Biodistribution and Lymphatic Tracking of the Main Neurotoxin of Micrurus fulvius Venom by Molecular Imaging
Abstract
:1. Introduction
2. Results
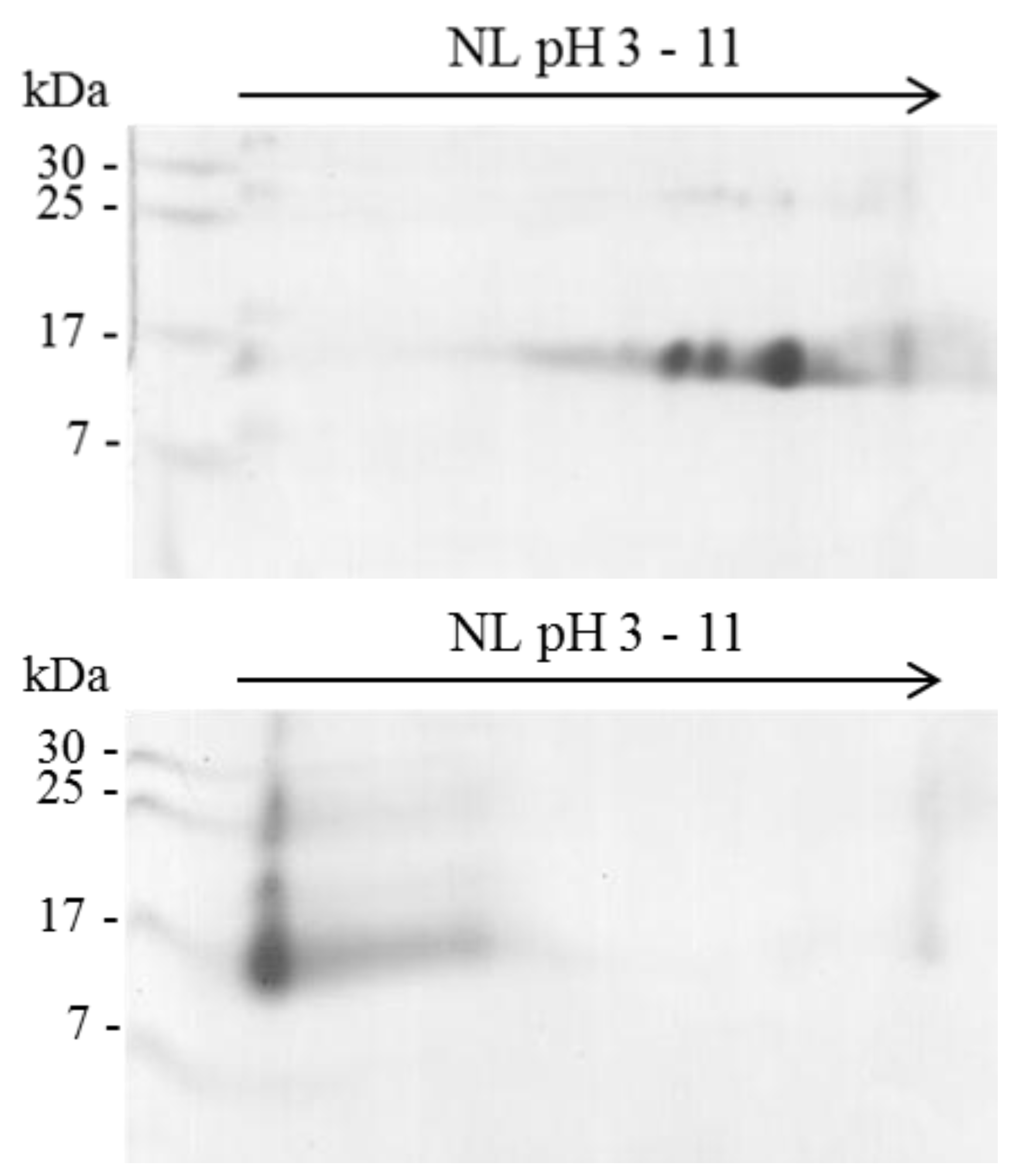
2.1. Isoelectric Point (pI) of β-NTx
2.2. Conjugation Efficiency
2.3. Changes in Biological Activities of B-NTx
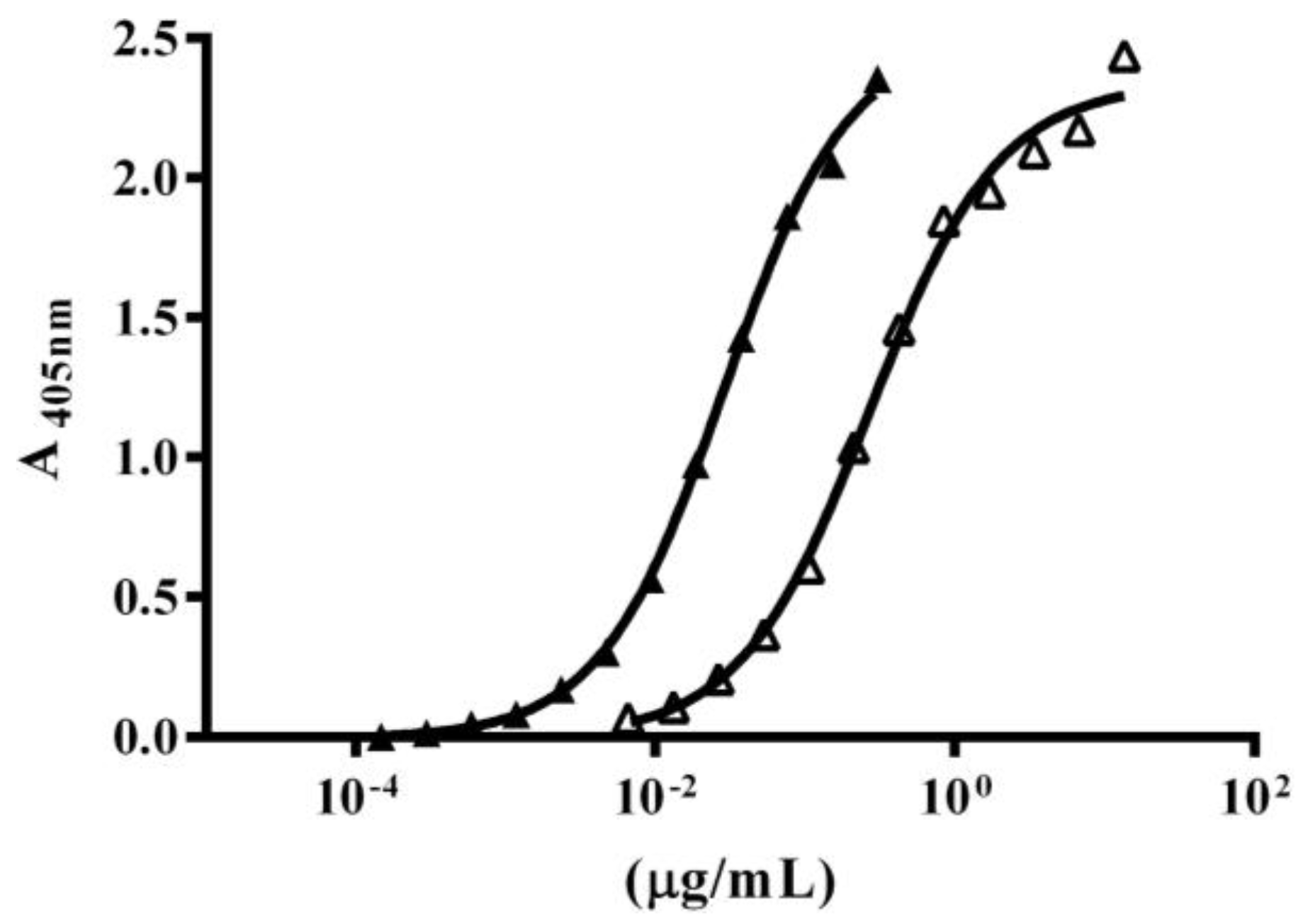
2.4. Immuno-Recognition of β-NTx-DTPA
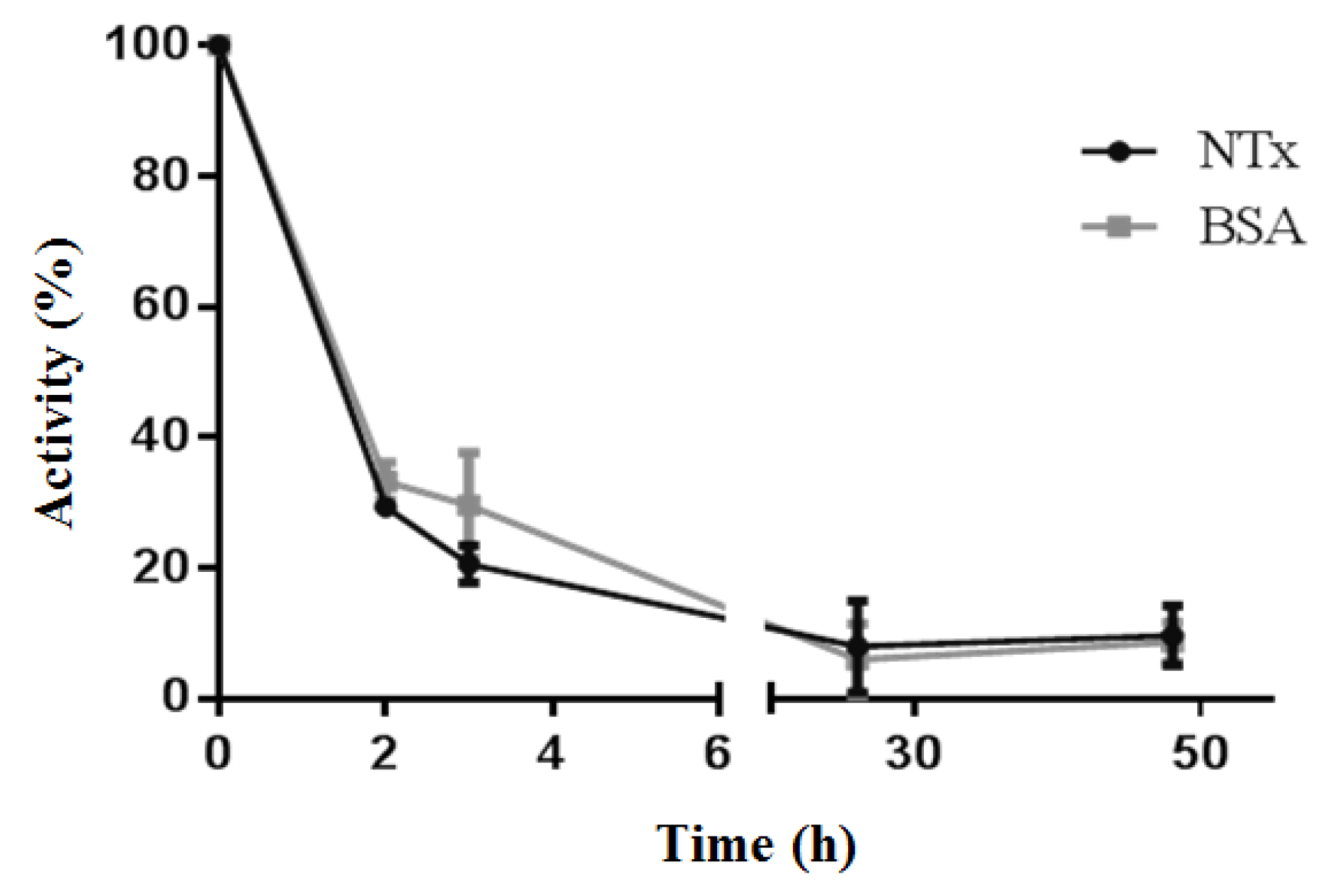
2.5. Radiolabeling Efficiency and Stability
2.6. β-NTx-DTPA-67Ga Disappearance from Injection Site
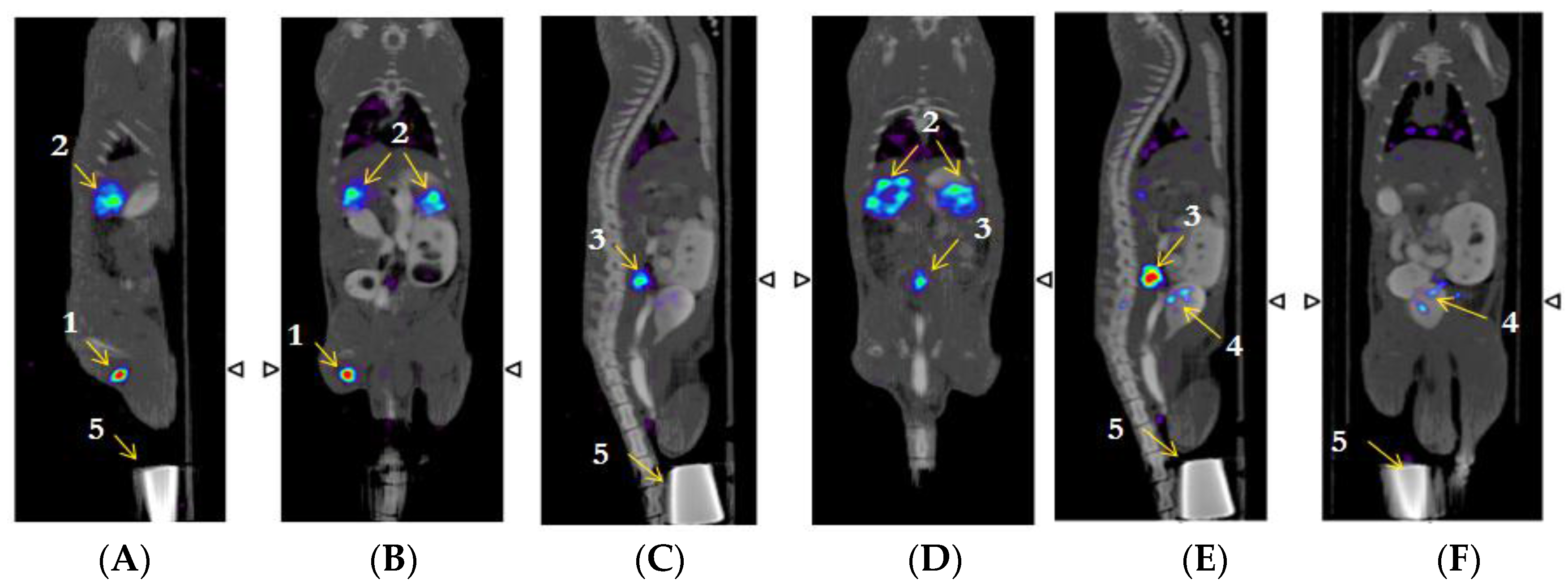
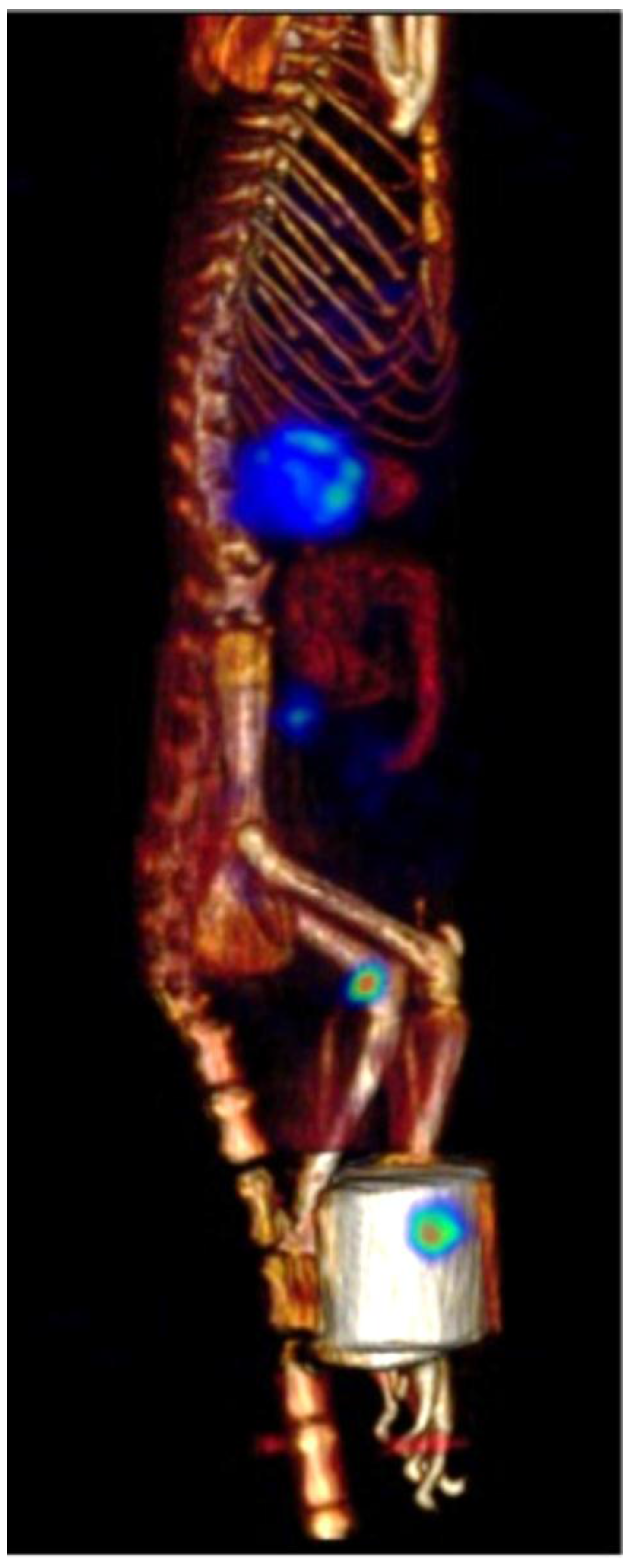
2.7. β-NTx-DTPA-67Ga Biodistribution by Molecular Imaging
3. Discussion
4. Experimental Section
4.1. Reagents and Venom
4.2. Animals
4.3. Bioconjugation of β-NTx or BSA with DTPA Chelator
4.4. Bidimensional Electrophoresis (2D-PAGE)
4.5. Atomic Absorption Spectroscopy
4.6. Biological Assays
4.7. Radiolabeling
4.8. Imaging Studies
4.9. Biodistribution
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roze, J.A. Coral Snakes of the Americas: Biology, Identification, and Venoms; Krieger Publishing Company: Malabar, FL, USA, 1996. [Google Scholar]
