Detection and Quantification of ADP-Ribosylated RhoA/B by Monoclonal Antibody
Abstract
:1. Introduction
2. Results
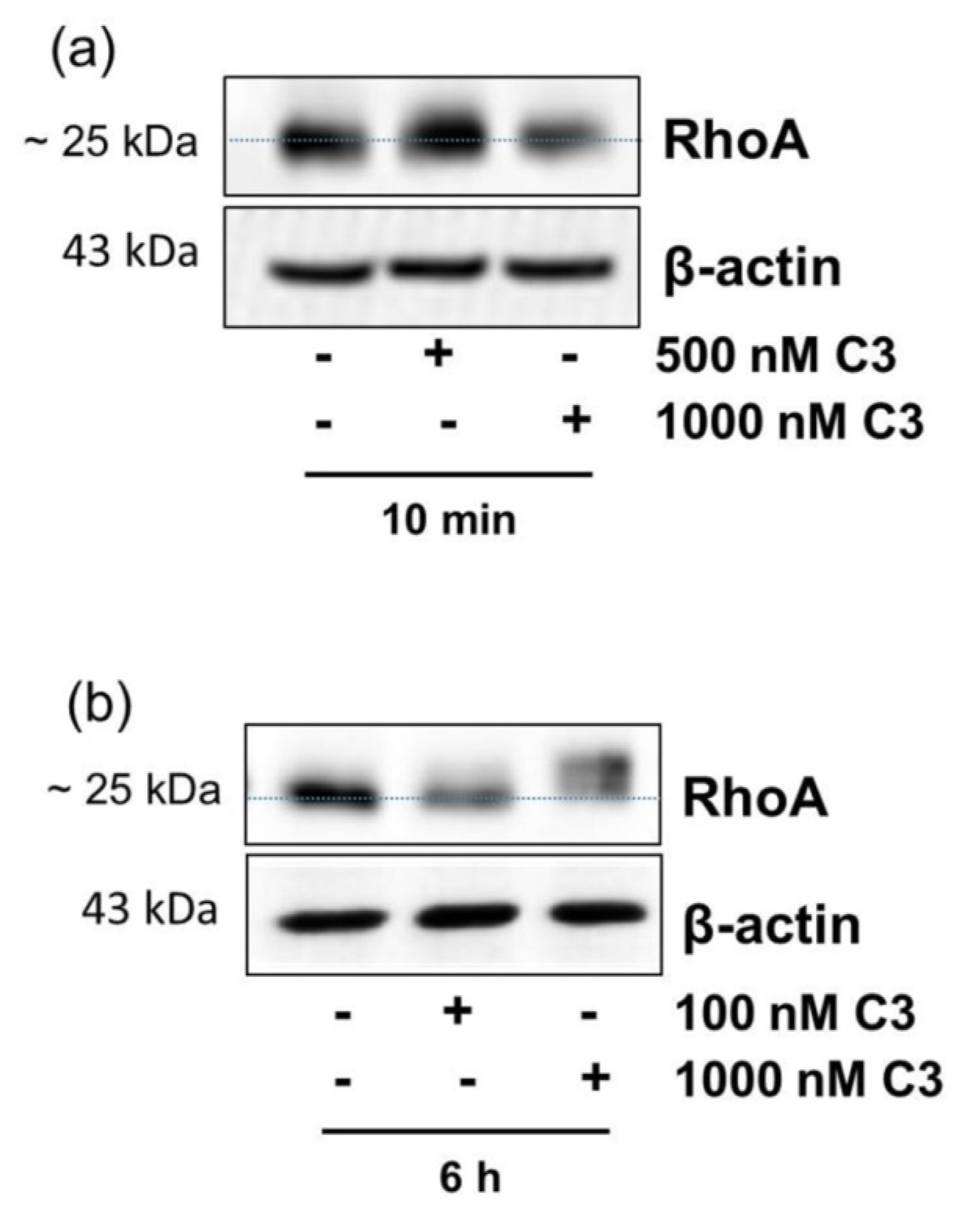
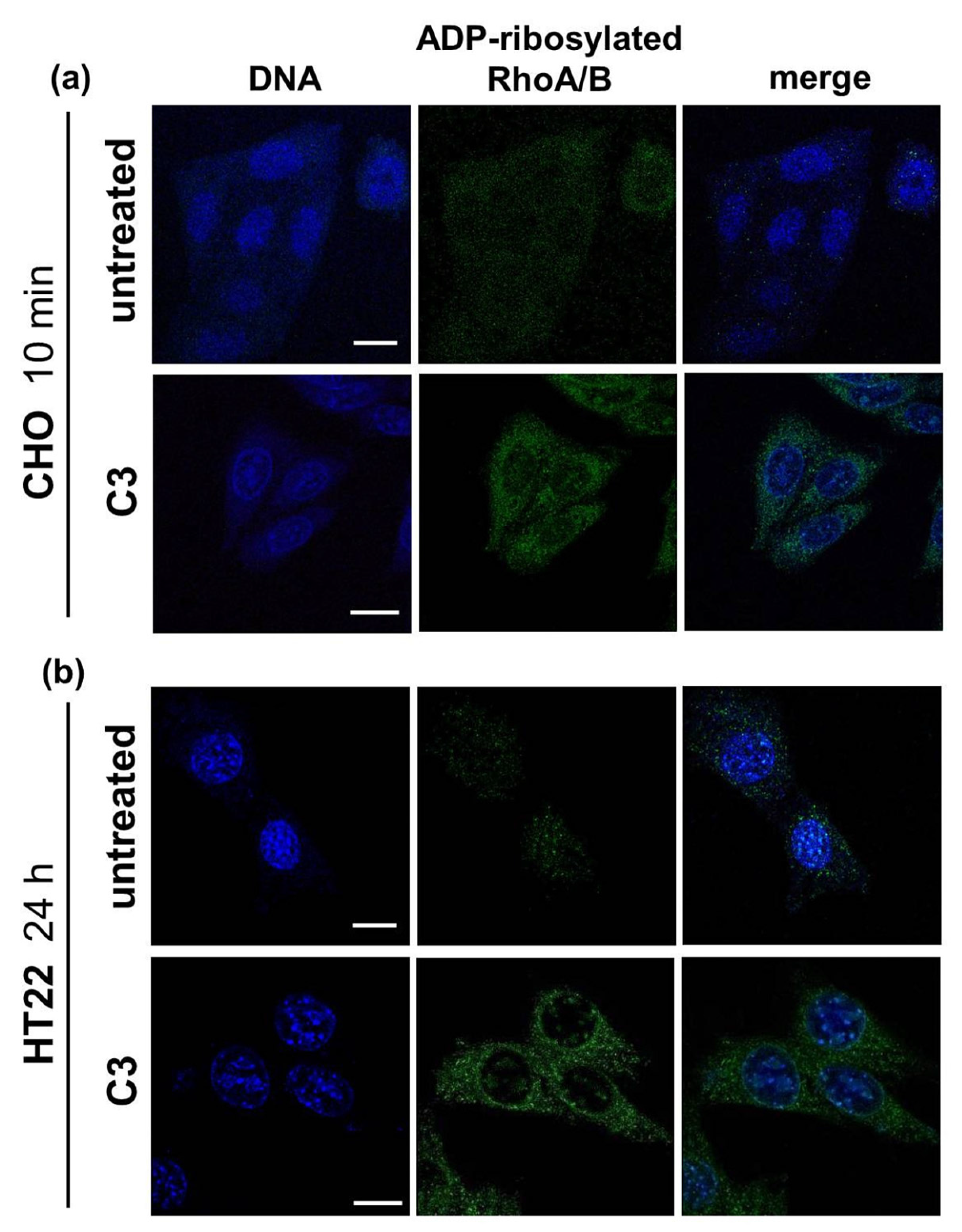
2.1. Detection of C3-Catalyzed ADP-Ribosylation of RhoA in CHO Cells within 10 min
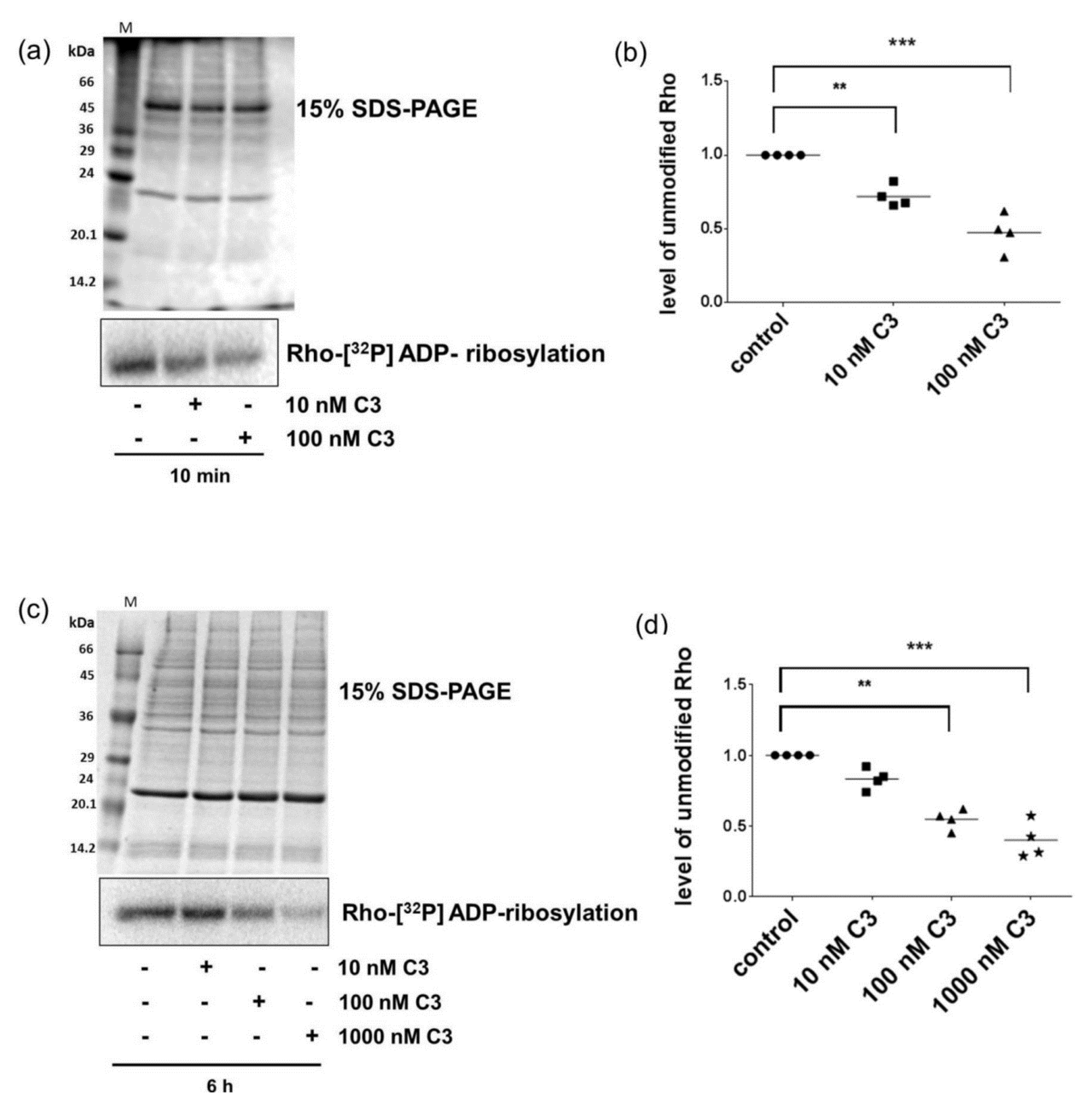
2.2. Sequential [32P]-ADP-Ribosylation Confirmed Uptake of C3 within Minutes
2.3. Mass Spectrometric Detection of ADP-Ribosylated RhoA
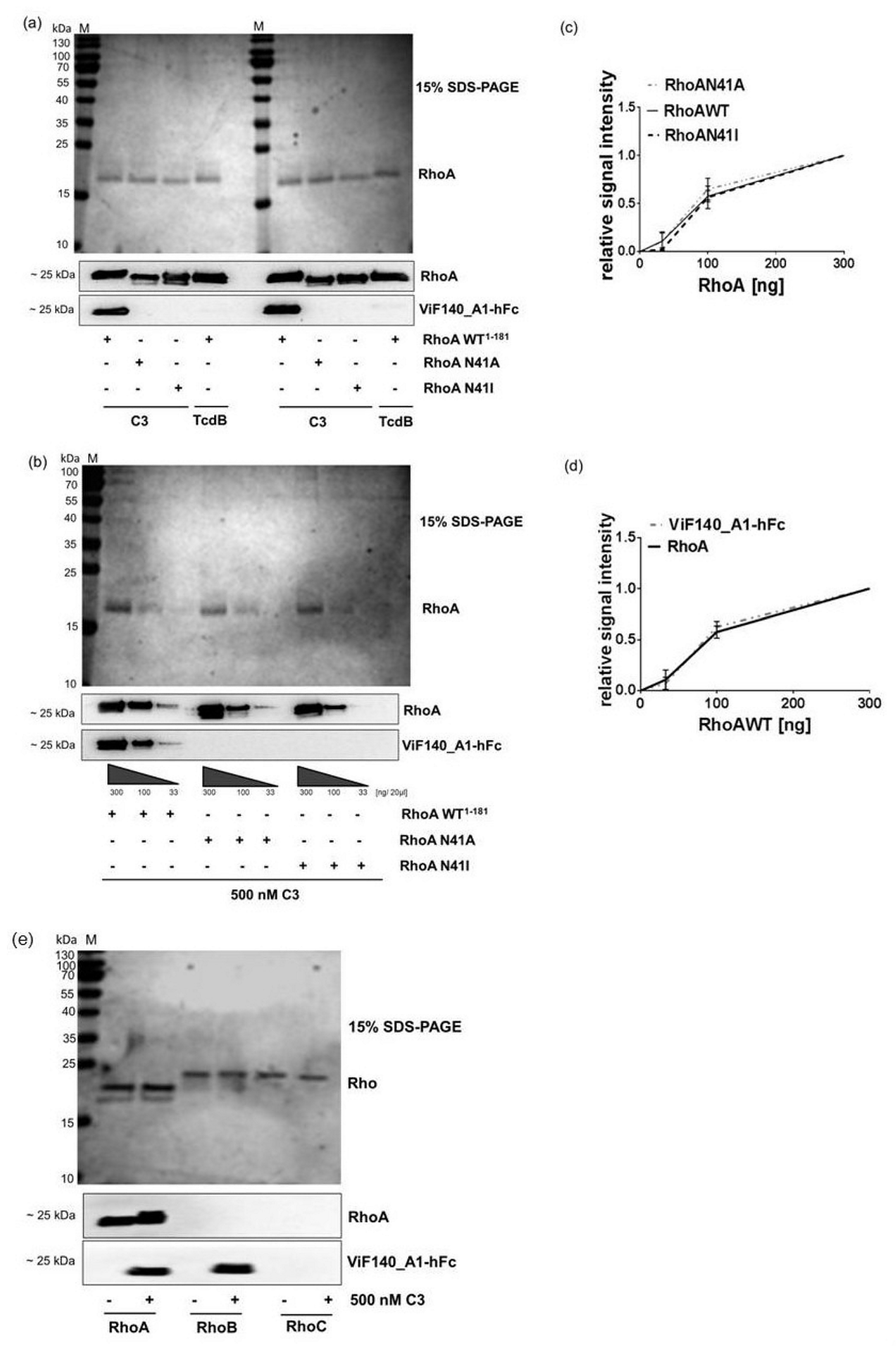
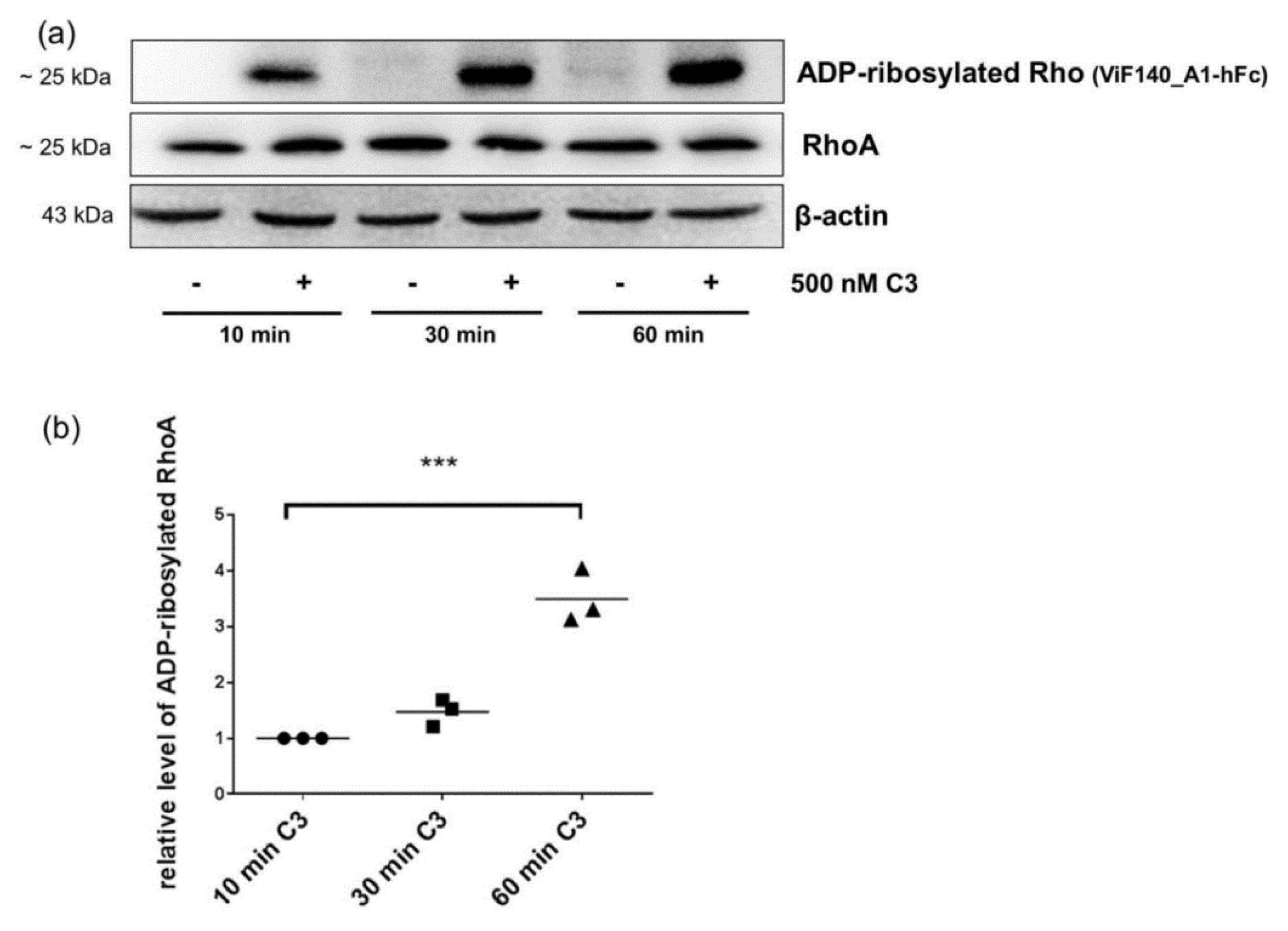
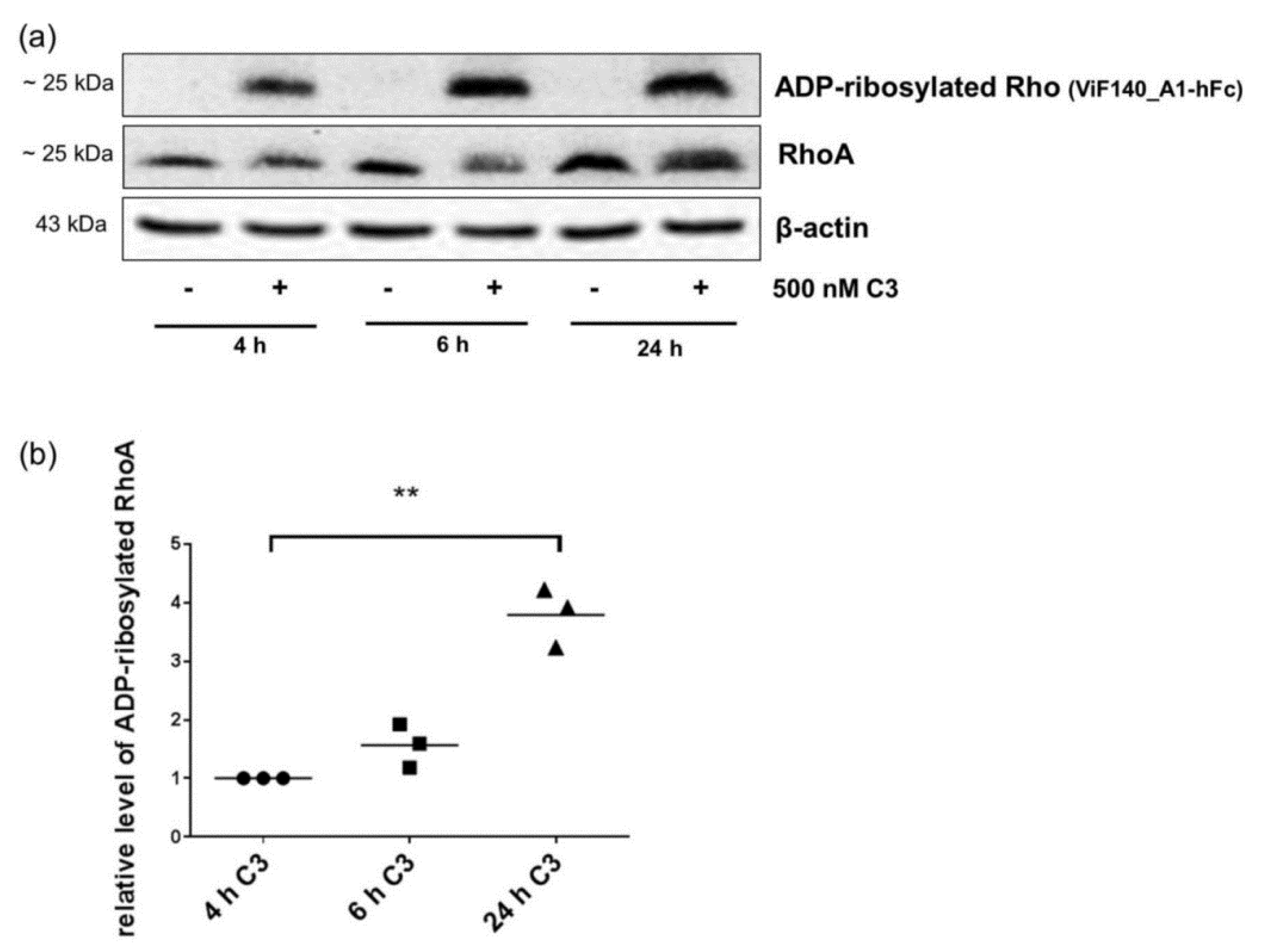
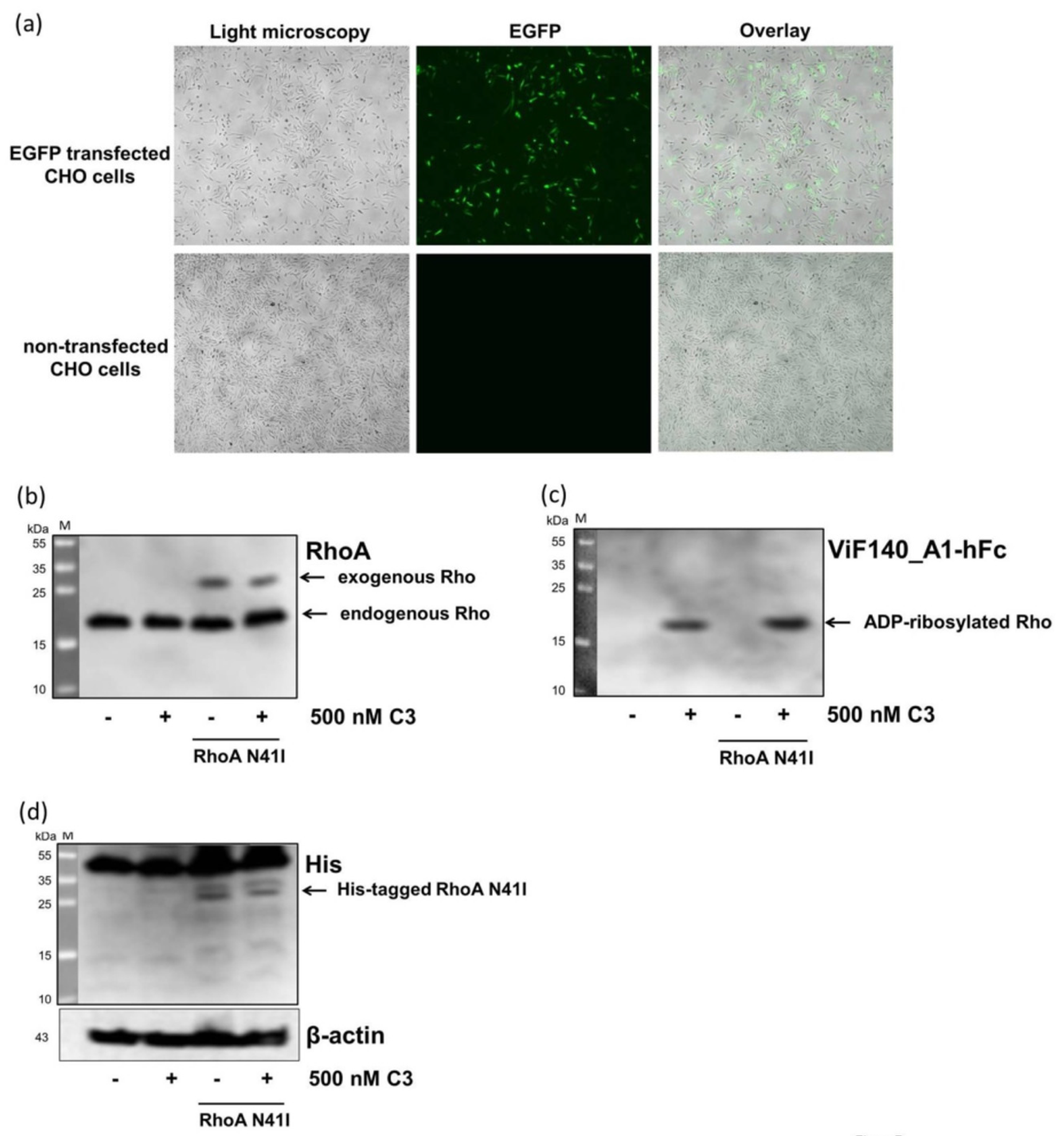
2.4. Detection and Quantification of Adp-Ribosylated Rhoa/B by Monoclonal Antibody
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Expression and Purification of Recombinant C3 Proteins
4.3. Expression and Purification of Recombinant Rho protein
4.4. Western Blot Analysis
4.5. ADP-Ribosylation of Rho in Murine Cells
4.6. ADP-Ribosylation of Recombinant Rho Proteins
4.7. Glucosylation of Recombinant Rho Proteins
4.8. Transfection of CHO Cells
4.9. LC-MS Analysis
4.10. Generation of A Specific Antibody Recognizing ADP-Ribosylated RhoA
4.11. Production of scFv-Fc (Yumabs)
4.12. Immunocytochemistry
4.13. Confocal Laser Scanning Microscopy
