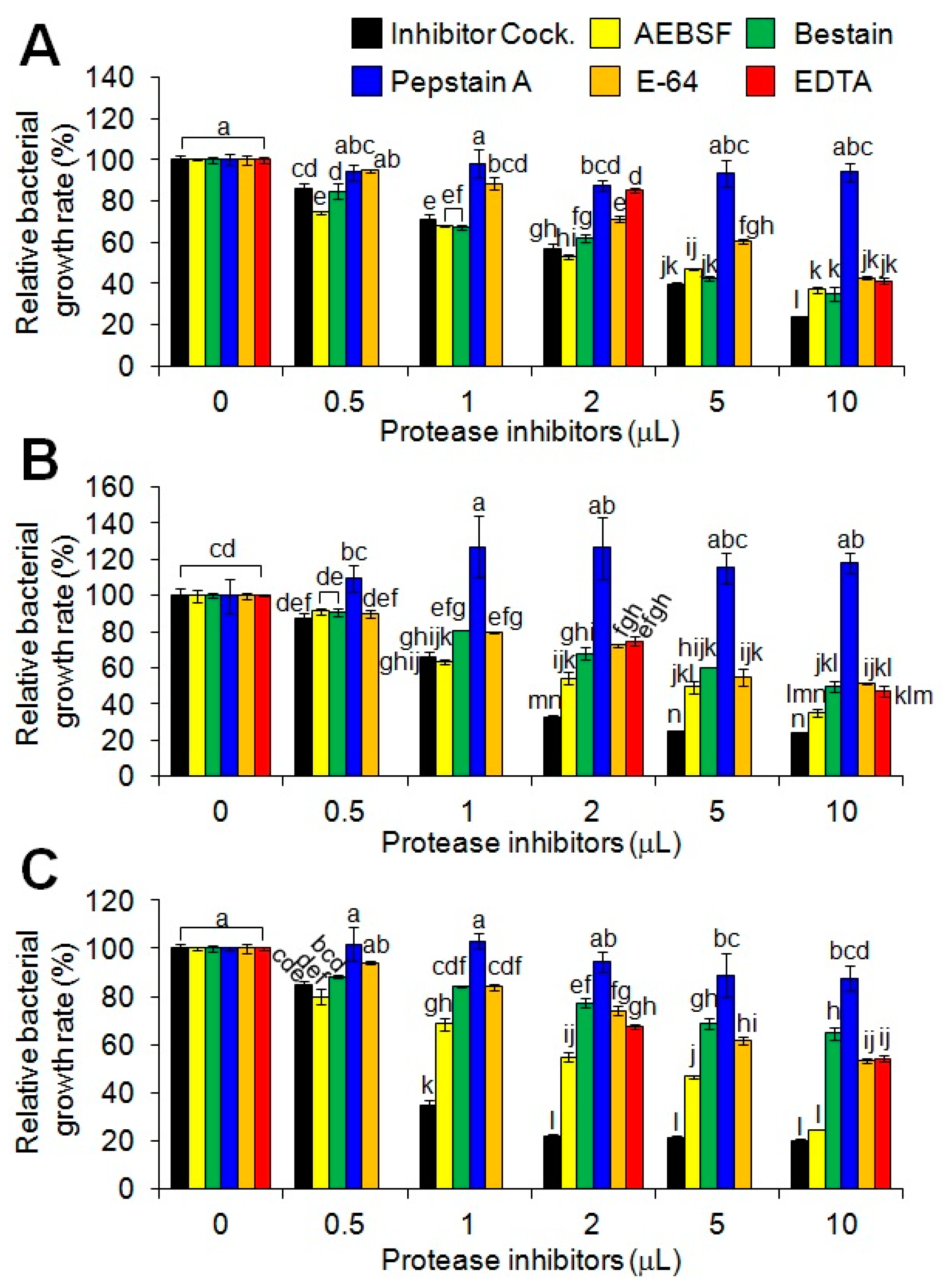
2.2. Anti-Salmonella Activity Modulation of the Synthetic MP-V1 Using Various Protease Inhibitors
2.3. Construction of the E. coli Secretion System to Efficiently Produce Active MP-V1
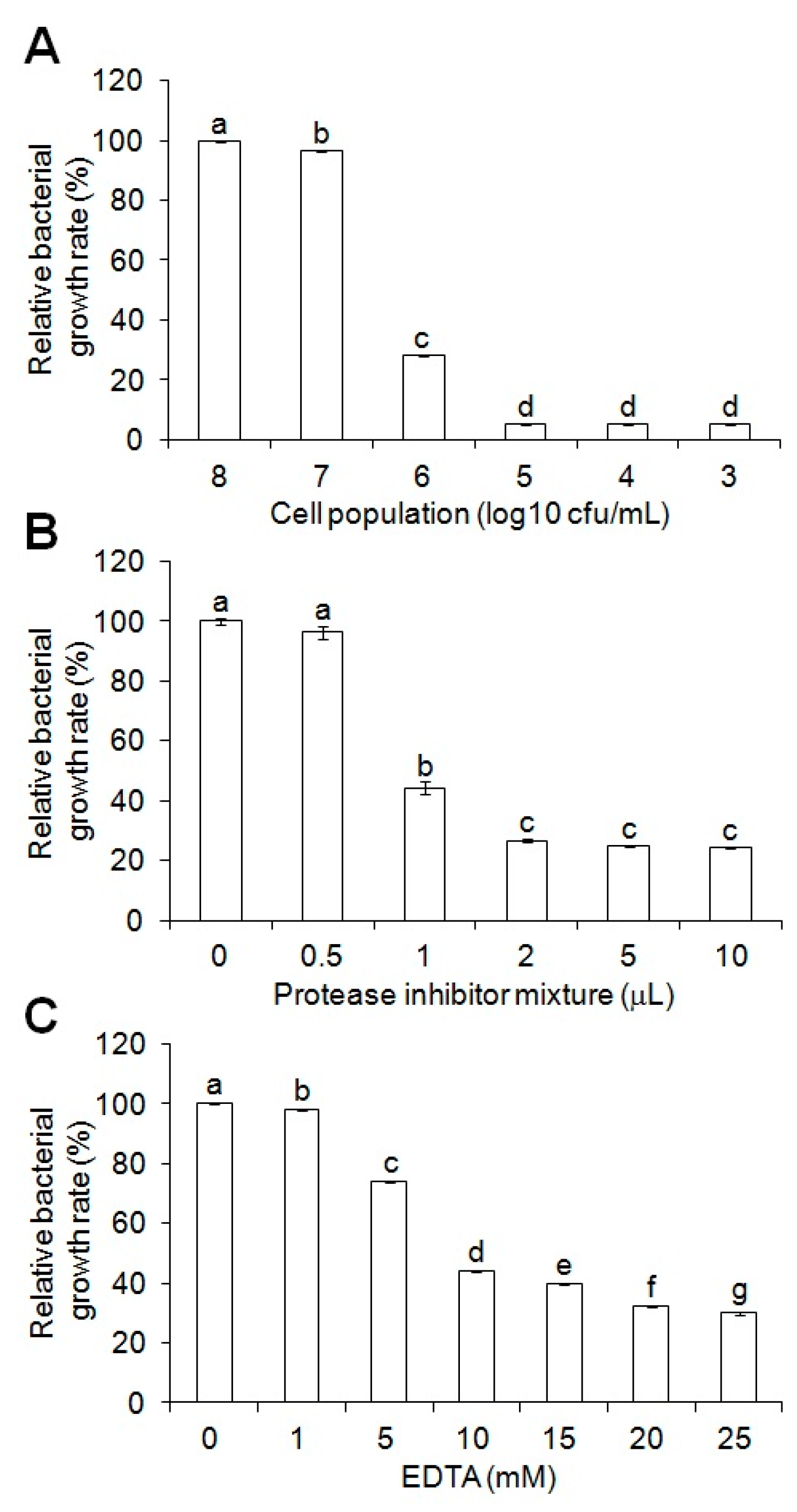
2.4. Anti-Salmonella Activity Modulation of the Cell-Free Supernatant Using Protease Inhibitors
3. Discussion
3.1. Protease Inhibitors Can Modulate the Anti-Salmonella Activity of MP-V1 through Avoidance of the Inoculum Effect
3.2. Efficient Production of Active MP-V1 Using the OmpA SS-Mediated E. coli Secretion System
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Analysis of Minimal Inhibitory Concentration (MIC)
5.3. Examination of Antimicrobial Activity Depending on Protease Inhibitor
5.4. Plasmid Construction for the Secretion of MP-V1 and Transformation into a General Host Strain
5.5. Antimicrobial Activity Analysis of Cell-Free Supernatant from the E. coli Secretion System
5.6. Scanning-Electron Microscope (SEM) Analysis
5.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dougan, G.; John, V.; Palmer, S.; Mastroeni, P. Immunity to salmonellosis. Immunol. Rev. 2011, 240, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Patro, S.; Purohit, S.; Jain, S.; Senapati, S.; Dey, N. Effective control of Salmonella infections by employing combinations of recombinant antimicrobial human beta-defensins hBD-1 and hBD-2. Antimicrob. Agents Chemother. 2014, 58, 6896–6903. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.-H.; Lee, B.; Kim, D.; Lee, J.-K.; Kim, S.; Bae, J.; Park, Y.; Lee, K. Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar Typhimurium. Biomaterials 2016, 104, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.; Rothwell, L.; Galyov, E.E.; Barrow, P.A.; Burnside, J.; Wigley, P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 2000, 146, 3217–3226. [Google Scholar] [CrossRef] [PubMed]
- McClelland, M.; Sanderson, K.E.; Spieth, J.; Clifton, S.W. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 2001, 413, 852. [Google Scholar] [CrossRef] [PubMed]
- Parkhill, J.; Dougan, G.; James, K.D.; Thomson, N.R. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 2001, 413, 848. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Bonomo, R.A. Overview: Global and local impact of antibiotic resistance. Infect. Dis. Clin. 2016, 30, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551. [Google Scholar] [CrossRef] [PubMed]
