Impact of Dendrimer Terminal Group Chemistry on Blockage of the Anthrax Toxin Channel: A Single Molecule Study
Abstract
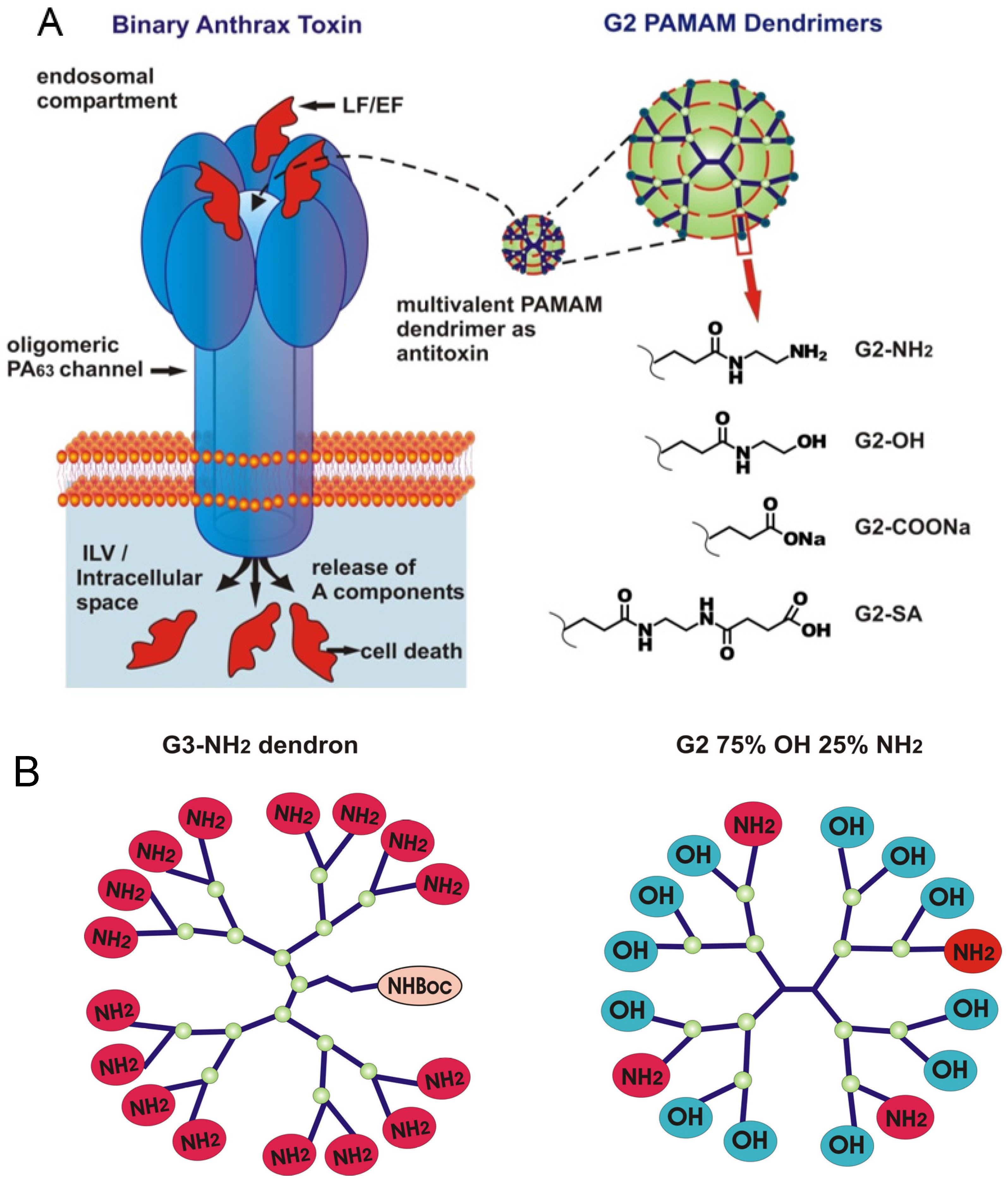
:1. Introduction
2. Results
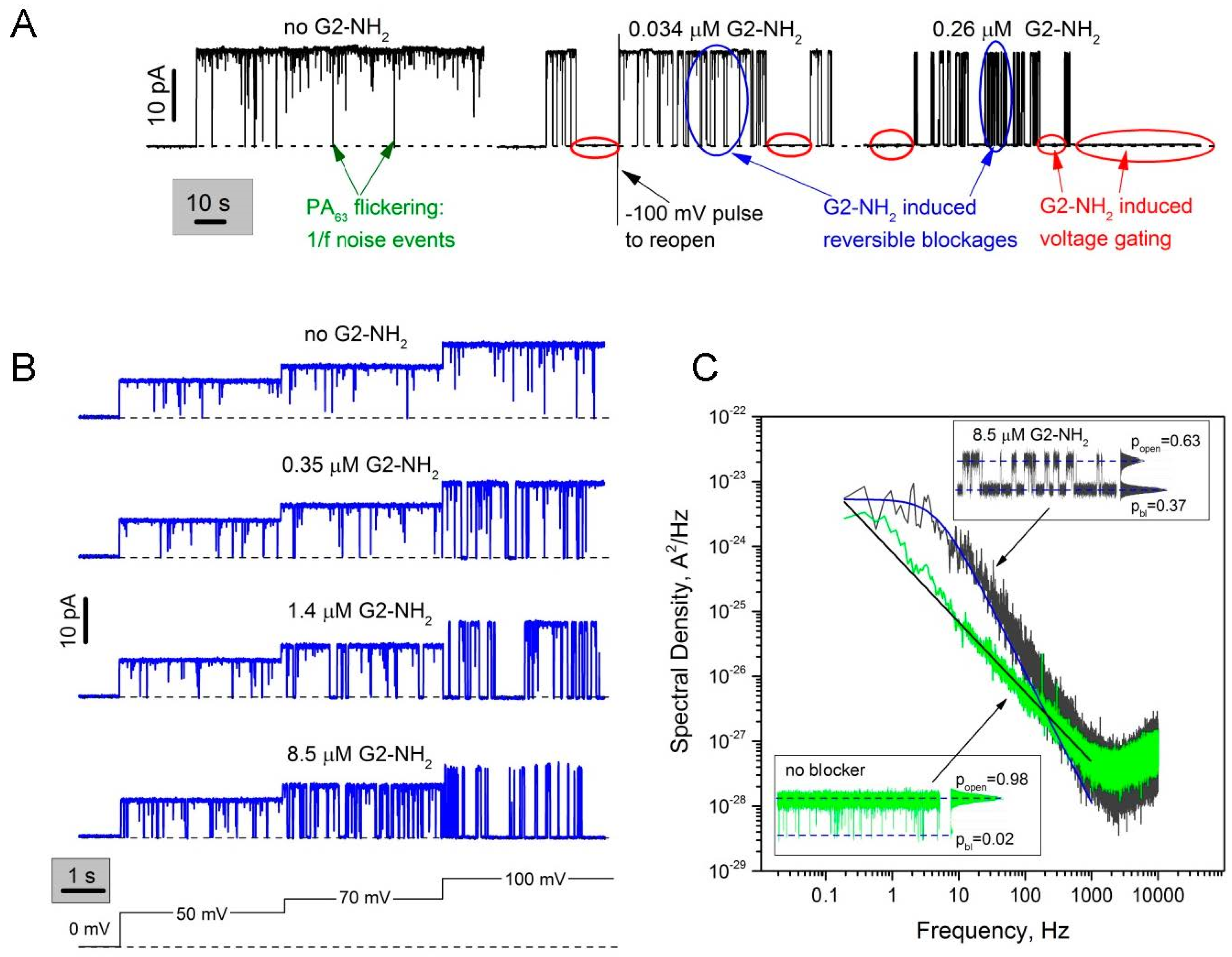
2.1. Two Modes of G2-NH2 PAMAM Dendrimer Inhibition of PA63 Channel
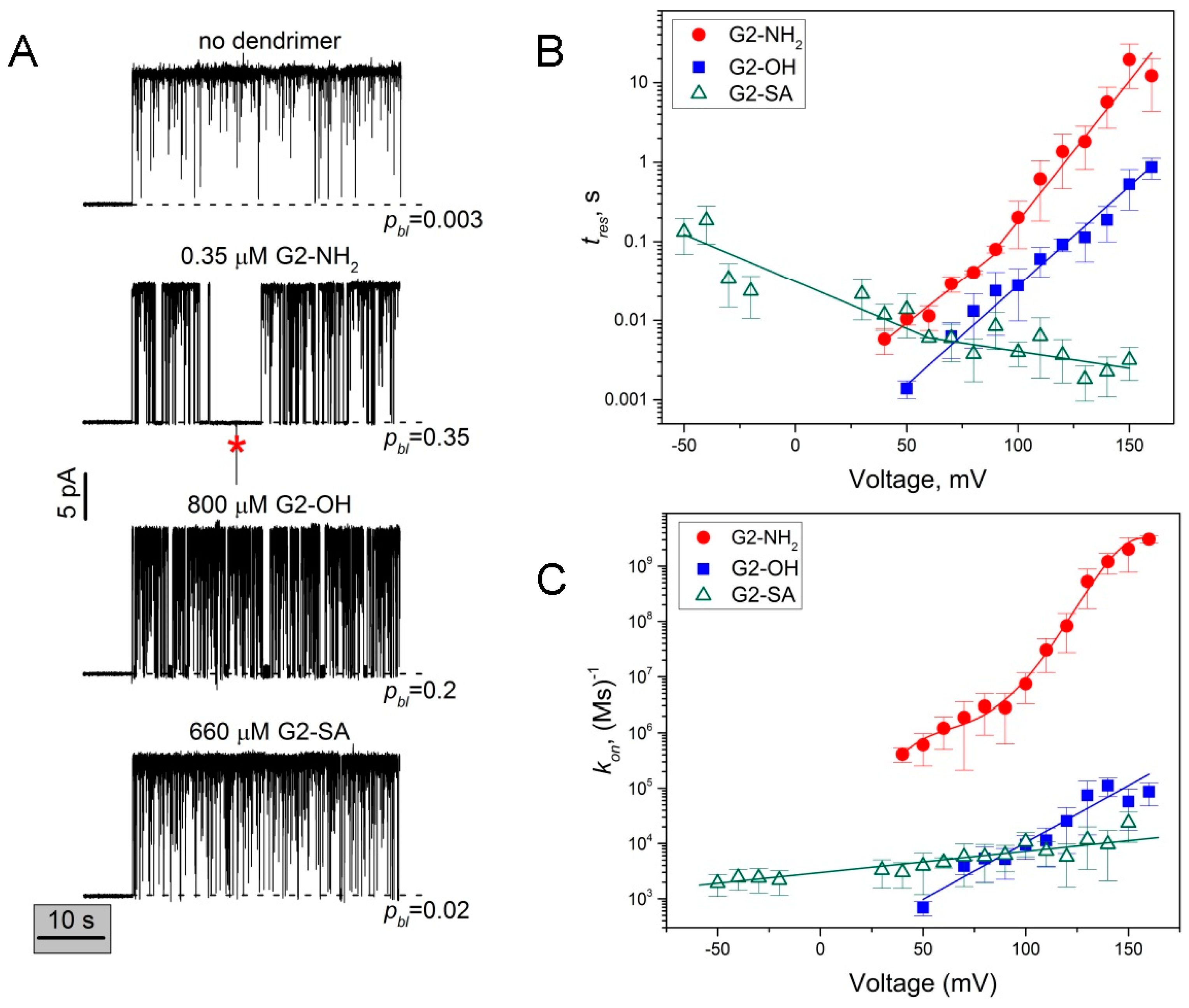
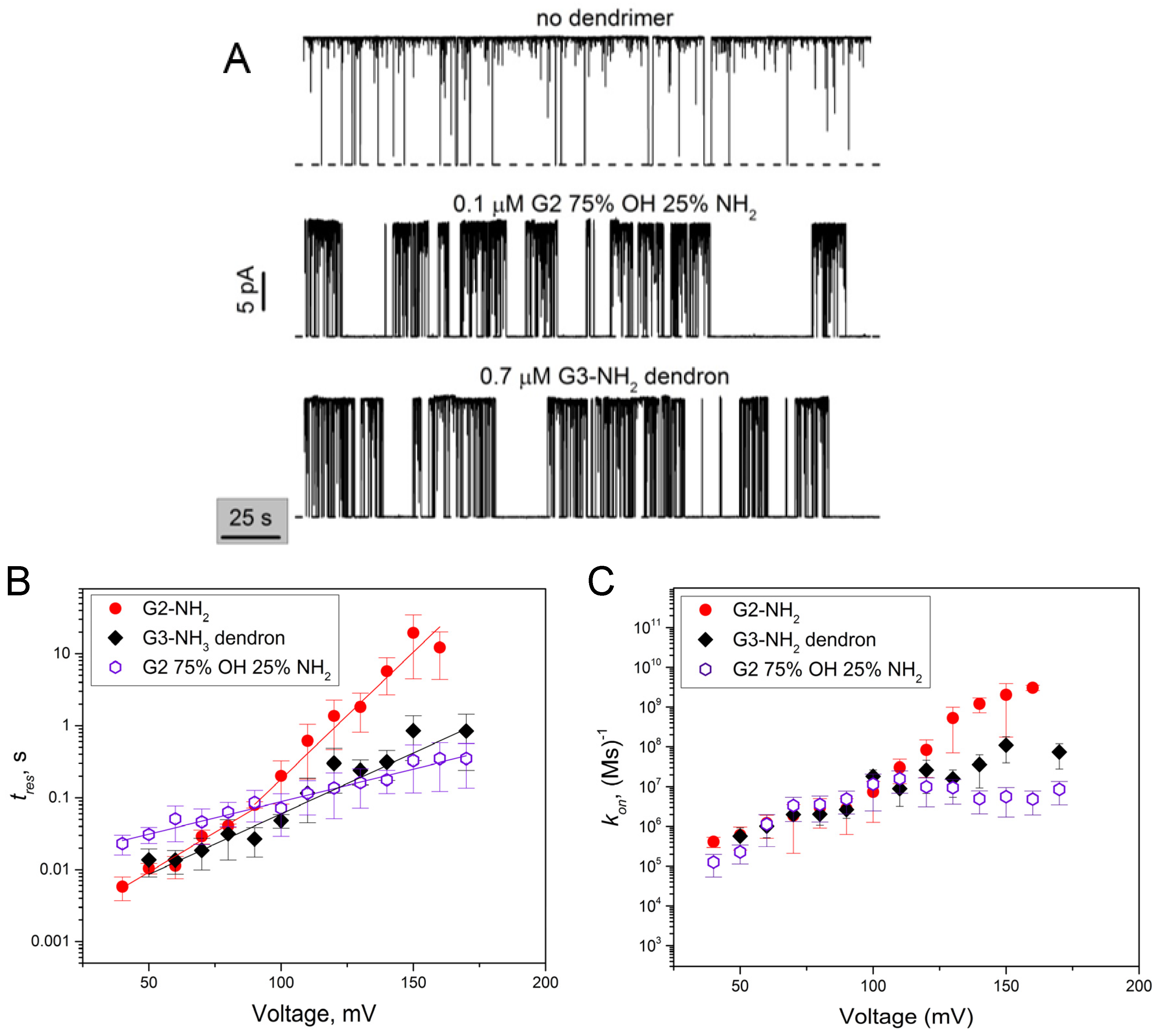
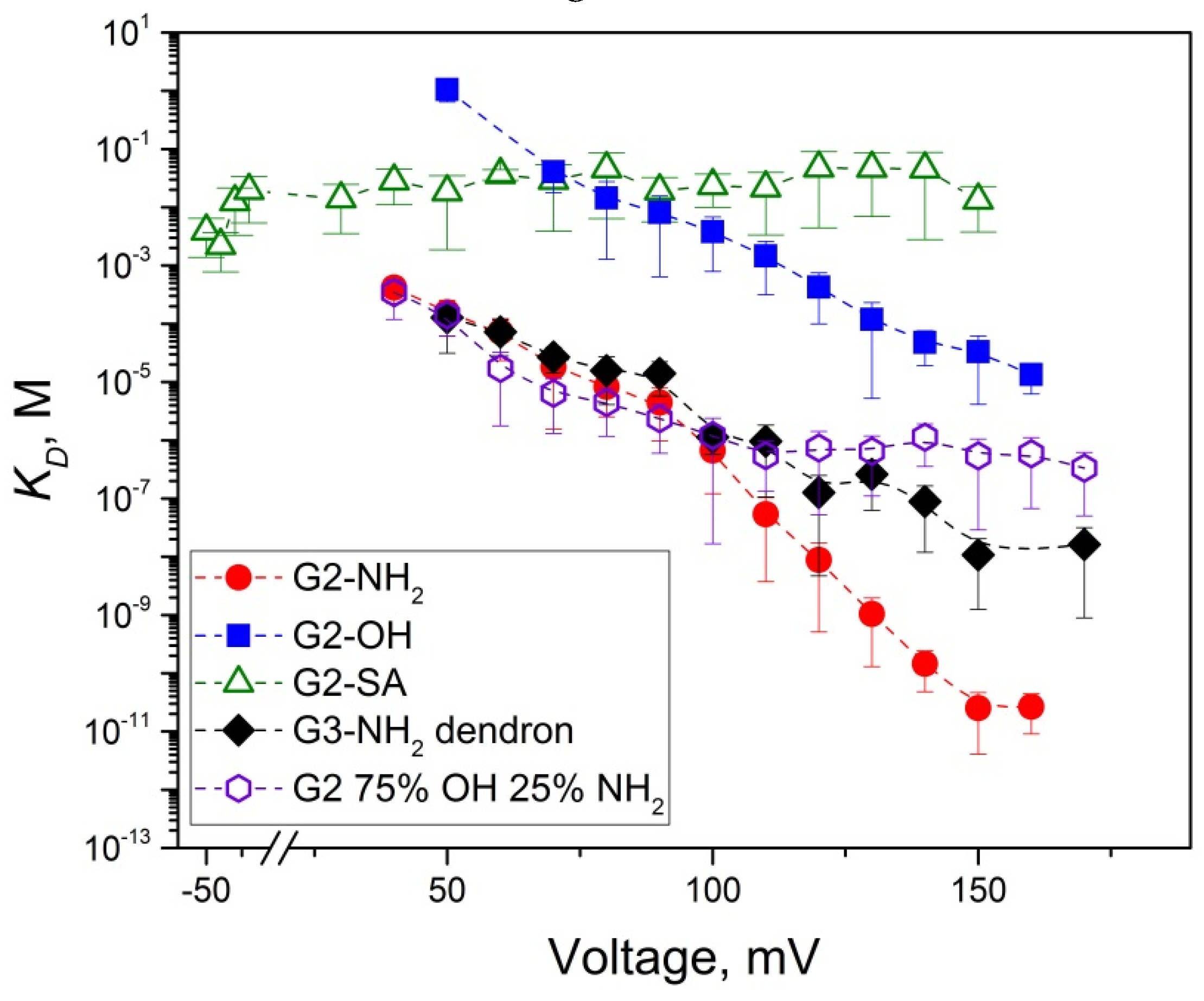
2.2. Role of PAMAM Dendrimer Surface Chemistry in PA63 Blockage
2.3. The Rate Constants of Dendrimer’s First Mode of Binding Reaction are Voltage Dependent
2.4. PA63 Blockage by Imperfect Cationic PAMAM Dendrimers
3. Discussion
3.1. Two Modes of G2-PAMAM Dendrimer Inhibition of PA63 Channel
3.2. Voltage Dependence of the Reversible Dendrimer/PA63 Interaction
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Channel Reconstitution into Planar Lipid Bilayers
5.3. Reproducibility of the Experiments and Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamini, G.; Nestorovich, E.M. Multivalent inhibitors of channel-forming bacterial toxins. Curr. Top. Microbiol. Immunol. 2016. [Google Scholar] [CrossRef]
- Karginov, V.A.; Nestorovich, E.M.; Moayeri, M.; Leppla, S.H.; Bezrukov, S.M. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc. Natl. Acad. Sci. USA 2005, 102, 15075–15080. [Google Scholar] [CrossRef] [PubMed]
- Nestorovich, E.M.; Karginov, V.A.; Popoff, M.R.; Bezrukov, S.M.; Barth, H. Tailored ß-cyclodextrin blocks the translocation pores of binary exotoxins from C. botulinum and C. perfringens and protects cells from intoxication. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Roeder, M.; Nestorovich, E.M.; Karginov, V.A.; Schwan, C.; Aktories, K.; Barth, H. Tailored cyclodextrin pore blocker protects mammalian cells from clostridium difficile binary toxin CDT. Toxins (Basel) 2014, 6, 2097–2114. [Google Scholar] [CrossRef] [PubMed]
- Forstner, P.; Bayer, F.; Kalu, N.; Felsen, S.; Fortsch, C.; Aloufi, A.; Ng, D.Y.; Weil, T.; Nestorovich, E.M.; Barth, H. Cationic PAMAM dendrimers as pore-blocking binary toxin inhibitors. Biomacromolecules 2014, 15, 2461–2474. [Google Scholar] [CrossRef] [PubMed]
- Nestorovich, E.M.; Bezrukov, S.M. Obstructing toxin pathways by targeted pore blockage. Chem. Rev. 2012, 112, 6388–6430. [Google Scholar] [CrossRef] [PubMed]