- Kitchens, C.S.; Van Mierop, L.H.S. Envenomation by the eastern coral snake (Micrurus fulvius fulvius): A study of 39 victims. JAMA 1987, 258, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Schauben, J.; Thundiyil, J.; Kunisaki, T.; Sollee, D.; Lewis-Younger, C.; Bernsteine, J.; Weismane, R. Review of Eastern coral snake (Micrurus fulvius fulvius) exposures managed by the Florida Poison Information Center Network: 1998–2010. Clin. Toxicol. (Phila.) 2013, 51, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Vergara, I.; Pedraza-Escalona, M.; Paniagua, D.; Restano-Cassulini, R.; Zamudio, F.; Batista, C.V.; Possani, L.D.; Alagón, A. Eastern coral snake Micrurus fulvius venom toxicity in mice is mainly determined by neurotoxic phospholipases A2. J. Proteomics 2014, 105, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.F.; Jacobsen, B. Subcutaneous absorption of biotherapeutics: Knowns and unknowns. Drug Metab. Dispos. 2014, 42, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.H.; Charman, S. Lymphatic transport of proteins after subcutaneous administration. J. Pharm. Sci. 2000, 89, 297–310. [Google Scholar] [CrossRef]
- Supersaxo, A.; Hein, W.R.; Steffen, H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 1990, 7, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, D.; Jiménez, L.; Romero, C.; Vergara, I.; Calderón, A.; Benard, M.; Bernas, M.J.; Rilo, H.; de Roodt, A.; D´Suze, G.; et al. Lymphatic route of transport and pharmacokinetics of Micrurus fulvius (coral snake) venom in sheep. Lymphology 2012, 45, 144–153. [Google Scholar] [PubMed]