- Steckbeck, J.D.; Deslouches, B.; Montelaro, R.C. Antimicrobial peptides: New drugs for bad bugs? Expert Opin. Biol. Ther. 2014, 14, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Zhuang, Z.-J.; Lin, C.-Y.; Chen, W.-J. Novel antimicrobial peptides with promising activity against multidrug resistant Salmonella enterica serovar Choleraesuis and its stress response mechanism. J. Appl. Microbiol. 2016, 121, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, L.; Wang, Y. The antimicrobial peptide cathelicidin-BF could be a potential therapeutic for Salmonella typhimurium infection. Microbiol. Res. 2015, 171, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 603. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Xia, Y.; Liu, C. Research on Peptide Toxins with Antimicrobial Activities. Ann. Pharmacol. Pharm. 2016, 1, 1006. [Google Scholar]
- Ramirez-Carreto, S.; Jimenez-Vargas, J.M.; Rivas-Santiago, B.; Corzo, G.; Possani, L.D.; Becerril, B.; Ortiz, E. Peptides from the scorpion Vaejovis punctatus with broad antimicrobial activity. Peptides 2015, 73, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.J.A.; Estrela, A.B.; Nascimento, A.K.L.; Melo, M.M.A.; Torres-Rego, M.; Lima, E.O.; Rocha, H.A.O.; Carvalho, E.; Silva-Junior, A.A.; Fernandes-Pedrosa, M.F. Characterization of TistH, a multifunctional peptide from the scorpion Tityus stigmurus: Structure, cytotoxicity and antimicrobial activity. Toxicon 2016, 119, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.-P.S.; Hancock, R.E.W. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Hetru, C.; Letellier, L.; Ziv, O.; Hoffmann, J.A.; Yechiel, S. Androctonin, a hydrophilic disulphide-bridged non-haemolytic anti-microbial peptide: A plausible mode of action. Biochem. J. 2000, 345, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Oguiura, N.; Boni-Mitake, M.; Affonso, R.; Zhang, G. In Vitro antibacterial and hemolytic activities of crotamine, a small basic myotoxin from rattlesnake Crotalus durissus. J. Antibiot. 2011, 64, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Kao, P.-H.; Fu, Y.