- Krantz, B.A.; Melnyk, R.A.; Zhang, S.; Juris, S.J.; Lacy, D.B.; Wu, Z.; Finkelstein, A.; Collier, R.J. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science 2005, 309, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Bachmeyer, C.; Orlik, F.; Barth, H.; Aktories, K.; Benz, R. Mechanism of C2-toxin inhibition by fluphenazine and related compounds: Investigation of their binding kinetics to the C2II-channel using the current noise analysis. J. Mol. Biol. 2003, 333, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Orlik, F.; Schiffler, B.; Benz, R. Anthrax toxin protective antigen: Inhibition of channel function by chloroquine and related compounds and study of binding kinetics using the current noise analysis. Biophys. J. 2005, 88, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Beitzinger, C.; Bronnhuber, A.; Duscha, K.; Riedl, Z.; Huber-Lang, M.; Benz, R.; Hajós, G.; Barth, H. Designed azolopyridinium salts block protective antigen pores in vitro and protect cells from anthrax toxin. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronhardt, A.; Beitzinger, C.; Barth, H.; Benz, R. Chloroquine Analog Interaction with C2- and Iota-Toxin in Vitro and in Living Cells. Toxins (Basel) 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.P.; Ficker, M.; Christensen, J.B.; Trohopoulos, P.N.; Moghimi, S.M. Dendrimers in medicine: Therapeutic concepts and pharmaceutical challenges. Bioconjug. Chem. 2015, 26, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Nestorovich, E.M.; Karginov, V.A.; Berezhkovskii, A.M.; Bezrukov, S.M. Blockage of anthrax PA63 pore by a multicharged high-affinity toxin inhibitor. Biophys. J. 2010, 99, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bezrukov, S.M.; Liu, X.; Karginov, V.A.; Wein, A.N.; Leppla, S.H.; Popoff, M.R.; Barth, H.; Nestorovich, E.M. Interactions of high-affinity cationic blockers with the translocation pores of B. anthracis, C. botulinum, and C. perfringens binary toxins. Biophys. J. 2012, 103, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Frechet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Bielinska, A.U.; Mecke, A.; Keszler, B.; Beals, J.L.; Shi, X.; Balogh, L.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconjug. Chem. 2004, 15, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Redemann, C.T.; Szoka, F.C., Jr. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem. 1996, 7, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Nestorovich, E.M.; Bezrukov, S.M. Designing inhibitors of anthrax toxin. Expert Opin. Drug Discov. 2014, 9, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Duesbery, N.S.; Webb, C.P.; Leppla, S.H.; Gordon, V.M.; Klimpel, K.R.; Copeland, T.D.; Ahn, N.G.; Oskarsson, M.K.; Fukasawa, K.; Paull, K.D.; et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 1998, 280, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Bernardi, L.; Napolitani, G.; Mock, M.; Montecucco, C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 2000, 352, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Levinsohn, J.L.; Newman, Z.L.; Hellmich, K.A.; Fattah, R.; Getz, M.A.; Liu, S.; Sastalla, I.; Leppla, S.H.; Moayeri, M. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Leppla, S.H. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1982, 79, 3162–3166. [Google Scholar] [CrossRef] [PubMed]
- Leppla, S.H. Bacillus anthracis calmodulin-dependent adenylate cyclase: Chemical and enzymatic properties and interactions with eucaryotic cells. Adv. Cycl. Nucleotide Protein Phosphorylation Res. 1984, 17, 189–198. [Google Scholar]
- Petosa, C.; Collier, R.J.; Klimpel, K.R.; Leppla, S.H.; Liddington, R.C. Crystal structure of the anthrax toxin protective antigen. Nature 1997, 385, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Kintzer, A.F.; Thoren, K.L.; Sterling, H.J.; Dong, K.C.; Feld, G.K.; Tang, I.I.; Williams, E.R.; Berger, J.M.; Krantz, B.A. The protective antigen component of anthrax toxin forms functional octameric complexes. J. Mol. Biol. 2009, 392, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Kintzer, A.F.; Sterling, H.J.; Tang, I.I.; Williams, E.R.; Krantz, B.A. Anthrax toxin receptor drives protective antigen oligomerization and stabilizes the heptameric and octameric oligomer by a similar mechanism. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Mogridge, J.; Cunningham, K.; Collier, R.J. Stoichiometry of anthrax toxin complexes. Biochemistry 2002, 41, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Pilpa, R.M.; Bayrhuber, M.; Marlett, J.M.; Riek, R.; Young, J.A. A receptor-based switch that regulates anthrax toxin pore formation. PLoS Pathog. 2011, 7. [Google Scholar] [CrossRef] [PubMed]
