Abstract
Tropidolaemus wagleri and Cryptelytrops purpureomaculatus are venomous pit viper species commonly found in Malaysia. Tandem mass spectrometry analysis of the crude venoms has detected different proteins in T. wagleri and C. purpureomaculatus. They were classified into 13 venom protein families consisting of enzymatic and nonenzymatic proteins. Enzymatic families detected in T. wagleri and C. purpureomaculatus venom were snake venom metalloproteinase, phospholipase A2, l-amino acid oxidase, serine proteases, 5′-nucleotidase, phosphodiesterase, and phospholipase B. In addition, glutaminyl cyclotransferase was detected in C. purpureomaculatus. C-type lectin-like proteins were common nonenzymatic components in both species. Waglerin was present and unique to T. wagleri—it was not in C. purpureomaculatus venom. In contrast, cysteine-rich secretory protein, bradykinin-potentiating peptide, and C-type natriuretic peptide were present in C. purpureomaculatus venom. Composition of the venom proteome of T. wagleri and C. purpureomaculatus provides useful information to guide production of effective antivenom and identification of proteins with potential therapeutic applications.
1. Introduction
Snake venom is a highly complex mixture of proteins and polypeptides with a myriad of biological activities. It functions as an important tool for defense against predators, prey immobilization, and facilitation of prey digestion [1]. There are two families of terrestrial venomous snake that cause the majority of envenoming cases in Malaysia: Elapidae and Viperidae. Among the potentially dangerous snakes that belong to the viperid subfamily Crotalinae (pit vipers) are Tropidolaemus wagleri (Temple or Wagler’s pit viper) and Cryptelytrops purpureomaculatus (mangrove pit viper) [1]. Envenomation from these pit vipers is often associated with intense pain, local swelling, necrosis, hemorrhage, and blood pressure disruption [1].
Tropidolaemus wagleri is recognized as a primitive and unique pit viper, morphologically as well as biochemically [2,3]. Enzymatic properties of the venom highlight similarity of venom components to other vipers and include nonlethal enzymes such as phosphodiesterase, thrombin-like enzyme (TLE), l-amino acid oxidase (LAAO), and phospholipase A2 (PLA2) [4]. Lethal components of T. wagleri venom were identified as low molecular-weight toxins, namely waglerins-1, -2, -3, and -4 [5]. These toxins were classified as neurotoxins due to their competitive antagonism with the nicotinic acetylcholine receptor (nAChR) [6,7]. This feature is considered unusual for snakes of the family Viperidae, as the presence of postsynaptic neurotoxins is well-known in the venoms of elapid snakes [8]. Cryptelytrops purpureomaculatus is known to be very aggressive and is commonly found on mangrove mudflats on the coastal region of west peninsular Malaysia. The color of C. purpureomaculatus is variable, ranging from grayish to olive and brownish purple [1,9]. The venom is known to be nonfatal to humans but causes local swelling and pain [10,11]. The venom was found to have anticoagulant, thrombin-like, hemorrhagic, and other enzymatic activities, and it is more toxic than several other Asian arboreal viper species [12].
Information on the venom proteome is important for understanding and predicting the clinical consequences of envenomation and for formulating an effective antivenom that will target and neutralize venom components common to viper species [13]. At present, online protein database searches using UniProt [14] for “Tropidolaemus wagleri” and “Cryptelytrops purpureomaculatus” listed only five and four proteins, respectively. Hence, much is still unknown about the proteome of T. wagleri and C. purpureomaculatus venoms. Here we performed proteomic profiling and compared the crude venom composition of T. wagleri and C. purpureomaculatus using a shotgun-proteomics approach. Shotgun-proteomics and liquid chromatography tandem mass spectrometry (LC–MS/MS) have been described as a rapid, bottom-up proteomic techniques that allows direct analysis of simple and complex protein samples to generate a global profile of the total protein components [15,16].
2. Results and Discussion
2.1. The Venom Proteome of T. wagleri and C. purpureomaculatus
The venom proteomes of T. wagleri and C. purpureomaculatus have never been completely reported using proteomic techniques such as shotgun-proteomic and LC–MS/MS. Shotgun-proteomics and LC–MS/MS approaches have been used to characterize many other snake venom proteomes, including kraits and cobras [17,18].
2.1.1. T. wagleri Venom
Earlier studies on T. wagleri venom have demonstrated the presence of enzymatic proteins commonly occurring in pit viper venom such as phosphodiesterase, phosphomonoesterase, hydrolase, TLE, LAAO, and PLA2 [4]. The venom lethality was comparable to other pit viper species venom but distinct due to the lack of hemorrhagic activity [4,5]. The lethal toxins were then identified as low molecular-weight peptides; waglerins [4,5]. In the public protein database (UniProtKB), only five proteins were reviewed and listed under the heading of Tropidolaemus wagleri: waglerin-3 (WAG13_TROWA), SNACLEC trowaglerix subunit alpha (SLA_TROWA), waglerin-4 (WAG24_TROWA), SNACLEC trowaglerix subunit beta (SLB_TROWA), and NADH-ubiquinone oxidoreductase (NU4M_TROWA). In our study, mass spectrometry analysis of T. wagleri venom detected 13 different proteins that shared similar sequences with proteins in the database (SwissProt, Serpentes) (Table 1). Protein families that were not reported previously in the venom, (e.g., snake venom metalloproteinase (SVMP)) were detected in the present study (Table 2).

