EGA Protects Mammalian Cells from Clostridium difficile CDT, Clostridium perfringens Iota Toxin and Clostridium botulinum C2 Toxin
Abstract
:1. Introduction
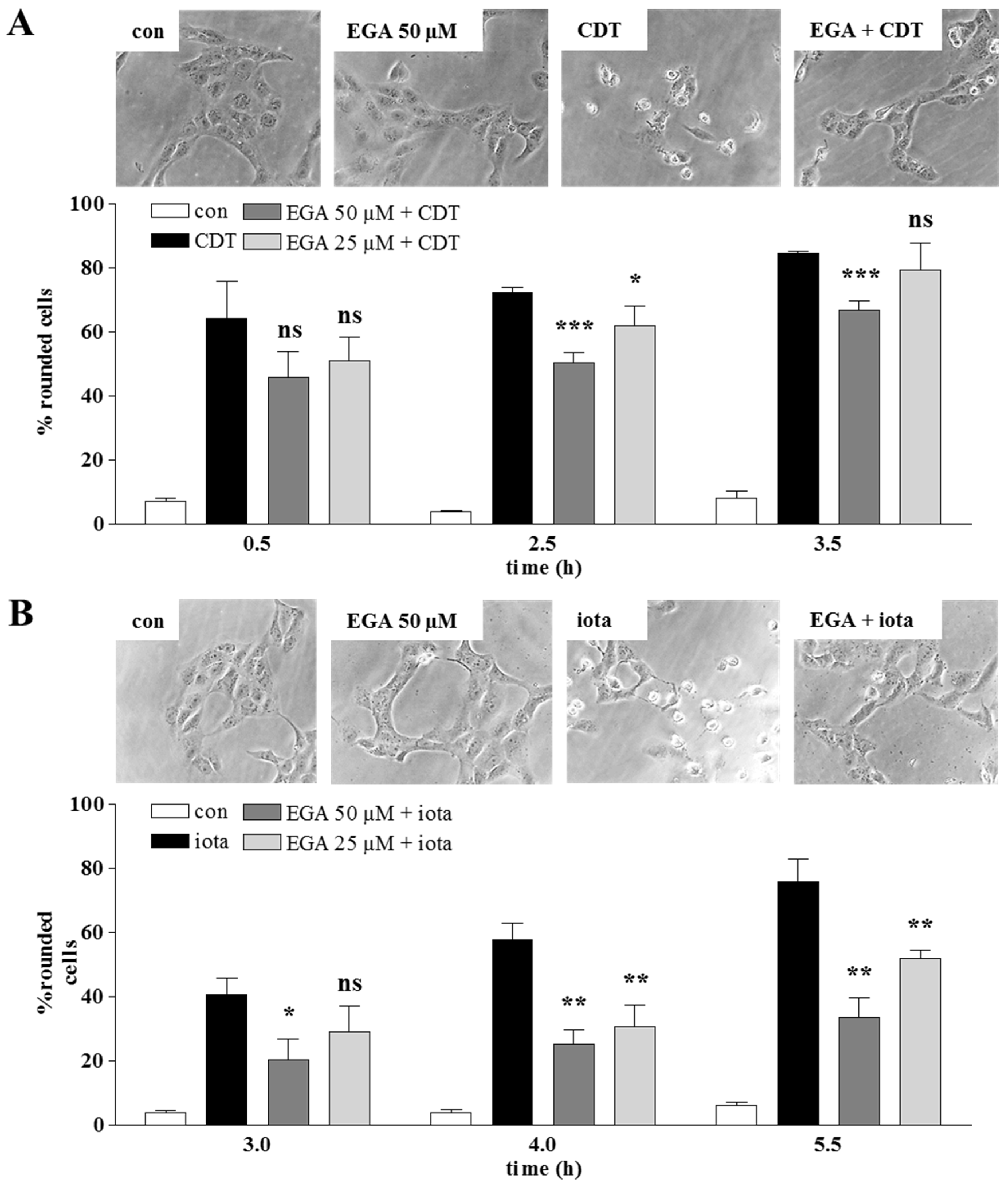
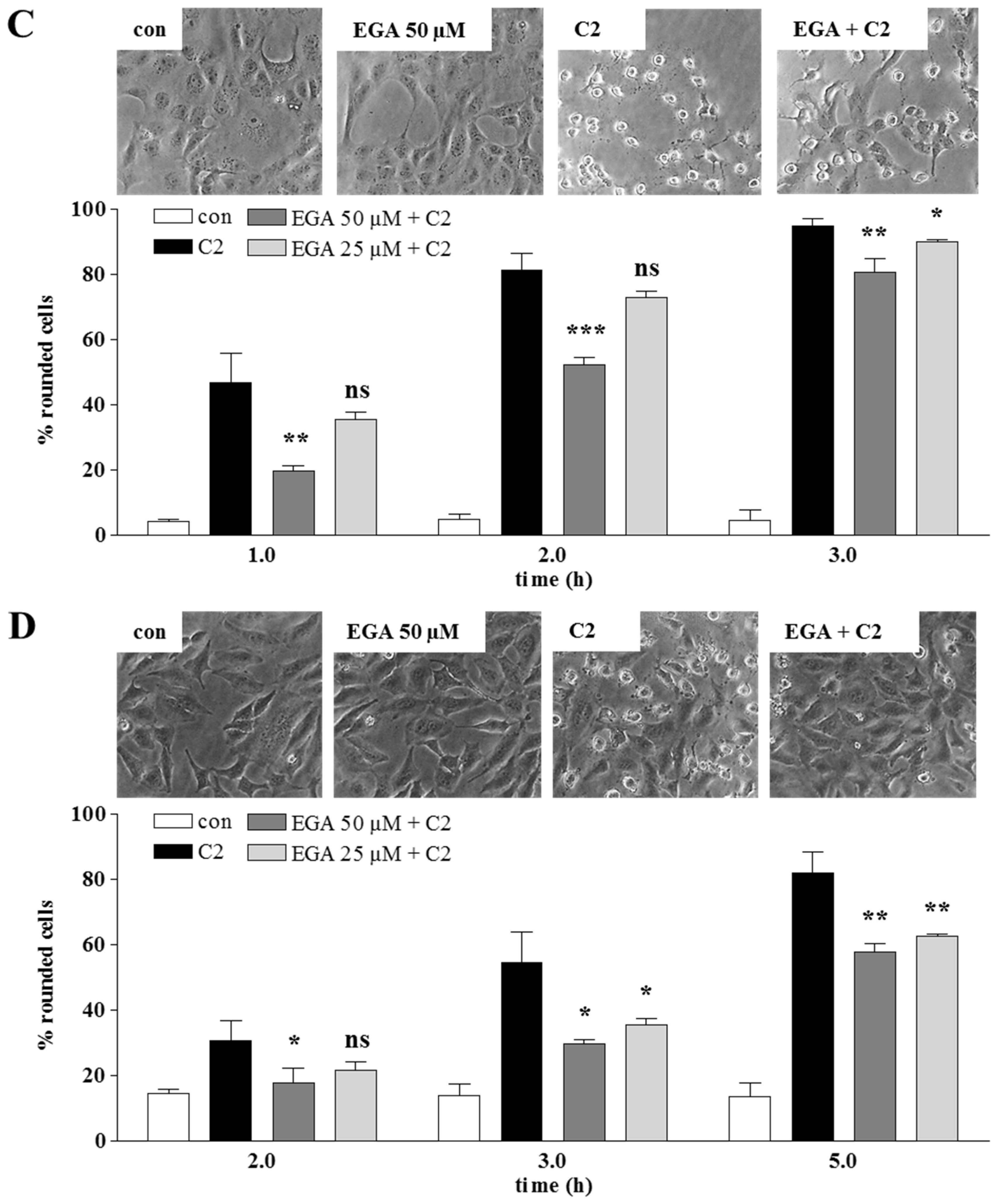
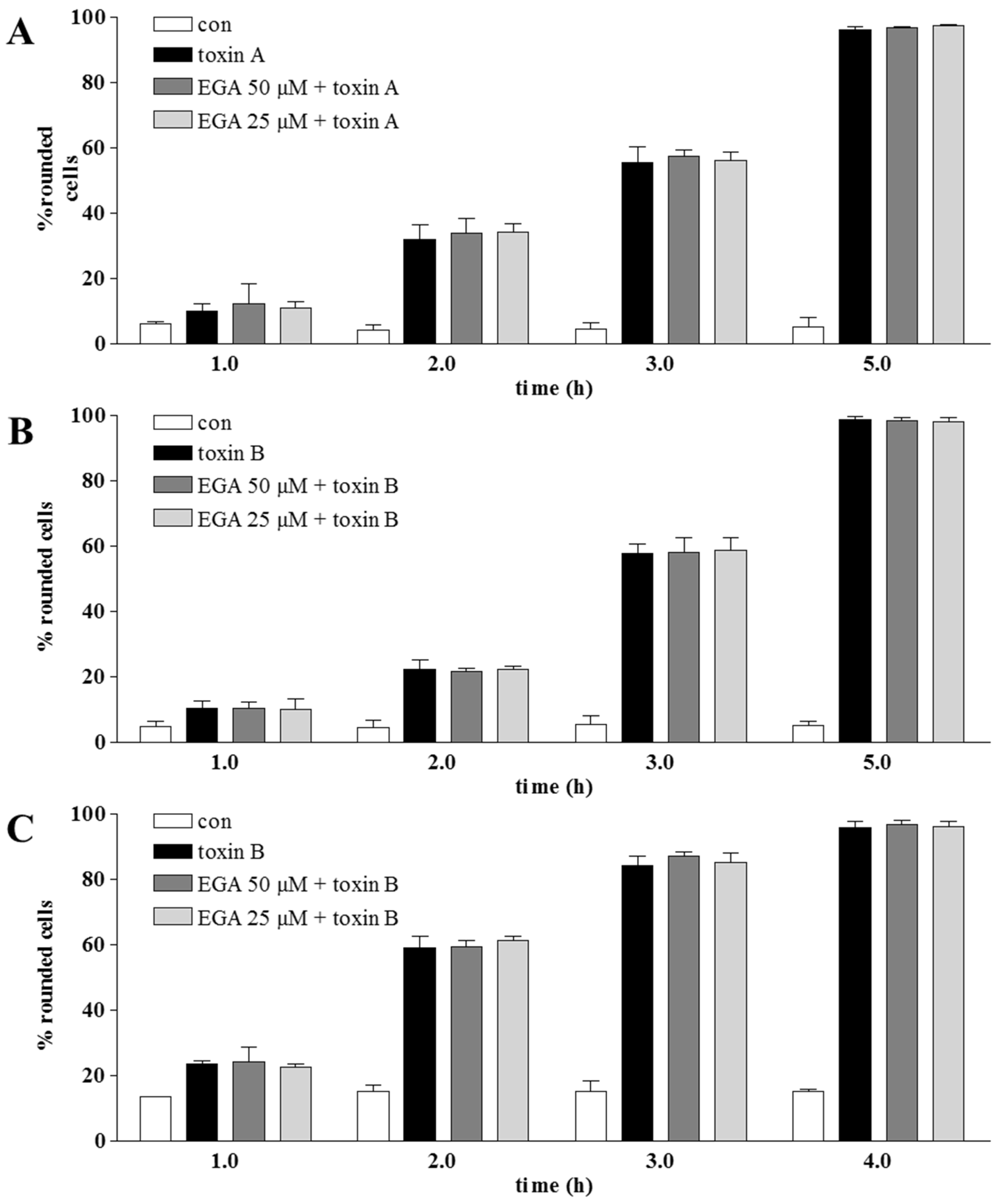
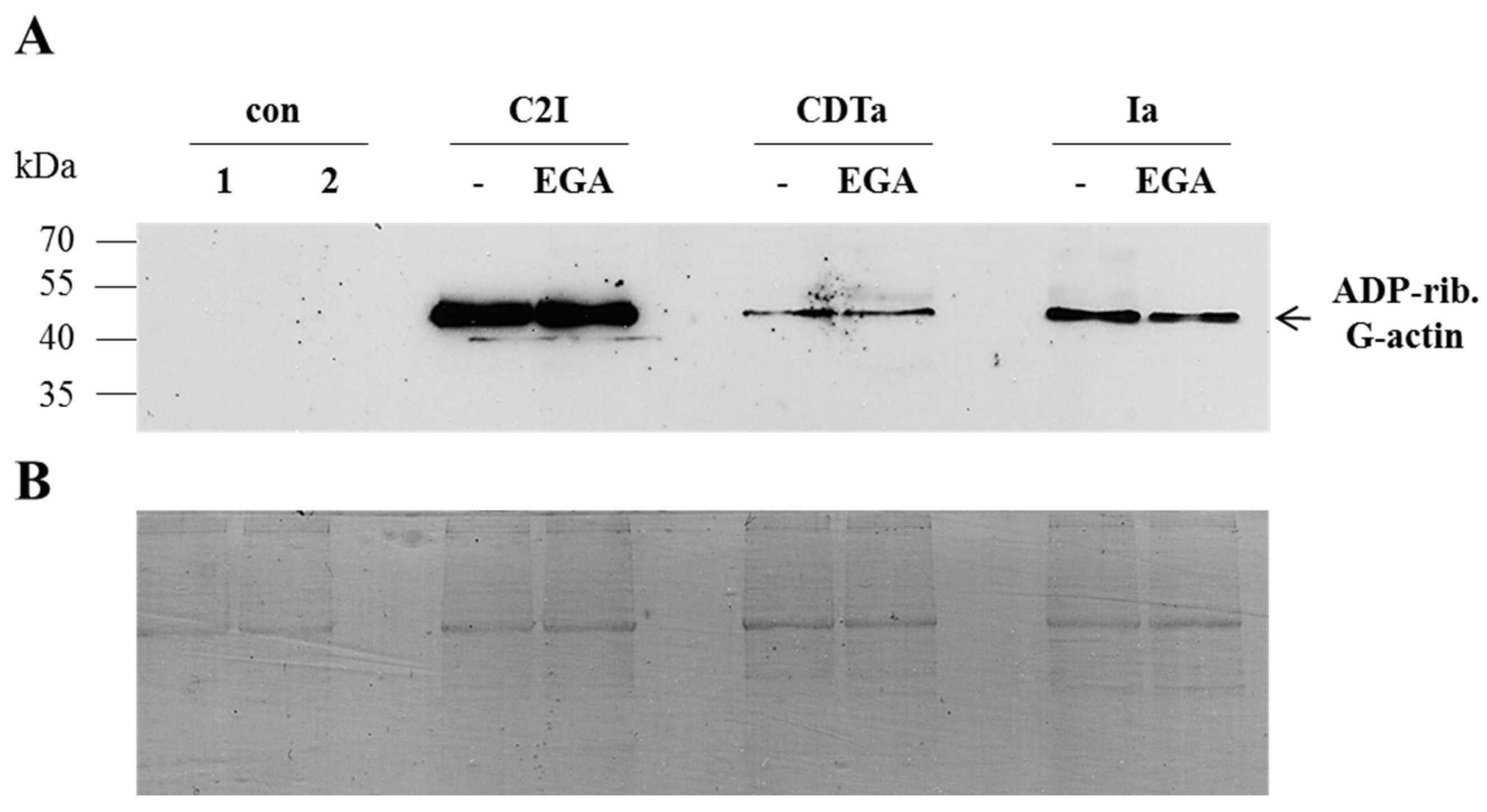
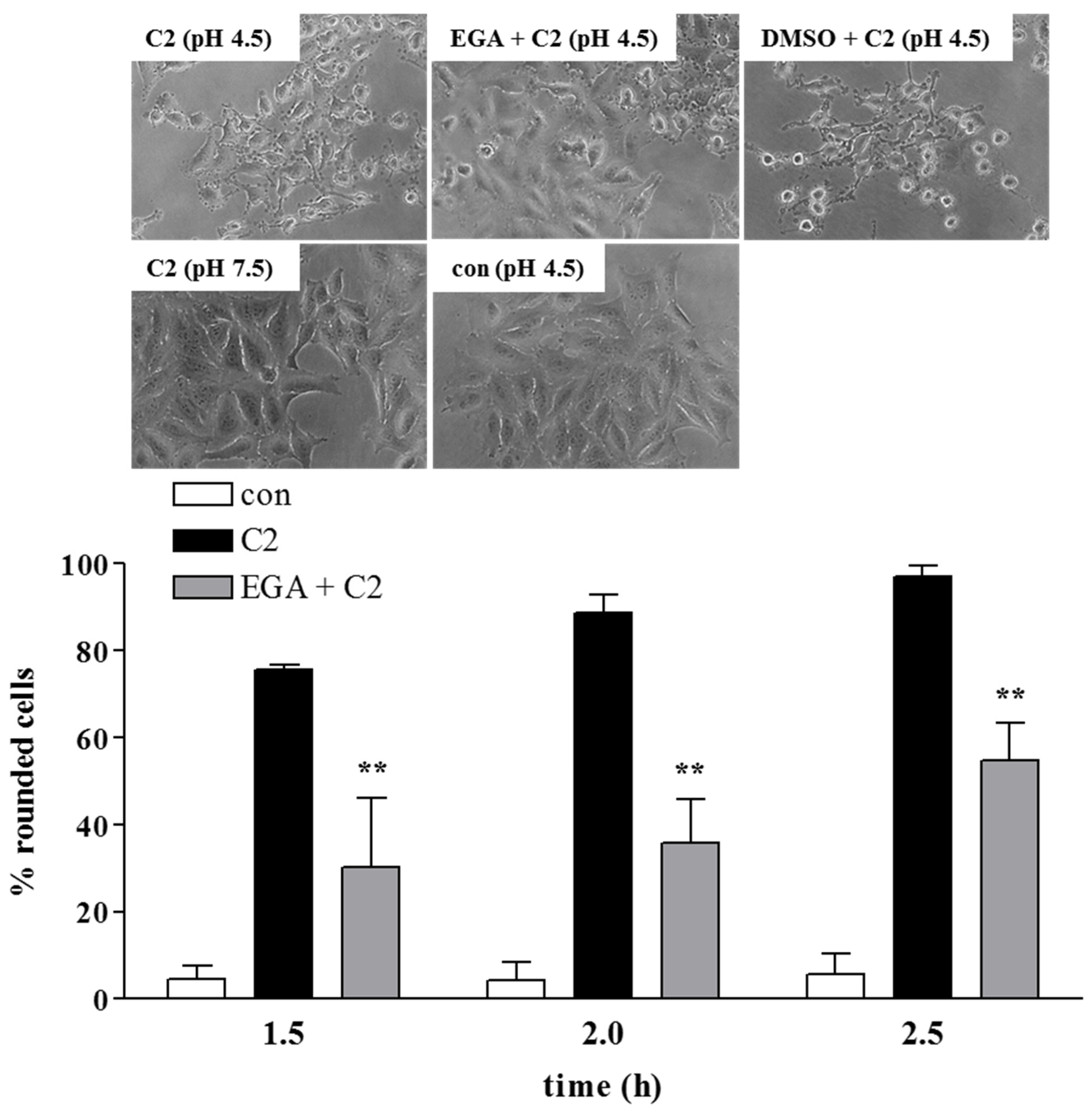
2. Results and Discussion
3. Experimental Section
3.1. Materials and Reagents
3.2. Cell Culture and Intoxication Assays
3.3. SDS-PAGE and Western Blotting
3.4. ADP-Ribosylation of Actin by C2I, CDTa and Ia in a Cell-Free System
3.5. Binding of C2 Toxin to Its Cell Surface Receptor
3.6. Toxin-Translocation across the Cytoplasmic Membrane of Living Cells
3.7. Reproducibility of the Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Popoff, M.R.; Rubin, E.J.; Gill, D.M.; Boquet, P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 1988, 56, 2299–2306. [Google Scholar] [PubMed]
- Perelle, S.; Gibert, M.; Bourlioux, P.; Corthier, G.; Popoff, M.R. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect. Immun. 1997, 65, 1402–1407. [Google Scholar] [PubMed]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes 2014, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, S.; Rupnik, M.; Gibert, M.; Brazier, J.; Duerden, B.; Popoff, M.R. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 2000, 186, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996, 9, 216–234. [Google Scholar] [PubMed]
- Stiles, B.G.; Wilkins, T.D. Purification and characterization of Clostridium perfringens iota toxin: Dependence on two nonlinked proteins for biological activity. Infect. Immun. 1986, 54, 683–688. [Google Scholar] [PubMed]
- Stiles, B.G.; Wilkins, T.D. Clostridium perfringens iota toxin: Synergism between two proteins. Toxicon Off. J. Int. Soc.Toxinol. 1986, 24, 767–773. [Google Scholar] [CrossRef]