- Abrami, L.; Brandi, L.; Moayeri, M.; Brown, M.J.; Krantz, B.A.; Leppla, S.H.; van der Goot, F.G. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell. Rep. 2013, 5, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, R.O.; Koehler, T.M.; Collier, R.J.; Finkelstein, A. Anthrax toxin: Channel-forming activity of protective antigen in planar phospholipid bilayers. Proc. Natl. Acad. Sci. USA 1989, 86, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pentelute, B.L.; Collier, R.J.; Zhou, Z.H. Atomic structure of anthrax protective antigen pore elucidates toxin translocation. Nature 2015, 521, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Udho, E.; Wu, Z.; Collier, R.J.; Finkelstein, A. Protein translocation through anthrax toxin channels formed in planar lipid bilayers. Biophys. J. 2004, 87, 3842–3849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Finkelstein, A.; Collier, R.J. Evidence that translocation of anthrax toxin’s lethal factor is initiated by entry of its N terminus into the protective antigen channel. Proc. Natl. Acad. Sci. USA 2004, 101, 16756–16761. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Panchal, R.G.; Bavari, S.; Nguyen, T.L.; Gussio, R.; Ribot, W.; Friedlander, A.; Chabot, D.; Reiner, J.E.; Robertson, J.W.; et al. Anthrax toxin-induced rupture of artificial lipid bilayer membranes. J. Chem. Phys. 2013, 139. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Teijido, O.; Hoogerheide, D.P.; Rostovtseva, T.K.; Berezhkovskii, A.M.; Bezrukov, S.M. Conductance hysteresis in the voltage-dependent anion channel. Eur. Biophys. J. 2015, 44, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Hellman, J.; Nikaido, H. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J. Biol. Chem. 1988, 263, 1182–1187. [Google Scholar] [PubMed]
- Robertson, K.M.; Tieleman, D.P. Molecular basis of voltage gating of OmpF porin. Biochem. Cell Biol. 2002, 80, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Electrophysiology of Unconventional Channels and Pores; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Bainbridge, G.; Gokce, I.; Lakey, J.H. Voltage gating is a fundamental feature of porin and toxin beta-barrel membrane channels. FEBS Lett. 1998, 431, 305–308. [Google Scholar] [CrossRef]