- Franc, B.; Acton, P.; Mari, C.; Hasegawa, B. Small-animal SPECT and SPECT/CT: Important tools for preclinical investigation. J. Nucl. Med. 2008, 49, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, A.; Saravis, C.A.; Kassis, A.I. Semipreparative isoelectrophoresis of DTPA-conjugated antibody leads to isolation of antibody isoforms with different immunoreactivities. J. Nuclear Biol. Med. (Turin, Italy: 1991) 1994, 38, 61–67. [Google Scholar]
- Lee, H.J.; Pardridge, W.M. Monoclonal antibody radiopharmaceuticals: Cationization, pegylation, radiometal chelation, pharmacokinetics, and tumor imaging. Bioconjug. Chem. 2003, 14, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Raubitschek, A.; Shively, J.E. A facile, water-soluble method for modification of proteins with DOTA. Use of elevated temperature and optimized pH to achieve high specific activity and high chelate stability in radiolabeled immunoconjugates. Bioconjug. Chem. 1994, 5, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Rivière, G.; Choumet, V.; Saliou, B.; Debray, M.; Bon, C. Absorption and elimination of viper venom after antivenom administration. J.Pharmacol. Exp. Ther. 1998, 285, 490–495. [Google Scholar] [PubMed]
- Pujatti, P.B.; Simal, C.J.R.; Santos, R.G.D. Preparation of crotalus venom radiolabeled with technetium-99m as a tool for biodistribution study. Braz. Arch. Biol. Technol. 2005, 48, 9–12. [Google Scholar] [CrossRef]
- Nunan, E.A.; Cardoso, V.N.; Moraes-Santos, T. Technetium-99m labeling of tityustoxin and venom from the scorpion Tityus serrulatus. Appl. Radiat. Isotopes 2002, 57, 849–852. [Google Scholar] [CrossRef]
- Arce-Bejarano, R.; Lomonte, B.; Gutiérrez, J.M. Intravascular hemolysis induced by the venom of the Eastern coral snake, Micrurus fulvius, in a mouse model: Identification of directly hemolytic phospholipases A2. Toxicon 2014, 90, 26–35. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: http://www.uniprot.org (accessed on 25 October 2014).
- Chang, C.C.; Su, M.J.; Lee, J.D.; Eaker, D. Effects of Sr2+ and Mg2+ on the phospholipase A and the presynaptic neuromuscular blocking actions of β-bungarotoxin, crotoxin and taipoxin. Naunyn Schmiedebergs. Arch. Pharmacol. 1977, 299, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Rossetto, O. How do presynaptic PLA2 neurotoxins block nerve terminals? Trends Biochem. Sci. 2000, 25, 266–270. [Google Scholar] [CrossRef]