- Sakurai, J.; Nagahama, M.; Oda, M.; Tsuge, H.; Kobayashi, K. Clostridium perfringens iota-toxin: Structure and function. Toxins 2009, 1, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Bärmann, M.; Ohishi, I.; Tsuyama, S.; Jakobs, K.H.; Habermann, E. Botulinum C2 toxin ADP-ribosylates actin. Nature 1986, 322, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, I.; Iwasaki, M.; Sakaguchi, G. Purification and characterization of two components of botulinum C2 toxin. Infect. Immun. 1980, 30, 668–673. [Google Scholar] [PubMed]
- Ohishi, I.; Tsuyama, S. ADP-ribosylation of nonmuscle actin with component I of C2 toxin. Biochem. Biophys. Res. Commun. 1986, 136, 802–806. [Google Scholar] [CrossRef]
- Barth, H.; Aktories, K.; Popoff, M.R.; Stiles, B.G. Binary bacterial toxins: Biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol. Mol. Biol. Rev. 2004, 8, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Aktories, K. New insights into the mode of action of the actin ADP-ribosylating virulence factors Salmonella enterica SpvB and Clostridium botulinum C2 toxin. Eur. J. Cell Biol. 2011, 90, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Blöcker, D.; Behlke, J.; Bergsma-Schutter, W.; Brisson, A.; Benz, R.; Aktories, K. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J. Biol. Chem. 2000, 275, 18704–18711. [Google Scholar] [CrossRef] [PubMed]
- Blöcker, D.; Behlke, J.; Aktories, K.; Barth, H. Cellular uptake of the binary Clostridium perfringens iota-toxin. Infect. Immun. 2001, 69, 2980–2987. [Google Scholar] [CrossRef] [PubMed]
- Stiles, B.G.; Hale, M.L.; Marvaud, J.C.; Popoff, M.R. Clostridium perfringens iota toxin: Binding studies and characterization of cell surface receptor by fluorescence-activated cytometry. Infect. Immun. 2000, 68, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Stiles, B.G.; Hale, M.L.; Marvaud, J.C.; Popoff, M.R. Clostridium perfringens iota toxin: Characterization of the cell-associated iota b complex. Biochem. J. 2002, 367, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, P.; Carette, J.E.; Bell, G.W.; Schwan, C.; Guttenberg, G.; Brummelkamp, T.R.; Aktories, K. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc. Natl. Acad. Sci. USA 2011, 108, 16422–16427. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, P.; Wilczek, C.; Nölke, T.; Guttenberg, G.; Hornuss, D.; Schwan, C.; Aktories, K. Identification of the cellular receptor of Clostridium spiroforme toxin. Infect. Immun. 2012, 80, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, P.; Hornuss, D.; Nölke, T.; Hemmasi, S.; Castonguay, J.; Picchianti, M.; Aktories, K. Clostridium difficile binary toxin CDT induces clustering of the lipolysis-stimulated lipoprotein receptor into lipid rafts. MBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Wigelsworth, D.J.; Ruthel, G.; Schnell, L.; Herrlich, P.; Blonder, J.; Veenstra, T.D.; Carman, R.J.; Wilkins, T.D.; van Nhieu, G.T.; Pauillac, S.; et al. CD44 promotes intoxication by the clostridial iota-family toxins. PLoS ONE 2012, 7, e51356. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, M.; Barth, H.; Blöcker, D.; Aktories, K. Binding of Clostridium botulinum C2 toxin to asparagine-linked complex and hybrid carbohydrates. J. Biol. Chem. 2000, 275, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Blöcker, D.; Pohlmann, K.; Haug, G.; Bachmeyer, C.; Benz, R.; Aktories, K.; Barth, H. Clostridium botulinum C2 toxin: Low pH-induced pore formation is required for translocation of the enzyme component C2I into the cytosol of host cells. J. Biol. Chem. 2003, 278, 37360–37367. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Benz, R.; Just, I.; Aktories, K. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes. Formation of cation-selective channels and inhibition of channel function by chloroquine. J. Biol. Chem. 1994, 269, 16706–16711. [Google Scholar] [PubMed]
- Bachmeyer, C.; Benz, R.; Barth, H.; Aktories, K.; Gilbert, M.; Popoff, M.R. Interaction of C2 toxin with lipid bilayer membranes and Vero cells: Inhibition of channel function by chloroquine and related compounds in vitro and intoxification in vivo. FASEB J. 2001, 15, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Blöcker, D.; Bachmeyer, C.; Benz, R.; Aktories, K.; Barth, H. Channel formation by the binding component of Clostridium botulinum C2 toxin: Glutamate 307 of C2II affects channel properties in vitro and pH-dependent C2I translocation in vivo. Biochemistry 2003b, 42, 5368–5377. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Neumeyer, T.; Sun, J.; Collier, R.J.; Benz, R.; Aktories, K. Amino acid residues involved in membrane insertion and pore formation of Clostridium botulinum C2 toxin. Biochemistry 2008, 47, 8406–8413. [Google Scholar] [CrossRef] [PubMed]
- Schleberger, C.; Hochmann, H.; Barth, H.; Aktories, K.; Schulz, G.E. Structure and action of the binary C2 toxin from Clostridium botulinum. J. Mol. Biol. 2006, 364, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Knapp, O.; Benz, R.; Gibert, M.; Marvaud, J.C.; Popoff, M.R. Interaction of the binding component of Clostridium perfringens iota-toxin with lipid bilayer membranes: Demonstration of channel formation by the activated binding component Ib and channel block by the enzyme component Ia. J. Biol. Chem. 2002, 277, 6143–6152. [Google Scholar] [CrossRef] [PubMed]
- Gibert, M.; Marvaud, J.C.; Pereira, Y.; Hale, M.L.; Stiles, B.G.; Boquet, P.; Lamaze, C.; Popoff, M.R. Differential requirement for the translocation of clostridial binary toxins: Iota toxin requires a membrane potential gradient. FEBS Lett. 2007, 581, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Haug, G.; Wilde, C.; Leemhuis, J.; Meyer, D.K.; Aktories, K.; Barth, H. Cellular uptake of Clostridium botulinum C2 toxin: Membrane translocation of a fusion toxin requires unfolding of its dihydrofolate reductase domain. Biochemistry 2003, 42, 15284–15291. [Google Scholar] [CrossRef] [PubMed]
- Haug, G.; Leemhuis, J.; Tiemann, D.; Meyer, D.K.; Aktories, K.; Barth, H. The host cell chaperone Hsp90 is essential for translocation of the binary Clostridium botulinum C2 toxin into the cytosol. J. Biol. Chem. 2003, 278, 32266–32274. [Google Scholar] [CrossRef] [PubMed]