- Teijido, O.; Rappaport, S.M.; Chamberlin, A.; Noskov, S.Y.; Aguilella, V.M.; Rostovtseva, T.K.; Bezrukov, S.M. Acidification asymmetrically affects voltage-dependent anion channel implicating the involvement of salt bridges. J. Biol. Chem. 2014, 289, 23670–23682. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Tan, W.; Colombini, M. On the role of VDAC in apoptosis: Fact and fiction. J. Bioenerg. Biomembr. 2005, 37, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Kullman, L.; Winterhalter, M.; Bezrukov, S.M. Transport of maltodextrins through maltoporin: A single-channel study. Biophys. J. 2002, 82, 803–812. [Google Scholar] [CrossRef]
- Blaustein, R.O.; Finkelstein, A. Diffusion limitation in the block by symmetric tetraalkylammonium ions of anthrax toxin channels in planar phospholipid bilayer membranes. J. Gen. Physiol. 1990, 96, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, R.O.; Finkelstein, A. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. Effects on macroscopic conductance. J. Gen. Physiol. 1990, 96, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Karginov, V.A.; Nestorovich, E.M.; Yohannes, A.; Robinson, T.M.; Fahmi, N.E.; Schmidtmann, F.; Hecht, S.M.; Bezrukov, S.M. Search for cyclodextrin-based inhibitors of anthrax toxins: Synthesis, structural features, and relative activities. Antimicrob. Agents Chemother. 2006, 50, 3740–3753. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.S.; Blaustein, R.O. Preventing voltage-dependent gating of anthrax toxin channels using engineered disulfides. J. Gen. Physiol. 2008, 132, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M.; Robinson, T.M.; Leppla, S.H.; Karginov, V.A. In vivo efficacy of beta-cyclodextrin derivatives against anthrax lethal toxin. Antimicrob. Agents Chemother. 2008, 52, 2239–2241. [Google Scholar] [CrossRef] [PubMed]
- French, R.J.; Shoukimas, J.J. An ion’s view of the potassium channel. The structure of the permeation pathway as sensed by a variety of blocking ions. J. Gen. Physiol. 1985, 85, 669–698. [Google Scholar] [CrossRef] [PubMed]