- Slinkin, M.A.; Klibanov, A.L.; Torchilin, V.P. Terminal-modified polylysine-based chelating polymers: Highly efficient coupling to antibody with minimal loss in immunoreactivity. Bioconjug. Chem. 1991, 2, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.A.; Damphousse, D.J.; Heminway, S.J.; Liu, S.; Edwards, D.S.; Looby, R.J.; Carroll, T.R. Biological evaluation of 99mTc-labeled cyclic glycoprotein IIb/IIIa receptor antagonists in the canine arteriovenous shunt and deep vein thrombosis models: Effects of chelators on biological properties of [99mTc] chelator-peptide conjugates. Bioconjug. Chem. 1996, 7, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.; Seifert, S.; Cain, J. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann. Emerg. Med 2001, 37, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.A.; Theakston, R.D.G. The management of snake bite. Bull. World Health Org. 1983, 61, 885–895. [Google Scholar] [PubMed]
- Seifert, S.; Boyer, L. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 1. Pharmacokinetics and pharmacodynamics of immunoglobulin antivenoms and related antibodies. Ann. Emerg. Med. 2001, 37, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Goodman and Gilman. Las Bases de la Farmacología, 9th ed.; McGraw-Hill-Interamericana: Ciudad de Mexico, Mexico, 1996; Chapter 3. [Google Scholar]
- Tang, L.; Meibohm, B. Pharmacokinetics of peptides and proteins. In Pharmacokinetics and Pharmacodynamics of Biotech Drugs: Principles and Case Studies in Drug Development; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Chapter 2. [Google Scholar]