4.14. Reproducibility of the Experiments and Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| C3bot | C3 exoenzyme derived from Clostridium botulinum |
| SDS | sodium dodecyl sulfate |
| PAGE | polyacrylamide gel electrophoresis |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
References
- Sekine, A.; Fujiwara, M.; Narumiya, S. Asparagine residue in the Rho gene product is the modification site for botulinum ADP-ribosyltransferase. J. Biol. Chem. 1989, 264, 8602–8605. [Google Scholar] [PubMed]
- Hall, A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu. Rev. Cell Biol. 1994, 10, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Ahnert-Hilger, G.; Höltje, M.; Grosse, G.; Pickert, G.; Mucke, C.; Nixdorf-Bergweiler, B.; Boquet, P.; Hofmann, F.; Just, I. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurones. J. Neurochem. 2004, 90, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rotsch, J.; Rohrbeck, A.; May, M.; Kolbe, T.; Hagemann, S.; Schelle, I.; Just, I.; Genth, H.; Huelsenbeck, S.C. Inhibition of macrophage migration by C. botulinum exoenzyme C3. Naunyn-Schmiedeberg's Arch. Pharmacol. 2012, 385, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Winton, M.J.; Dubreuil, C.I.; Lasko, D.; Leclerc, N.; McKerracher, L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. 2002, 277, 32820–32829. [Google Scholar] [CrossRef] [PubMed]
