2.1. Evolution of the SpoIISA Toxin in Bacilli
We found the presence of SpoIISA proteins to be restricted mostly to the Bacillus genus of the Firmicutes phylum (Firmicutes; Bacilli; Bacillales; Bacillaceae, Bacillus) (
Table S1). It is notable that there are many Bacillus species whose genomes appear to lack a SpoIISA homologue (
Table S1). Most of these are from unfinished genome projects, however (the genome assembly is presently in a form of individual contigs), and it is thus likely that SpoIISA proteins will be found in these genomes in the future. There are still a few species that are almost certainly missing SpoIISA.
Bacillus megaterium is one example: the complete genomes of multiple strains (
B. megaterium DSM 319; QM B1551; WSH-002) of this species (including 10 plasmids) are available [
9,
10], and none of them possess a SpoIISA protein (
Table S1). Either
B. megaterium inherited a SpoIISA protein from the common ancestor of the other SpoIISA-bearing species and subsequently lost it or this species split off from a bacilli subgroup before SpoIISA was obtained. The genomes of the alkaliphilic
B. pseudofirmus OF4 (one chromosome and two plasmids),
B. selenitireducens MLS10 (one chromosome) and
B. halodurans C-125 (one chromosome) all seem to be missing SpoIISA, as well [
11,
12,
13]. It is notable that
B. selenitireducens does not form spores. Interestingly, SpoIISA also appears in some species outside of the Bacillus genus and some even appear outside of the Bacillaceae family (
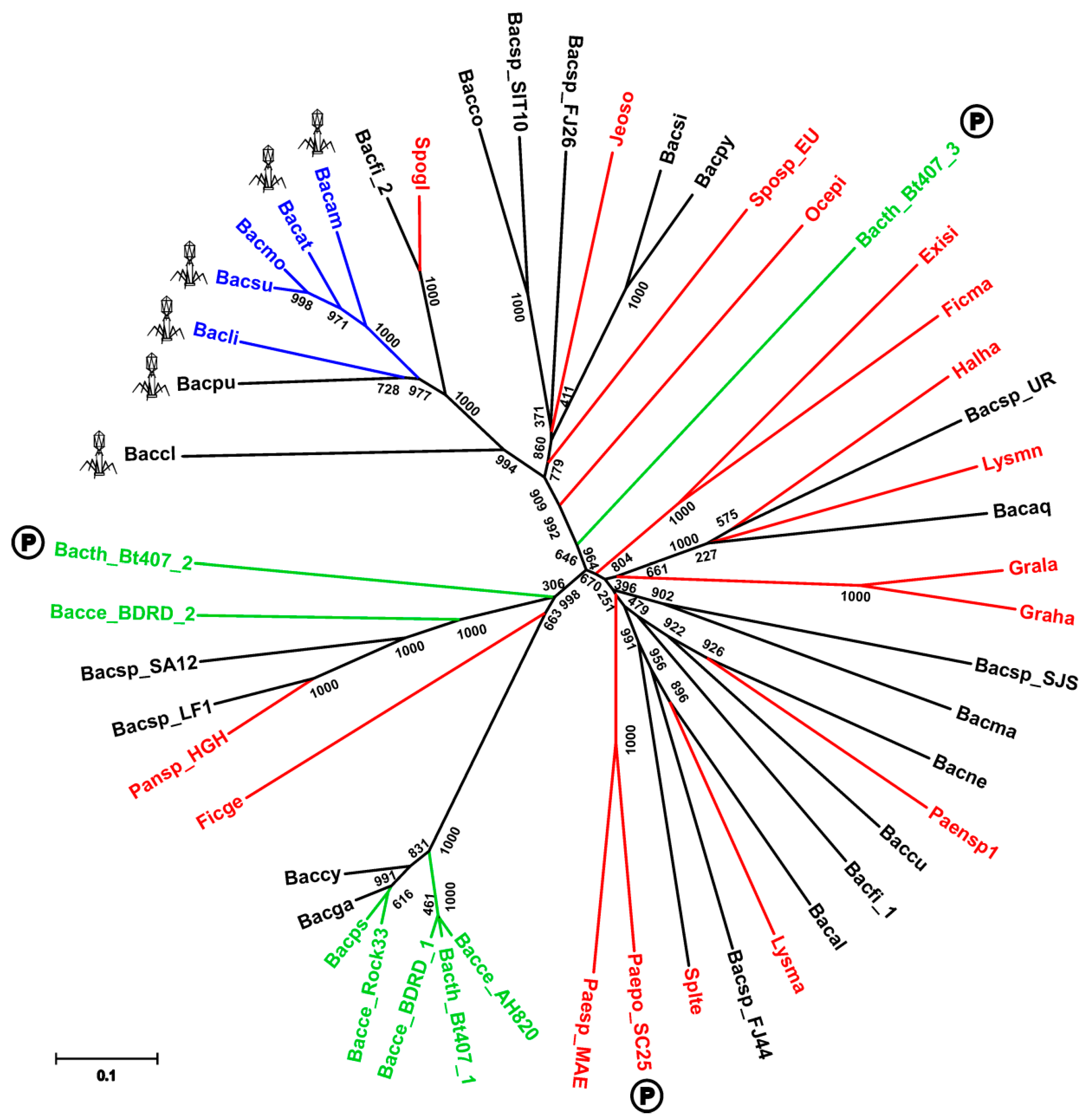
Table S1). The SpoIISA sequences from these non-bacillus species are quite dissimilar from each other, however, even if they come from closely related species (e.g.,
Lysinibacillus manganicus and
Lysinibacillus massiliensis), which is reflected by their failure to group together in the SpoIISA phylogenetic tree (
Figure 1).