- Gurnev, P.A.; Queralt-Martin, M.; Aguilella, V.M.; Rostovtseva, T.K.; Bezrukov, S.M. Probing tubulin-blocked state of VDAC by varying membrane surface charge. Biophys. J. 2012, 102, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Gurnev, P.A.; Yap, T.L.; Pfefferkorn, C.M.; Rostovtseva, T.K.; Berezhkovskii, A.M.; Lee, J.C.; Parsegian, V.A.; Bezrukov, S.M. Alpha-synuclein lipid-dependent membrane binding and translocation through the alpha-hemolysin channel. Biophys. J. 2014, 106, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Frechet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Ballauff, M.; Likos, C.N. Dendrimers in solution: Insight from theory and simulation. Angew. Chem. Int. Ed. Engl. 2004, 43, 2998–3020. [Google Scholar] [CrossRef] [PubMed]
- Huissmann, S.; Likos, C.N.; Blaak, R. Conformations of high-generation dendritic polyelectrolytes. J. Mater. Chem. 2010, 20, 10486–10494. [Google Scholar] [CrossRef]
- Maiti, P.K.; Lin, S.T.; Cagin, T.; Goddard, W.A. Effect of Solvent and pH on the Structure of PAMAM Dendrimers. Macromolecules 2005, 38, 979–991. [Google Scholar] [CrossRef]
- Huissmann, S.; Wynveen, A.; Likos, C.N.; Blaak, R. The effects of pH, salt and bond stiffness on charged dendrimers. J. Phys. Condens Matter 2010, 22, 232101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A., 3rd. PAMAM dendrimers undergo pH responsive conformational changes without swelling. J. Am. Chem. Soc. 2009, 131, 2798–2799. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, E.; Paulo, P.M. Deswelling and electrolyte dissipation in free diffusion of charged PAMAM dendrimers. J. Phys. Chem. Lett. 2014, 5, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.K.; Bagchi, B. Diffusion of flexible, charged, nanoscopic molecules in solution: Size and pH dependence for PAMAM dendrimer. J. Chem. Phys. 2009, 131, 214901. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.K.; Messina, R. Counterion Distribution and ζ-Potential in PAMAM Dendrimer. Macromolecules 2008, 41, 5002–5006. [Google Scholar] [CrossRef]
- Mecke, A.; Lee, I.; Baker, J.R., Jr.; Holl, M.M.; Orr, B.G. Deformability of poly(amidoamine) dendrimers. Eur. Phys. J. E. Soft Matter 2004, 14, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Porcar, L.; Hong, K.; Shew, C.Y.; Li, X.; Liu, E.; et al. Effect of counterion valence on the pH responsiveness of polyamidoamine dendrimer structure. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef] [PubMed]