- Fendrick, J.L.; Iglewski, W.J. Endogenous ADP-ribosylation of elongation factor 2 in polyoma virus-transformed baby hamster kidney cells. Proc. Natl. Acad. Sci. USA 1989, 86, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Rohrbeck, A.; Kolbe, T.; Hagemann, S.; Genth, H.; Just, I. Distinct biological activities of C3 and ADP-ribosyltransferase-deficient C3-E174Q. FEBS J. 2012, 279, 2657–2671. [Google Scholar] [PubMed]
- Rohrbeck, A.; Schröder, A.; Hagemann, S.; Pich, A.; Höltje, M.; Ahnert-Hilger, G.; Just, I. Vimentin mediates uptake of C3bot exoenzyme. PLoS ONE 2014, 6. [Google Scholar] [CrossRef]
- Paone, G.; Wada, A.; Stevens, L.A.; Matin, A.; Hirayama, T.; Levine, R.L.; Moss, J. ADP ribosylation of human neutrophil peptide-1 regulates its biological properties. Proc. Natl. Acad. Sci. USA 2002, 99, 8231–8235. [Google Scholar] [CrossRef] [PubMed]
- Paone, G.; Stevens, L.A.; Levine, R.L.; Bourgeois, C.; Steagall, W.K.; Gochuico, B.R.; Moss, J. ADP-ribosyltransferase-specific modification of human neutrophil peptide-1. J. Biol. Chem. 2006, 281, 17054–17060. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Frolov, A.; Hoffmann, R. Fragmentation behavior of Amadori-peptides obtained by non-enzymatic glycosylation of lysine residues with ADP-ribose in tandem mass spectrometry. J. Mass Spectrom. 