Moreover, most of them are part of an accessory genome. For example, SpoIISA from
Paenibacillus polymyxa SC2 is positioned on the plasmid PPSC2_p0465. It is thus more likely that some, possibly even all, SpoIISA proteins from non-bacillus species were obtained through horizontal gene transfer, rather than from a common ancestor of bacillus and non-bacillus SpoIISA-bearing species. This is corroborated by the observation that the non-bacillus species do not form a single separate cluster in the phylogenetic tree, but are intermixed with sequences from bacillus species. Nevertheless, most SpoIISA proteins identified in this study come from species that are closely related to
B. cereus or
B. subtilis (
Table S1), and the SpoIISA homologues can be grouped based on whether they originate from
B. subtilis relatives or
B. cereus relatives. SpoIISA similarity within each group is quite high (more than 90% for species from the
B. cereus group and more than 80% for species from the more diverse
B. subtilis group), but inter-group similarity is considerably lower (e.g., the similarity between the SpoIISA proteins from
B. cereus E33L and from
B. subtilis strain 168 is 45%, and the identity is only 20%). The similarity between the SpoIISA proteins from these two groups is thus comparable to the similarity between those located on chromosomes and those on plasmids (e.g., the similarity between
B. cereus E33L and
Paenibacillus polymyxa SC2 is 45.3%, and the identity is 19.6%).
Exactly one gene coding for SpoIISA was found in every available genome for strains from the
B. subtilis and
B. cereus groups. Moreover, the
spoIISA gene was always found nearby well-conserved genes from the core genome, suggesting that in these species,
spoIISA genes behave similarly as those from a very stable part of the core genome. There are two notable exceptions to this, however:
B. cereus BDRD-ST196 possesses two SpoIISA proteins, one of which (NCBI-Protein ID: EEL06055) shows very high similarity (more than 95%) to SpoIISA proteins from other
B. cereus strains and is positioned well inside the conserved core region of the genome, while the other one (NCBI-Protein ID: EEL06141) is only weakly similar to SpoIISA proteins from other
B. cereus strains (about 30% identity and 45% similarity). The closest known relative to this second one is the SpoIISA protein (NCBI-Protein ID: WP_016427205) from
Paenisporosarcina sp. HGH0030 (54% identity and 71% similarity). The second species with multiple SpoIISA proteins is
B. thuringiensis Bt407, which contains three SpoIISA proteins. Again, one protein (NCBI-Protein ID: EEM28840) is very similar (more than 95%) to SpoIISA from closely-related strains and species, while the other two (NCBI-Protein ID: EEM25308 and EEM24943) differ considerably (less than 30% identity and less than 55% similarity to SpoIISA proteins from closely-related strains and species). The closest homologue of EEM25308 is again SpoIISA from
Paenisporosarcina sp. HGH0030 (35% identity and 54% similarity), though they show only weak mutual similarity. The closest homologue of EEM24943 is SpoIISA from
B. simplex (NCBI-Protein ID: CEG32548), but again, their mutual similarity is quite low (29% identity and 55% similarity). Interestingly, this protein is more similar to the SpoIISA proteins from the
B. subtilis group (the identity to
B. amyloliquefaciens LFB112 is 26%, similarity 50%) than to those from the
B. cereus group (the identity to
B. cereus E33L is 19%, similarity 46%) (
Figure 1). Not only do EEM25308 and EEM24943 show low similarity to proteins from other species from the
B. cereus group, but the proteins encoded by their neighboring genes are also dissimilar, making it probable that these genes are part of a flexible genome, which arose from horizontal gene transfer. It was recently reported that EEM24943 (locus: AFV22040; locus tag: BTB_502p07350) and EEM25308 (locus: AFV21663; locus tag: BTB_502p03580) are both positioned on plasmid BTB_502p [
14]. The role that these multiple SpoIIS modules play in a cell is presently unknown, and many outstanding questions remain: Are all of these genes expressed or are the dissimilar ones nonfunctional pseudogenes? Are they all expressed during the same period in the cell cycle? In the same cell compartment? Do they interact with each other?