-S.; Lin, S.-R.; Chang, L.-S. Membrane-damaging activity of Taiwan cobra cardiotoxin 3 is responsible for its bactericidal activity. Toxicon 2011, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Guina, T.; Eugene, C.Y.; Wang, H.; Hackett, M.; Miller, S.I. A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to alpha-helical antimicrobial peptides. J. Bacteriol. 2000, 182, 4077–4086. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, K.L.; Crispell, E.K.; McBride, S.M. Antimicrobial peptide resistance mechanisms of gram-positive bacteria. Antibiotics 2014, 3, 461–492. [Google Scholar] [CrossRef] [PubMed]
- Band, V.I.; Weiss, D.S. Mechanisms of antimicrobial peptide resistance in Gram-negative bacteria. Antibiotics 2014, 4, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Bray, B.L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2003, 2, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Soundrarajan, N.; Cho, H.-S.; Ahn, B.; Choi, M.; Choi, H.; Cha, S.-Y.; Kim, J.-H.; Park, C.-K.; Seo, K.; Park, C. Green fluorescent protein as a scaffold for high efficiency production of functional bacteriotoxic proteins in Escherichia coli. Sci. Rep. 2016, 6, 20661. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Son, M.; Noh, E.-Y.; Kim, S.; Kim, C.; Yeo, J.-H.; Park, C.; Lee, K.W.; Bang, W.Y. MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities. Molecules 2016, 21, 512. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, M.; Parham, N.J.; Scott-Tucker, A.; Henderson, I.R. The general secretory pathway: A general misnomer? Trends Microbiol. 2004, 12, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Filloux, A. The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1694, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Abendroth, J.; Hol, W.G.; Sandkvist, M. Type II secretion: From structure to function. FEMS Microbiol. Lett. 2006, 255, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Karslake, J.; Maltas, J.; Brumm, P.; Wood, K.B. Population Density Modulates Drug Inhibition and Gives Rise to Potential Bistability of Treatment Outcomes for Bacterial Infections. PLoS Comput. Biol. 2016, 12, e1005098. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Smith, R.P.; Srimani, J.K.; Riccione, K.A.; Prasada, S.; Kuehn, M.; You, L. The inoculum effect and band-pass bacterial response to periodic antibiotic treatment. Mol. Syst. Biol. 2012, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Molla, M.N.; Cantor, C.R.; Collins, J.J. Bacterial charity work leads to population-wide resistance. Nature 2010, 467, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Hooper, N.M. Proteases: A primer. Essays Biochem. 2002, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Yamakawa, M. Production in Escherichia coli of moricin, a novel type antibacterial peptide from the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 1996, 220, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Piers, K.L.; Brown, M.H.; Hancock, R.E.W. Recombinant DNA procedures for producing small antimicrobial cationic peptides in bacteria. Gene 1993, 134, 7–13. [Google Scholar] [CrossRef]
- Xie, Y.-G.; Luan, C.; Zhang, H.-W.; Han, F.-F.; Feng, J.; Choi, Y.-J.; Groleau, D.; Wang, Y.-Z. Effects of thioredoxin: SUMO and intein on soluble fusion expression of an antimicrobial peptide OG2 in Escherichia coli. Protein Pept. Lett. 2013, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhang, F.; Liu, Z.; Ma, J.I.; Yang, J. Expression and characterization of cecropinXJ, a bioactive antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in Escherichia coli. Exp. Ther. Med. 2013, 5, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Akita, M.; Sasaki, S.; Matsuyama, S.-I.; Mizushima, S. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J. Biol. Chem. 1990, 265, 8164–8169. [Google Scholar] [PubMed]
- Hartl, F.-U.; Lecker, S.; Schiebel, E.; Hendrick, J.P.; Wickner, W. The binding cascade of SecB to SecA to SecYE mediates preprotein targeting to the E. coli plasma membrane. Cell 1990, 63, 269–279. [Google Scholar] [CrossRef]
- Lill, R.; Cunningham, K.; Brundage, L.A.; Ito, K.; Oliver, D.; Wickner, W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989, 8, 961. [Google Scholar] [PubMed]
- Lill, R.; Dowhan, W.; Wickner, W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell 1990, 60, 271–280. [Google Scholar] [CrossRef]
- Collier, D.N. SecB: A molecular chaperone of Escherichia coli protein secretion pathway. Adv. Protein Chem. 1993, 44, 151–193. [Google Scholar] [PubMed]