2010, 45, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Rohrbeck, A.; Just, I.; Pich, A. Proteome alterations of hippocampal cells caused by clostridium botulinum C3 exoenzyme. J. Proteome Res. 2015, 14, 4721–4733. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, A.; Kügler, J.; Wilke, S.; Schirrmann, T.; Hust, M. Construction of human antibody gene libraries and selection of antibodies by phage display. Methods Mol. Biol. 2014, 1060, 215–243. [Google Scholar] [PubMed]
- Kügler, J.; Wilke, S.; Meier, D.; Tomszak, F.; Frenzel, A.; Schirrmann, T.; Dübel, S.; Garritsen, H.; Hock, B.; Toleikis, L.; et al. Generation and analysis of the improved human HAL9/10 antibody phage display libraries. BMC Biotechnol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Hynds, D.L. Subcellular localization of Rho GTPases: Implications for axon regeneration. Neural Regen Res. 2015, 10, 1032–1033. [Google Scholar] [CrossRef] [PubMed]
- Lo Vasco, V.R.; Leopizzi, M.; Rocca, C.D. Ezrin-related Phosphoinositide pathway modifies RhoA and Rac1 in human osteosarcoma cell lines. J. Cell Commun. Signal. 2015, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.C. Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal. 1998, 10, 167–172. [Google Scholar] [CrossRef]