- Haug, G.; Aktories, K.; Barth, H. The host cell chaperone Hsp90 is necessary for cytotoxic action of the binary iota-like toxins. Infect. Immun. 2004, 72, 3066–3068. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Pust, S.; Kroll, C.; Barth, H. Cyclophilin A facilitates translocation of the Clostridium botulinum C2 toxin across membranes of acidified endosomes into the cytosol of mammalian cells. Cell. Microbiol. 2009, 11, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Kroll, C.; Ernst, K.; Schwan, C.; Popoff, M.R.; Fischer, G.; Buchner, J.; Aktories, K.; Barth, H. Membrane translocation of binary actin-ADP-ribosylating toxins from Clostridium difficile and Clostridium perfringens is facilitated by cyclophilin A and Hsp90. Infect. Immun. 2011, 79, 3913–3921. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Böhm, N.; Ernst, K.; Langer, S.; Schwan, C.; Aktories, K.; Popoff, M.R.; Fischer, G.; Barth, H. FK506-binding protein 51 interacts with Clostridium botulinum C2 toxin and FK506 blocks membrane translocation of the toxin in mammalian cells. Cell. Microbiol. 2012, 4, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.; Langer, S.; Kaiser, E.; Osseforth, C.; Michaelis, J.; Popoff, M.R.; Schwan, C.; Aktories, K.; Kahlert, V.; Malesevic, M.; et al. Cyclophilin-facilitated membrane translocation as pharmacological target to prevent intoxication of mammalian cells by binary clostridial actin ADP-ribosylating toxins. J. Mol. Biol. 2015, 427, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Gülke, I.; Pfeifer, G.; Liese, J.; Fritz, M.; Hofmann, F.; Aktories, K.; Barth, H. Characterization of the enzymatic component of the ADP-ribosyltransferase toxin CDTa from Clostridium difficile. Infect. Immun. 2001, 69, 6004–6011. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K.; Wegner, A. ADP-ribosylation of actin by clostridial toxins. J. Cell Biol. 1989, 109, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, J.; Schering, B.; Bärmann, M.; Aktories, K. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 1987, 225, 48–52. [Google Scholar] [CrossRef]
- Vandekerckhove, J.; Schering, B.; Bärmann, M.; Aktories, K. Botulinum C2 toxin ADP-ribosylates cytoplasmic beta/gamma-actin in arginine 177. J. Biol. Chem. 1988, 263, 696–700. [Google Scholar] [PubMed]