An amino acid sequence comparison of the functionally-characterized SpoIISA proteins from
B. subtilis PY79 (NCBI-Protein ID: AHA77284) and
B. cereus ATCC 14579 (NCBI-Protein ID: AAP09399) shows that these two proteins contain 41 identical and 88 similar residues. The similarity between the SpoIISA proteins from
B. subtilis PY79 and
Lysinibacillus massiliensis (NCBI-Protein ID: KGR89544) is even higher (
Figure 2), with 45 identical and 94 similar residues. Since a multiple sequence alignment of all of the SpoIISA proteins in our set showed that only a very few positions have identical or similar residues (
Figure S1), it seems likely that the high level of similarity observed by comparing only these two SpoIISA proteins is not due to purifying selection, but is more likely a remnant of ancestral similarity. This assumes, of course, that all aligned sequences are from functional proteins. Since the presence of nonfunctional SpoIISA proteins could mask functionally-important positions, we aligned only those protein sequences containing the conserved, functionally-important arginine (Arg
38 in
B. subtilis) and the three
N-terminal transmembrane helices, which were shown to be indispensable for SpoIISA functionality [
5,
15]. It is likely, however, that some essential features of SpoIISA are still undiscovered, and thus, we cannot be sure that all nonfunctional proteins were excluded from our final alignment. Comparison of the SpoIISA proteins from 56 different bacterial species revealed only four positions containing invariant residues. According to the
B. subtilis numbering, they are Arg
38, Tyr
42, Asp
78 and Asp
225; eight positions possessed similar residues: Ile
37, Lys
39, Phe
82, Lys
89, Ile
143, Leu
186, Val
221 and Leu
232 (
Figure S1). Most of these conserved residues seem to be located in the second and third transmembrane regions and in the
C-terminal helix. Arg
38, found near the beginning of the second transmembrane region, is important for oligomerization [
15], and its substitution by glutamine results in loss of toxicity [
5]. The function of the other conserved residues identified is presently unknown. The conserved aspartate (Asp
78) in the third transmembrane region (
Figure S1) may have an important functional role. It has been shown that an aspartate found in the transmembrane region of some receptors and transporters plays an important role in their operation [
16,
17,
18,
19]. Interestingly, a conserved aspartate (Asp
225) is also present in the SpoIISA
C-terminal α-helix. Some transmembrane prediction tools suggest that this helix is located in the membrane, but others do not [
15]. Structural studies also failed to confirm the transmembrane localization of this helix, but its temporary presence in the membrane still cannot be ruled out [
7]. The two conserved aspartates could play a role in SpoIISA assembly or multimerization, or they could be involved in some other process. Further functional and structural studies of the SpoIISA protein with substituted conserved aspartates could throw more light on their function.
2.2. Evolution of the SpoIISB and SpoIISC Antitoxins in Bacilli
The sequence similarity of the SpoIISB and SpoIISC proteins is relatively high within the
B. subtilis and
B. cereus groups, but quickly decreases with growing evolutionary distance outside of these groups. Consequently, only SpoIISB and SpoIISC from the
B. subtilis and
B. cereus groups could be reliably identified and only sequences from these two groups were used in our study. We found SpoIISB proteins (hereafter SpoIISB
sub) in all
B. subtilis strains examined (
Table S3), and the identity of SpoIISB
sub between all of these strains is more than 96%. The identity of SpoIISB
sub from
B. subtilis to its homologues from closely-related species from the
B. subtilis group (
B. vallismortis,
B. tequilensis,
B. sp. MSP13,
B. mojavensis and various
B. atrophaeus strains) is also quite high, above 94%, with nearly all of the differences occurring in the first loop of the
N-terminal part, which has been shown to be unnecessary for antitoxin activity [
7]. Moving to more distantly-related species, SpoIISB
sub identity drops to 85% for various strains of
B. amyloliquefaciens and is around 70%–75% for SpoIISB
sub from
B. pumilus,
B. altitudinis,
B. xiamenensis,
B. sp. DW5-4,
B. sp. NSP9.1,
B. safensis,
B. sp. WP8,
B. sp. BT1B_CT2,
B. sonorensis and
B. licheniformis. The
N-terminal part again seems to harbor the substitutions. The shared, conserved regions could be identified by comparing the SpoIISB
sub proteins from the most distantly-related species (the identity of SpoIISB
sub from
B. subtilis strain 168 to
B. firmus and
B. sp. J33 is 57%–59%). The most highly-conserved regions correspond approximately to the SpoIISB
sub secondary structure elements (
Figure 3). Two of the most highly-conserved regions have been shown to be functionally important for antitoxin activity [
7]. The first of these is β-strand β1′, whose three lysines are nearly invariant. This strand forms a two-stranded intermolecular antiparallel β-sheet with strand β1 of SpoIISA, and deletion of this part abolishes SpoIISB
sub antitoxin activity [
7]. The second is a positively-charged
C-terminal region comprising 2–4 tandem lysine or arginine residues (in
B. subtilis K
52RKK
55). Although none of these four residues is invariant, they are always substituted with other hydrophilic residues (
Figure 3). The interaction of this region with SpoIISA could not be inferred from the SpoIISAB complex structure, since it was not resolved, but its importance was clearly demonstrated by a deletion experiment: deletion of the last four
C-terminal SpoIISB
sub residues obliterates the protein’s antitoxin activity [
7]. Other conserved regions, whose function has not presently been identified, include β-strand β2′ and α-helix α1′ (the
N-terminal edge of this helix is the most highly conserved region of the whole protein).