- Halon, A.; Donizy, P.; Surowiak, P.; Matkowski, R. ERM/Rho protein expression in ductal breast cancer: A 15 year follow-up. Cell Oncol. 2013, 36, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Carranza, D.C.; Chen, I.Y.; Kolodney, M.S. Isoprenylation is necessary for the full invasive potential of RhoA overexpression in human melanoma cells. J. Investig. Dermatol. 2002, 119, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Xu, J. Factors influencing RhoA protein distribution in the nucleus. Mol. Med. Rep. 2011, 4, 1115–1119. [Google Scholar] [PubMed]
- Guilluy, C.; Dubash, A.D.; García-Mata, R. Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus. Nat. Protoc. 2011, 6, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Dubash, A.D.; Guilluy, C.; Srougi, M.C.; Boulter, E.; Burridge, K.; García-Mata, R. The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PLoS ONE 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yang, X.; Chen, Y.; Chen, M. Nuclear translocation of small G protein RhoA via active transportation in gastric cancer cells. Oncol. Rep. 2013, 30, 1878–1882. [Google Scholar] [PubMed]
- Tao, Y.; Chen, Y.C.; Lan, T.; Qian, H.; Wang, Y.; Jiang, L. LPS-induced nuclear translocation of RhoA is dependent on NF-κB in the human lung cancer cell line A549. Oncol. Lett. 2012, 3, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yang, X.; Liu, Y.; Chen, Y.; Chen, C.M. Bilobol inhibits the lipopolysaccharide-induced expression and distribution of RhoA in HepG2 human hepatocellular carcinoma cells. Oncol. Lett. 2015, 10, 962–966. [Google Scholar] [PubMed]
- Fahrer, J.; Kuban, J.; Heine, K.; Rupps, G.; Kaiser, E.; Felder, E.; Benz, R.; Barth, B.H. Selective and specific internalization of clostridial C3 ADP-ribosyltransferases into macrophages and monocytes. Cell Microbiol. 2010, 12, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, H.; Elima, K.; Henttinen, T.; Irjala, H.; Salmi, M.; Jalkanen, S. Molecular identification of PAL-E, a widely used endothelial-cell marker. Blood 2005, 106, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Gonzalez, A.M.; Debiase, P.J.; Trejo, H.E.; Goldman, R.D.; Flitney, F.W.; Jones, J.C. Recruitment of vimentin to the cell surface by β3 integrin and plectin mediates adhesion strength. J. Cell Sci. 2009, 122, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Hizal, D.B.; Tabb, D.L.; Chaerkady, R.; Chen, L.; Lewis, N.E.; Nagarajan, H.; Sarkaria, V.; Kumar, A.; Wolozny, D.; Colao, J.; et al. Proteomic analysis of Chinese hamster ovary (CHO) cells. J. Proteome Res. 2012, 11, 5265–5276. [Google Scholar] [CrossRef] [PubMed]