- Schering, B.; Bärmann, M.; Chhatwal, G.S.; Geipel, U.; Aktories, K. ADP-ribosylation of skeletal muscle and non-muscle actin by Clostridium perfringens iota toxin. Eur. J. Biochem. 1988, 171, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Preiss, J.C.; Hofmann, F.; Aktories, K. Characterization of the catalytic site of the ADP-ribosyltransferase Clostridium botulinum C2 toxin by site-directed mutagenesis. J. Biol. Chem. 1998, 273, 29506–29511. [Google Scholar] [CrossRef] [PubMed]
- Perelle, S.; Domenighini, M.; Popoff, M.R. Evidence that Arg-295, Glu-378, and Glu-380 are active-site residues of the ADP-ribosyltransferase activity of iota toxin. FEBS Lett. 1996, 2–3, 191–194. [Google Scholar] [CrossRef]
- Tsuge, H.; Nagahama, M.; Oda, M.; Iwamoto, S.; Utsunomiya, H.; Marquez, V.E.; Katunuma, N.; Nishizawa, M.; Sakurai, J. Structural basis of actin recognition and arginine ADP-ribosylation by Clostridium perfringens iota-toxin. Proc. Natl. Acad. Sci. USA 2008, 105, 7399–7404. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.; Aktories, K. ADP-ribosylated actin caps the barbed ends of actin filaments. J. Biol. Chem. 1988, 263, 13739–13742. [Google Scholar] [PubMed]
- Weigt, C.; Just, I.; Wegner, A.; Aktories, K. Nonmuscle actin ADP-ribosylated by botulinum C2 toxin caps actin filaments. FEBS Lett. 1989, 246, 181–184. [Google Scholar] [CrossRef]
- Wiegers, W.; Just, I.; Müller, H.; Hellwig, A.; Traub, P.; Aktories, K. Alteration of the cytoskeleton of mammalian cells cultured in vitro by Clostridium botulinum C2 toxin and C3 ADP-ribosyltransferase. Eur. J. Cell Biol. 1991, 54, 237–245. [Google Scholar] [PubMed]
- Schwan, C.; Stecher, B.; Tzivelekidis, T.; van Ham, M.; Rohde, M.; Hardt, W.D.; Wehland, J.; Aktories, K. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009, 5, e1000626. [Google Scholar] [CrossRef] [PubMed]