- Mora, J.; Mora, R.; Lomonte, B.; Gutiérrez, J.M. Effects of Bothrops asper snake venom on lymphatic vessels: Insights into a hidden aspect of envenomation. PLoS Negl. Trop. Dis. 2008, 2. [Google Scholar] [CrossRef] [PubMed]
- Van Helden, D.F.; Thomas, P.A.; Dosen, P.J.; Imtiaz, M.S.; Laver, D.R.; Isbister, G.K. Pharmacological approaches that slow lymphatic flow as a snakebite first aid. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Hnatowich, D.J.; Childs, R.L.; Lanteigne, D.; Najafi, A. The preparation of DTPA-coupled antibodies radiolabeled with metallic radionuclides: An improved method. J. Immunol. Methods 1983, 65, 147–157. [Google Scholar] [CrossRef]
- Chan, C.; Scollard, D.A.; McLarty, K.; Smith, S.; Reilly, R.M. A comparison of 111In-or 64Cu-DOTA-trastuzumab Fab fragments for imaging subcutaneous HER2-positive tumor xenografts in athymic mice using microSPECT/CT or microPET/CT. EJNMMI Res. 2011, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Organ | β-NTx-DTPA-67Ga | BSA-DTPA-67Ga | 67Ga |
|---|---|---|---|
| % Dose/g Tissue | % Dose/g Tissue | % Dose/g Tissue | |
| Kidneys | 3.15 (±0.77) | 1.96 (±0.69) | 1.82 (±0.19) |
| Heart | 0.13 (±0.03) | 0.08 (±0.02) | 0.04 (±0.01) |
| Lung | 0.47 (±0.20) | 0.14 (±0.06) | 0.04 (±0.01) |