The identity of the SpoIISC proteins (hereafter SpoIISC
sub) between the various
B. subtilis strains is more than 93%. The identity of
B. subtilis SpoIISC
sub to its homologues from closely-related species (
B. vallismortis,
B. tequilensis,
B. sp. MSP13,
B. mojavensis) is comparable, from 90%–97%. The identity to its homologues from various
B. atrophaeus strains, which is also a close relative, is lower, around 90%, but almost all substitutions are for residues that are functionally similar (96% positives). Moving to more distant relatives, the
B. subtilis/
B. amyloliquefaciens identity is 87%, with most substitutions concentrated in the
C-terminal half of the protein, the region corresponding to the middle part of the α1′-helix in SpoIISB
sub. The identity drops to 75% for SpoIISC
sub homologues from
B. licheniformis WX-02 and
B. sp. CPSM8, and the substitutions are now distributed equally throughout the protein (
Figure 3). The identity of
B. subtilis SpoIISC
sub to its homologues from the more distantly-related members of the
B. subtilis group (
B. pumilus,
B. sp. M 2–6,
B.
altitudinis 41KF2b,
B. xiamenensis) is 68%, and the identity to the most distant SpoIISC
sub homologs (from
B. firmus and
B. sp. J33) is 55%–60%. Since the identities of the SpoIISC
sub proteins from various species of the
B. subtilis group do not differ much from the identities observed for the SpoIISB
sub proteins, it seems likely that both the SpoIISB
sub and SpoIISC
sub proteins from all of these organisms were inherited vertically and not obtained through horizontal gene transfer. In contrast to SpoIISB
sub, only a few SpoIISC
sub residues are invariant. Two conserved lysines are present at the
N-terminus (K
2K
3 in
B. subtilis BSn5), and a conserved serine and tyrosine appear in the central part of the protein (S
19XY
21). A
C-terminal stretch of 3–4 positively-charged residues (Lys or Arg) is also well conserved (
Figure 3).
SpoIISB
sub proteins show only limited similarity to SpoIISC
sub proteins. For example, the SpoIISB
sub and SpoIISC
sub proteins from
B. subtilis strain 168 are only 14.3% identical (39.3% similar). They also differ in length: SpoIISB
sub from the
B. subtilis 168 strain is 56 residues long, while its SpoIISC
sub is only 45 residues. Since the exact position of the start and stop codons was not identified, however, the real proteins could be slightly longer or shorter than this. SpoIISB
sub and SpoIISC
sub do share two stretches of positively-charged residues near their
C- and
N-terminal ends (according to
B. subtilis strain 168 numbering, the conserved, shared residues are K
15XXKIL(LV)KK
22 at the
N-terminus and K
52R(K)KK
55 at the
C-terminus). The central parts of these proteins are also well conserved (Y
31XV(L)
SXH(Y)T(S)X
RI(V)
40), with two invariant residues, a serine (Ser
34) and an arginine (Arg
39) (
Figure 3). However, comparing SpoIISB
sub and SpoIISC
sub from multiple species reveals that only the first three of the four
N-terminal positively-charged residues are conserved (according to
B. subtilis strain 168 numbering K
15X
varKXXK). Even more strikingly, only one of the conserved positively-charged residues at the
C-terminus (K
53) is completely conserved across all proteins (
Figure 3). As noted, deletion of the last four SpoIISB
sub residues considerably decreased sporulation efficiency. It is thus possible that it was the deletion of just this universally-conserved residue that was responsible for this effect. Measuring the sporulation efficiency of a point mutant in this lysine would be needed to corroborate this hypothesis, however. Of the two remaining residues common to both SpoIISB
sub and SpoIISC
sub, Ser
34 was conserved in all studied SpoIISB
sub and SpoIISC
sub proteins, but Arg
39 was not; the significance of this is presently unclear. Finally, for whatever reason, conservation of the SpoIISC
sub proteins is more relaxed than the conservation of the SpoIISB
sub proteins. This is most clearly seen by comparing the SpoIISB
sub and SpoIISC
sub proteins from
B. subtilis and
B. pumilus. The similarity between the SpoIISB
sub proteins from the two species is 81.4% (65.1% identity), and the similarity between the SpoIISC
sub proteins is only 58.1% (32.6% identity).