- Duong, F.; Wickner, W. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997, 16, 2756–2768. [Google Scholar] [CrossRef] [PubMed]
- Dalbey, R.E.; Wickner, W. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J. Biol. Chem. 1985, 260, 15925–15931. [Google Scholar] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]



| Strains or Plasmids | Genotypes or Phenotypes | Sources |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| Top10 | F-mcrA Δ(mrr-hsdRMS-mcrBC) F80lacZ ΔM15 ΔlacX74 recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Salmonella | ||
| HJL331 | Salmonella Typhimurium HJL331, Wild type, SmR (isolated from swine) | Chonbok National University, Korea |
| HJL462 | Salmonella Gallinarum HJL462, Wild type, NaR (isolated from chicken) | Chonbok National University, Korea |
| HJL390 | Salmonella Enteritidis HJL390, Wild type, CmR (isolated from swine) | Chonbok National University, Korea |
| Plasmids | ||
| T-vector | Cloning vector; pUCori AmpR | Promega |
| pMMP319 | A T-vector derivative harboring OmpA SS | This study This study |
| pMMP320 | A T-vector derivative harboring OmpA SS::MP-V1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.J.; Kim, S.W.; Lee, C.W.; Bae, C.-H.; Yeo, J.-H.; Kim, I.-S.; Gal, S.W.; Hur, J.; Jung, H.-K.; Kim, M.-J.; et al. Anti-Salmonella Activity Modulation of Mastoparan V1—A Wasp Venom Toxin—Using Protease Inhibitors, and Its Efficient Production via an Escherichia coli Secretion System. Toxins 2017, 9, 321. https://doi.org/10.3390/toxins9100321
Ha YJ, Kim SW, Lee CW, Bae C-H, Yeo J-H, Kim I-S, Gal SW, Hur J, Jung H-K, Kim M-J, et al. Anti-Salmonella Activity Modulation of Mastoparan V1—A Wasp Venom Toxin—Using Protease Inhibitors, and Its Efficient Production via an Escherichia coli Secretion System. Toxins. 2017; 9(10):321. https://doi.org/10.3390/toxins9100321
Chicago/Turabian StyleHa, Yeon Jo, Sam Woong Kim, Chae Won Lee, Chang-Hwan Bae, Joo-Hong Yeo, Il-Suk Kim, Sang Wan Gal, Jin Hur, Ho-Kyoung Jung, Min-Ju Kim, and et al. 2017. "Anti-Salmonella Activity Modulation of Mastoparan V1—A Wasp Venom Toxin—Using Protease Inhibitors, and Its Efficient Production via an Escherichia coli Secretion System" Toxins 9, no. 10: 321. https://doi.org/10.3390/toxins9100321