| Liver | 1.42 (±0.75) | 0.64 (±0.02) | 0.17 (±0.03) |
| Diaphragm | 0.10 (±0.04) | 0.04 (±0.01) | 0.03 (±0.01) |
| Stomach | 0.21 (±0.10) | 0.16 (±0.07) | 0.04 (±0.01) |
| Spleen | 0.76 (±0.30) | 0.60 (±0.11) | 0.32 (±0.19) |
| Sample | % Dose | % Dose | % Dose |
| Urine | 32.1 (±3.0) | 33.7 (±12.0) | 61.3 (±4.5) |
| Feces | 2.23 (±0.66) | 3.1 (±1.6) | 5.47 (±2.0) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara, I.; Castillo, E.Y.; Romero-Piña, M.E.; Torres-Viquez, I.; Paniagua, D.; Boyer, L.V.; Alagón, A.; Medina, L.A. Biodistribution and Lymphatic Tracking of the Main Neurotoxin of Micrurus fulvius Venom by Molecular Imaging. Toxins 2016, 8, 85. https://doi.org/10.3390/toxins8040085
Vergara I, Castillo EY, Romero-Piña ME, Torres-Viquez I, Paniagua D, Boyer LV, Alagón A, Medina LA. Biodistribution and Lymphatic Tracking of the Main Neurotoxin of Micrurus fulvius Venom by Molecular Imaging. Toxins. 2016; 8(4):85. https://doi.org/10.3390/toxins8040085
Chicago/Turabian StyleVergara, Irene, Erick Y. Castillo, Mario E. Romero-Piña, Itzel Torres-Viquez, Dayanira Paniagua, Leslie V. Boyer, Alejandro Alagón, and Luis Alberto Medina. 2016. "Biodistribution and Lymphatic Tracking of the Main Neurotoxin of Micrurus fulvius Venom by Molecular Imaging" Toxins 8, no. 4: 85. https://doi.org/10.3390/toxins8040085