- Hellevik, T.; Martinez, I.; Olsen, R.; Toh, B.H.; Webster, P.; Smedsrod, B. Transport of residual endocytosed products into terminal lysosomes occurs slowly in rat liver endothelial cells. Hepatology 1998, 28, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.; Berg, T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem. J. 1989, 257, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.J.; Poulter, N.S.; Haining, E.J.; Rappoport, J.Z. Clathrin-mediated endocytosis regulates occludin, and not focal adhesion, distribution during epithelial wound healing. Biol. Cell 2012, 104, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Höltje, M.; Hoffmann, A.; Hofmann, F.; Mucke, C.; Große, G.; van Rooijen, N.; Kettenmann, H.; Just, I.; Ahnert-Hilger, G. Role of Rho GTPase in astrocyte morphology and migratory response during in vitro wound healing. J. Neurochem. 2005, 95, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Jäger, V.; Büssow, K.; Wagner, A.; Weber, S.; Hust, M.; Frenzel, A.; Schirrmann, T. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol. 2013, 13. [Google Scholar] [CrossRef] [PubMed]

| Identified Peptide | Identified Modification | Calculated m/z of the Peptide | C3 | C3-E174Q | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide Sequence | Modification | MH+ (Da) | IonScore | m/z (Da) | RT (min) | IonScore | m/z (Da) | RT (min) | IonScore | m/z (Da) | RT (min) |
| DQFPEVYVPTVFENYVADIEVDGK | - | 2773 | 75 | 1387 | 157 | 78 | 1387 | 157 | 80 | 925 | 157 |
| DQFPEVYVPTVFEnYVADIEVDGK | N14(ADP-Ribosyl) | 3314 | 31 | 1105 | 169 | - | - | - | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohrbeck, A.; Fühner, V.; Schröder, A.; Hagemann, S.; Vu, X.-K.; Berndt, S.; Hust, M.; Pich, A.; Just, I. Detection and Quantification of ADP-Ribosylated RhoA/B by Monoclonal Antibody. Toxins 2016, 8, 100. https://doi.org/10.3390/toxins8040100
Rohrbeck A, Fühner V, Schröder A, Hagemann S, Vu X-K, Berndt S, Hust M, Pich A, Just I. Detection and Quantification of ADP-Ribosylated RhoA/B by Monoclonal Antibody. Toxins. 2016; 8(4):100. https://doi.org/10.3390/toxins8040100
Chicago/Turabian StyleRohrbeck, Astrid, Viola Fühner, Anke Schröder, Sandra Hagemann, Xuan-Khang Vu, Sarah Berndt, Michael Hust, Andreas Pich, and Ingo Just. 2016. "Detection and Quantification of ADP-Ribosylated RhoA/B by Monoclonal Antibody" Toxins 8, no. 4: 100. https://doi.org/10.3390/toxins8040100