- Schwan, C.; Kruppke, A.S.; Nölke, T.; Schumacher, L.; Koch-Nolte, F.; Kudryashev, M.; Stahlberg, H.; Aktories, K. Clostridium difficile toxin CDT hijacks microtubule organization and reroutes vesicle traffic to increase pathogen adherence. Proc. Natl. Acad. Sci. USA 2014, 111, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Heine, K.; Pust, S.; Enzenmüller, S.; Barth, H. ADP-ribosylation of actin by Clostridium botulinum C2 toxin in mammalian cells results in delayed caspase-dependent apoptotic cell death. Infect. Immun. 2008, 76, 4600–4608. [Google Scholar] [CrossRef] [PubMed]
- Hilger, H.; Pust, S.; von Figura, G.; Stiles, B.G.; Popoff, M.R.; Barth, H. Long-lived nature of Clostridium perfingens iota toxin in the cytosol of mammalian cells correlates with toxin-induced apoptosis. Infect. Immun. 2009, 77, 5593–5601. [Google Scholar] [CrossRef] [PubMed]
- Heinlen, L.; Ballard, J.D. Clostridium difficile infection. Am. J. Med. Sci. 2010, 340, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Just, I.; Selzer, J.; Wilm, M.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Just, I.; Wilm, M.; Selzer, J.; Rex, G.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 1995, 270, 13932–13936. [Google Scholar] [CrossRef] [PubMed]
- Jank, T.; Aktories, K. Structure and mode of action of clostridial glucosylating toxins: The ABCD model. Trends Microbiol. 2008, 16, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Geric, B.; Rupnik, M.; Gerding, D.N.; Grabnar, M.; Johnson, S. Distribution of Clostridium difficile variant toxinotypes and strains with binary toxin genes among clinical isolates in an American hospital. J. Med. Microbiol. 2004, 53, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Decre, D.; Barbut, F.; Burghoffer, B.; Petit, J.C. Prevalence and characterization of a binary toxin (actin-specific ADP-ribosyltransferase) from Clostridium difficile. J. Clin. Microbiol. 2004, 42, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Killgore, G.E.; Thompson, A.; Owens, R.C., Jr.; Kazakova, S.V.; Sambol, S.P.; Johnson, S.; Gerding, D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 2005, 353, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Willey, B.; Low, D.E.; Staempfli, H.R.; McGeer, A.; Boerlin, P.; Mulvey, M.; Weese, J.S. Characterization of Clostridium difficile strains isolated from patients in Ontario, Canada, from 2004 to 2006. J. Clin. Microbiol. 