Since
spoIISBsub and
spoIISCsub are positioned adjacently on the chromosome, exhibiting sequence similarity, it is likely that they are the result of a gene duplication event. Further evidence for this comes from the recent report that both proteins have similar functions [
8]: they are both able to interact with and neutralize the toxicity of SpoIISA. An earlier report [
5] showed that a sporulation defect was still observed when only
spoIISBsub (and not
spoIISCsub) was inactivated [
5]. The most likely reason for the earlier observation was that the insertion of the tetracycline resistance cassette into codon 17 of
spoIISBsub might have disrupted a potential
spoIISCsub promoter positioned inside the
spoIISBsub gene. The SpoIISC protein also did not compensate for
mut14, which deactivated
spoIISBsub, but
mut14 is a frameshift mutation caused by the deletion of 2 bp after codon 52 of
spoIISBsub [
5], and this mutation would also be expected to affect a
spoIISCsub promotor or regulatory region. Alternatively,
spoIISCsub might have been deactivated by its own, independent mutation, a possibility that was not explored in the original report [
5]. A final possibility is that SpoIISC
sub is expressed at a different stage of the cell cycle and therefore would not have been available to compensate for the loss of SpoIISB
sub. As noted earlier, different gene expression profiles could be one reason why there are two
spoIIS antitoxin genes present. Generally, after gene duplication, one of the copies becomes redundant and usually goes through a pseudogenization process in which it neutrally accepts nonsynonymous or even deleterious mutations and subsequently becomes nonfunctional or is lost. It may also obtain a new function, different from that of the other copy or the copies may also undergo a division of labor, with each copy retaining a different sub-function of the original, ancestral function. We thus searched for differences between the amino acid sequences of SpoIISB
sub and SpoIISC
sub, which could indicate either a division of labor or the gain of a new function. We found one position, conserved in both proteins, that contains dissimilar residues. A polar residue (arginine, lysine or glutamine; Arg
23 in
B. subtilis) in SpoIISB
sub is replaced by a nonpolar residue (valine or leucine) in SpoIISC
sub. The implication of this change remains to be investigated, but it could make SpoIISC
sub bind less (
Figure 4). The alignment in
Figure 4 shows that the SpoIISB
sub proteins contain more (twelve) conserved, non-shared positions than SpoIISC
sub (only two). Since these proteins have no reported function other than inhibiting SpoIISA, it is difficult to imagine how an ancestral function could be subdivided between them. It is possible (although it has not yet been investigated) that they differ in binding strength to SpoIISA and that this difference could explain why SpoIISB
sub contains more conserved positions than SpoIISC
sub. For example, SpoIISB
sub might contain more conserved positions than SpoIISC
sub because more of its residues are involved in SpoIISA interactions, resulting in stronger binding. It is not clear what advantage the presence of two inhibitors of different binding strengths would give to a cell, suggesting that SpoIISC
sub might have an additional function. The most likely possibility is that SpoIISC
sub (or SpoIISB
sub) when bound to SpoIISA can regulate the expression of the
spoIIS operon, making it serve as the third regulatory component of a three-component TA system [
20,
21,
22]. However, the SpoIIS cluster, with its two antitoxin proteins, would differ from other known three-component systems, which are formed by a toxin, an antitoxin and a regulatory protein, in that no regulatory proteins have yet been found that also exhibit antitoxin capabilities. On the contrary, it has been shown that PasC, the third component of the plasmid addiction system from