- Porcar, L.; Liu, Y.; Verduzco, R.; Hong, K.; Butler, P.D.; Magid, L.J.; Smith, G.S.; Chen, W. Structural investigation of PAMAM dendrimers in aqueous solutions using small-angle neutron scattering: Effect of generation. J. Phys. Chem. B 2008, 112, 14772–14778. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kerkeni, B.; Egami, T.; Do, C.; Liu, Y.; Wang, Y.; Porcar, L.; Hong, K.; Smith, S.C.; Liu, E.L.; et al. Structured water in polyelectrolyte dendrimers: Understanding small angle neutron scattering results through atomistic simulation. J. Chem. Phys. 2012, 136, 144901. [Google Scholar] [CrossRef] [PubMed]
- Cakara, D.; Kleimann, J.; Borkovec, M. Microscopic protonation equilibria of poly(amidoamine) dendrimers from macroscopic titrations. Macromolecules 2003, 36, 4201–4207. [Google Scholar] [CrossRef]
- Huang, Q.R.; Dubin, P.L.; Moorefield, C.N.; Newkome, G.R. Counterion binding on charged spheres: Effect of pH and ionic strength on the mobility of carboxyl-terminated dendrimers. J. Phys. Chem. B 2000, 104, 898–904. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; de Paoli Lacerda, S.H.; McNeil, S.E. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Böhme, U.; Klenge, A.; Hänel, B.; Scheler, U. Counterion condensation and effective charge of PAMAM dendrimers. Polymers 2011, 3, 812–819. [Google Scholar] [CrossRef]
- Bustamante, J.O.; Michelette, E.R.; Geibel, J.P.; Hanover, J.A.; McDonnell, T.J.; Dean, D.A. Dendrimer-assisted patch-clamp sizing of nuclear pores. Pflugers Arch. 2000, 439, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Kinns, H.; Mitchell, N.; Astier, Y.; Madathil, R.; Howorka, S. Nanoscale protein pores modified with PAMAM dendrimers. J. Am. Chem. Soc. 2007, 129, 9640–9649. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Harrington, L.; Li, Q.; Cheley, S.; Davis, B.G.; Bayley, H. Single-molecule interrogation of a bacterial sugar transporter allows the discovery of an extracellular inhibitor. Nat. Chem. 2013, 5, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Ficici, E.; Andricioaei, I.; Howorka, S. Dendrimers in nanoscale confinement: The interplay between conformational change and nanopore entrance. Nano Lett. 2015, 15, 4822–4828. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.; Kanchi, S.; Ayappa, K.G.; Maiti, P.K. pH controlled gating of toxic protein pores by dendrimers. Nanoscale 2016, 8, 13045–13058. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Gurnev, P.A.; Chen, M.Y.; Bezrukov, S.M. Membrane lipid composition regulates tubulin interaction with mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2012, 287, 29589–29598. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Epand, R.M. PAMAM dendrimers and model membranes: Differential scanning calorimetry studies. Int. J. Pharm. 2005, 305, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Mecke, A.; Uppuluri, S.; Sassanella, T.M.; Lee, D.K.; Ramamoorthy, A.; Baker, J.R., Jr.; Orr, B.G.; Banaszak Holl, M.M. Direct observation of lipid bilayer disruption by poly(amidoamine) dendrimers. Chem. Phys. Lipids 2004, 132, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Baytekin, B.; Werner, N.; Luppertz, F.; Engeser, M.; Brüggemann, J.; Bitter, S.; Henkel, R.; Felder, T.; Schalley, C.A. How useful is mass spectrometry for the characterization of dendrimers? “Fake defects” in the ESI and MALDI mass spectra of dendritic compounds. Int. J. Mass Spectrom. 2006, 249–250, 138–148. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
| PA63/PAMAM Dendrimer Binding Reaction, IC50 | ||||||
|---|---|---|---|---|---|---|
| G2-NH2 | G2-OH | G2-SA | G2-COONa | G2 75% OH 25% NH2 | G3-NH2 Dendron | |
| 0.1 M KCl | 7.2 ± 4.7 nM | 142 ± 36 nM | 879 ± 50 µM | >14 mM | 122 ± 35 nM | 16.4 ± 4.0 nM |
| 1 M KCl | 5.1 ± 2.6 mM | >30 mM | 1.7 ± 0.2 mM | not determined | 7.7 ± 0.2 mM | 7.8 ± 1.0 mM |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamini, G.; Kalu, N.; Nestorovich, E.M. Impact of Dendrimer Terminal Group Chemistry on Blockage of the Anthrax Toxin Channel: A Single Molecule Study. Toxins 2016, 8, 337. https://doi.org/10.3390/toxins8110337
Yamini G, Kalu N, Nestorovich EM. Impact of Dendrimer Terminal Group Chemistry on Blockage of the Anthrax Toxin Channel: A Single Molecule Study. Toxins. 2016; 8(11):337. https://doi.org/10.3390/toxins8110337
Chicago/Turabian StyleYamini, Goli, Nnanya Kalu, and Ekaterina M. Nestorovich. 2016. "Impact of Dendrimer Terminal Group Chemistry on Blockage of the Anthrax Toxin Channel: A Single Molecule Study" Toxins 8, no. 11: 337. https://doi.org/10.3390/toxins8110337