2008, 46, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Collery, M.M.; Kelly, M.L.; Cartman, S.T.; Cockayne, A.; Minton, N.P. Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J. Infect. Dis. 2014, 209, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A.; Collier, R.J. Anthrax toxin: Receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 2007, 76, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, E.J.; Ho, C.L.; Balaji, K.; Clemens, D.L.; Deng, G.; Wang, Y.E.; Elsaesser, H.J.; Tamilselvam, B.; Gargi, A.; Dixon, S.D.; et al. Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses. Proc. Natl. Acad. Sci. USA 2013, 110, E4904–E4912. [Google Scholar] [CrossRef] [PubMed]
- Tehran, D.A.; Zanetti, G.; Leka, O.; Lista, F.; Fillo, S.; Binz, T.; Shone, C.C.; Rossetto, O.; Montecucco, C.; Paradisi, C.; et al. A novel inhibitor prevents the peripheral neuroparalysis of botulinum neurotoxins. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnell, L.; Mittler, A.-K.; Sadi, M.; Popoff, M.R.; Schwan, C.; Aktories, K.; Mattarei, A.; Tehran, D.A.; Montecucco, C.; Barth, H. EGA Protects Mammalian Cells from Clostridium difficile CDT, Clostridium perfringens Iota Toxin and Clostridium botulinum C2 Toxin. Toxins 2016, 8, 101. https://doi.org/10.3390/toxins8040101
Schnell L, Mittler A-K, Sadi M, Popoff MR, Schwan C, Aktories K, Mattarei A, Tehran DA, Montecucco C, Barth H. EGA Protects Mammalian Cells from Clostridium difficile CDT, Clostridium perfringens Iota Toxin and Clostridium botulinum C2 Toxin. Toxins. 2016; 8(4):101. https://doi.org/10.3390/toxins8040101
Chicago/Turabian StyleSchnell, Leonie, Ann-Katrin Mittler, Mirko Sadi, Michel R. Popoff, Carsten Schwan, Klaus Aktories, Andrea Mattarei, Domenico Azarnia Tehran, Cesare Montecucco, and Holger Barth. 2016. "EGA Protects Mammalian Cells from Clostridium difficile CDT, Clostridium perfringens Iota Toxin and Clostridium botulinum C2 Toxin" Toxins 8, no. 4: 101. https://doi.org/10.3390/toxins8040101