Thiobacillus ferrooxidans plasmid pTF-FC2, is not capable of neutralizing the toxicity of the PasB toxin on its own [
20].
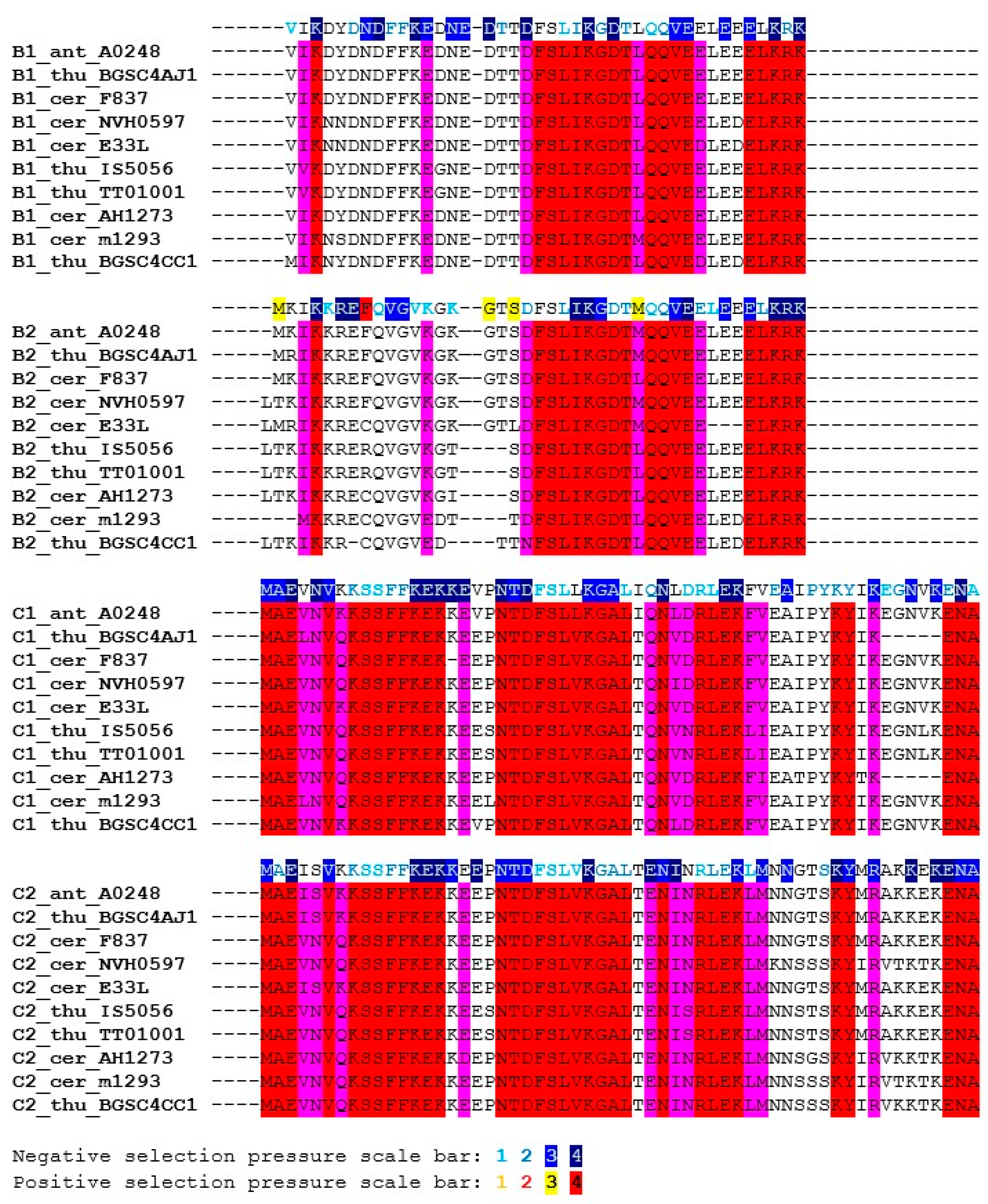
SpoIISB
cer proteins are present in all members of the
B. cereus group. Some
B. cereus strains possess two SpoIISB
cer (SpoIISB1
cer, SpoIISB2
cer,) and two SpoIISC
cer (SpoIISC1
cer, SpoIISC2
cer) proteins. The differences between them will be explored in
Section 2.3 below. The identity between SpoIISB
cer from various
B. cereus strains and from closely-related species like
B. anthracis and
B. thuringiensis is more than 80% (similarity is more than 90%). The identity between SpoIISB
cer proteins from more distantly-related members of the
B. cereus group (e.g., between
B. cereus E33L and
B. mycoides Rock1-4 SpoIIB
cer) is more than 70% (the similarity is more than 85%). The most conserved part is an eight-residue central region, including residues D
19FSLIKGD
26 (
B. cereus m1293 numbering) and the
C-terminal region. The
N-terminal region, except for the highly-conserved K
3 and a F
9FK
11 motif, is less conserved (
Figure 5). Similarly, SpoIISC
cer proteins are present in all members of the
B. cereus group. The identity between SpoIISC
cer from various
B. cereus strains and from closely-related species like
B. anthracis and
B. thuringiensis is more than 80% (similarity is more than 90%). The identity between SpoIISC
cer proteins from more distantly-related members of the
B. cereus group (e.g., between
B. cereus E33L and
B. mycoides Rock1-4 SpoIISC
cer) is more than 60% (the similarity is more than 80%). The SpoIISC
cer proteins are all about 16 residues longer than their corresponding SpoIISB
cer proteins (e.g.,
B. cereus m1293 SpoIISB
cer is 41 residues long, while its SpoIISC
cer is 58 residues). The conservation pattern in SpoIISC
cer is very similar to that of SpoIISB
cer: the lower similarity at the
N-terminus and the higher similarity at the
C-terminus. Both SpoIISB
cer and SpoIISC
cer proteins have an
N-terminal polar residue (K
3 in
B. cereus m1293), an FFK motif (F
9FK
11), a DFSLI(V)KG motif (D
19FSLIKG
25) and a conserved
C-terminal region. The SpoIISC
cer proteins contain an additional 12 residues at the
C-terminus (
Figure 5), but this region is not especially well conserved. Like their
B. subtilis counterparts, the SpoIISC
cer proteins are less tightly conserved than the corresponding SpoIISB
cer proteins. For example, the similarity between the SpoIISB
cer proteins from
B. cereus m1293 and
B. cereus AH620 is 97.6% (87.8% identity), while the similarity between the corresponding SpoIISC
cer proteins is 89.7% (84.5% identity). Since the genes coding for SpoIISB
cer and SpoIISC
cer proteins are positioned next to each other on the chromosome and show a high degree of sequence similarity, it is likely that they are the result of a gene duplication event. A search for signs of either division of labor or gain of function yielded three interesting positions. In SpoIISB
cer, a conserved aspartate (Asp
26 in
B. cereus m1293) appears after the DFSLI(V)KG motif, but in SpoIISC
cer, an alanine is found instead (
Figure 6). The following residue in SpoIISB
cer, a conserved threonine (Thr
27 in
B. cereus m1293), is replaced by leucine in SpoIISC
cer. Finally, a negatively-charged glutamate appears 10 positions downstream of this threonine in SpoIISB
cer (Glu
37 in
B. cereus m1293), but is replaced by the aromatic phenylalanine (or the aliphatic leucine) in SpoIISC
cer (
Figure 6). We also examined the selection pressure acting upon the SpoIISB
cer and SpoIISC
cer proteins, but no significant differences could be inferred (
Figure 5 and
Figure 6). Only a weak negative selection signal was detected in the highly-conserved central region. The extremely high level of conservation in this region, which is almost invariant even at the DNA level, provides only a low level of statistical support for negative selection, which is the most likely reason for this weak signal.
2.3. Evolution of the SpoIIS Module
It might be speculated that SpoIISA acts as a holin, forming a pore in the cytoplasmic membrane through which some lytic protein, e.g., an amidase, is released to lyse the cell wall peptidoglycan. Intriguingly, the
B. subtilis spoIIS cluster partially overlaps the
N-acetylmuramoyl-
l-alanine amidase (XlyA) of prophage PBSX (although in the opposite direction). Inspection of the gene arrangement in various species reveals, however, that the
spoIIS cluster is positioned near the PBSX prophage only in species from the
B. subtilis group and that this connection is missing in the more distantly-related
spoIIS cluster-bearing species. Although an evolutionary relationship, or some past or present interaction, between the
spoIIS cluster and the PBSX prophage cannot be ruled out, expression of the
spoIIS cluster from
B. subtilis and
B. cereus in
E. coli cells shows that SpoIISA is able to induce cell lysis without the help of XlyA or any other prophage protein [
6].
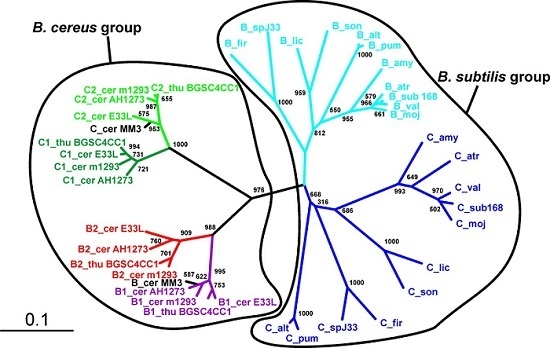
The overall similarity between SpoIISB and SpoIISC from species of
B. cereus and
B. subtilis groups is very low, with no invariant residues and only three positions containing relatively similar residues (
Figure 7). Thus, they either shared a common ancestor and afterwards evolved beyond recognition or they originated independently. Common ancestry seems the more probable explanation, since it is hard to imagine that so potent of a toxin as SpoIISA could exist in a viable cell without an inhibitor. A given SpoIISB or SpoIISC protein from a species belonging to one of the two groups is more similar to another SpoIISB or SpoIISC (either one) from the same group than it is to its corresponding orthologue from a species from the second group. This can be most clearly seen in the phylogenetic tree of SpoIISB and SpoIISC (
Figure 8) where the SpoIIB
sub and SpoIISC
sub proteins from the
B. subtilis group are positioned on a common branch, which is isolated from the branch occupied by the SpoIISB
cer and SpoIISC
cer proteins from the
B. cereus group. It thus seems very likely that the SpoIISB and SpoIISC proteins are the result of two independent gene duplication events, which took place after the
B. cereus and
B. subtilis groups separated (
Figure 9, upper part). Although the SpoIISB and SpoIISC proteins in a given organism are clearly paralogues, because the gene duplication events appear to have happened after the
B. subtilis and
B. cereus groups separated, the SpoIISB and SpoIISC proteins from species belonging to different groups are not clearly orthologues. Interestingly, species (and strains) from the
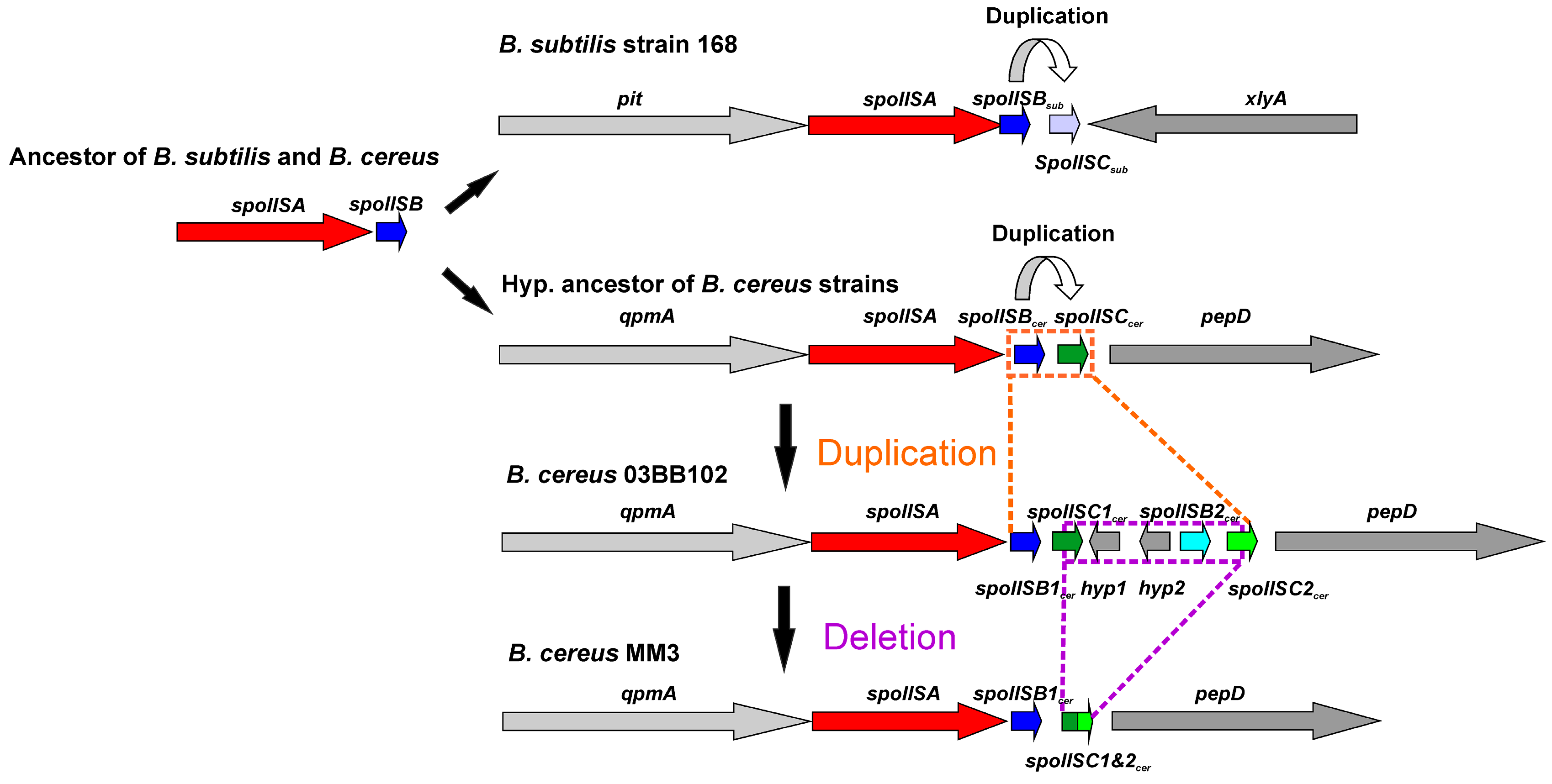
B. cereus group have two different architectures for the SpoIIS module. There is a shorter one, consisting of the
spoIISA gene followed by the
spoIISBcer and
spoIISCcer genes, and a longer one comprising the
spoIISA gene followed by the
spoIISB1cer and
spoIISC1cer genes, then two genes for unknown hypothetical proteins and, finally, the
spoIISB2cer and
spoIISC2cer genes. Although one might expect that the longer form is the result of duplicating the shorter form, the opposite seems to be true. As the phylogenetic tree (
Figure 8) shows, SpoIISB
cer from the short form corresponds to SpoIISB1
cer from the long form, while SpoIISC
cer from the short form corresponds to SpoIISC2
cer (or at least the
C-terminal part of it) from the long form. The most parsimonious explanation for these observations is that the middle part of the longer form was deleted (
Figure 9, lower part). Since the intergenic regions between
spoIISB1cer and
spoIISC1cer and
spoIISB2cer and
spoIISC2cer, as well as the
N-terminal parts of
spoIISC1cer and
spoIISC2cer are identical in the longer form, it is difficult to determine the exact position of the cleavage site. If the cleavage site was positioned inside the intergenic region, the deleted part would comprise the entirety of the
spoIISC1cer and
spoIISB2cer genes, leaving the complete sequences of the
spoIISB1cer and
spoIISC2cer genes in the short form. Alternatively, if the cleavage site was positioned inside
spoIISC1cer, then the
C-terminal part of
spoIISC1cer, all of
spoIISB2cer and the
N-terminal part of
spoIISC2cer would be deleted.
SpoIISBcer of the short form would then correspond to
spoIISB1cer, and
spoIISCcer would have been formed by the fusion of the
C-terminal part of
spoIISC1cer and the
N-terminal part of
spoIISC2cer from the longer form (
Figure 9). Notably, we found signs of nonfunctionalization in the
spoIISC1cer and
spoIISB1cer genes in some strains of
B. cereus, including deletions, nonsense mutations and reading-frame shifts. It is thus possible that these two genes were either pseudogenes (or were on their way to becoming ones) even before they were deleted. The high mutual sequence similarity between the
spoIISB1cer/
spoIISC1cer and
spoIISB2cer/
spoIISC2cer pairs and the presence of pseudogenes in the middle part of this region thus poised it for deletion.