Table 1.
List of detected proteins in Malaysian T. wagleri from in-solution digests by LC–MS/MS. Please refer to Supplementary File 1 for a complete list of peptides and m/z values.

Table 2.
List of detected proteins in Malaysian C. purpureomaculatus from in-solution digests by LC–MS/MS. Please refer to Supplementary File 2 for a complete list of peptides and m/z values.
2.1.2. C. purpureomaculatus Venom
The enzymatic activities detected in C. purpureomaculatus venom are due to the presence of TLE, phospholipase A, arginine ester hydrolase, arginine amidase, protease, 5′-nucleotidase, acetylcholinesterase, and alkaline phosphomonoesterase [19]. The venom may exhibit its lethality through hemorrhagic, edema-inducing, and thrombin-like activity [19]. There were only four proteins that were deposited under Cryptelytrops purpureomaculatus in UniProt, namely, TLE purpurase (VSPP_TRIPP), SNACLEC purpureotin subunit alpha (SLA_TRIPP) and beta (SLB_TRIPP), and CRVP (CRVP_TRIPP). In this study, 51 different venom proteins with sequence similarity with proteins in the public protein database were detected (Table 1). Several proteins that have never been reported previously for C. purpureomaculatus such as SVMP-disintegrin, glutaminyl-peptide cyclotransferase (QPCT), bradykinin-potentiating and C-type natriuretic peptide (BCNP), and phospholipase B (PLB) were detected in this study (Table 2).
2.2. Venom Composition of T. wagleri and C. purpureomaculatus
The venom composition of T. wagleri and C. purpureomaculatus was divided into enzymatic and nonenzymatic protein families (Table 3 and Table 4). Enzymatic protein families were found to be the major venom protein family in T. wagleri and C. purpureomaculatus venom with 62% and 73% of the total proteins, respectively (Figure 1 and Figure 2). Nonenzymatic protein families of T. wagleri and C. purpureomaculatus venom were detected at 38% and 27% of the total proteins, respectively.

Table 3.
Enzymatic and nonenzymatic proteins from T. wagleri crude venom.

Table 4.
Enzymatic and nonenzymatic proteins from C. purpureomaculatus crude venom.
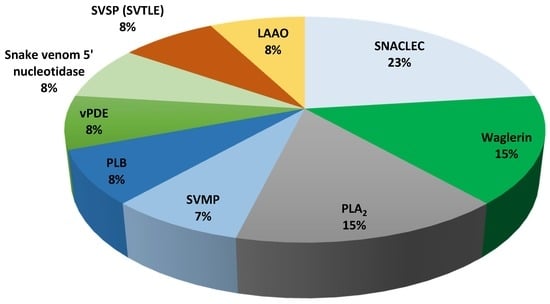
Figure 1.
Relative abundance of venom proteins that were detected using LC–MS/MS from Malaysian T. wagleri venom. PLB: phospholipase B, vPDE: venom phosphodiesterase, SVTLE: snake venom thrombin-like enzyme, SVSP: snake venom serine protease, SNACLEC: snake venom C-type lectin, LAAO: l-amino acid oxidase, PLA2: phospholipase A2, SVMP: snake venom metalloproteinase.
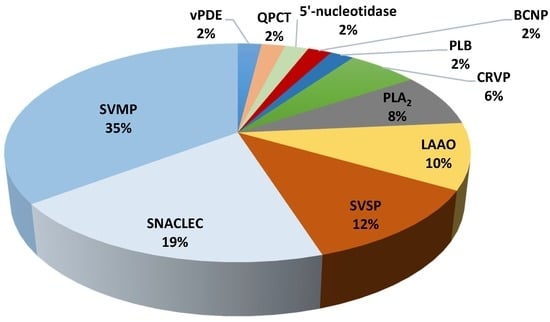
Figure 2.
Relative abundance of venom proteins that were detected using LC–MS/MS from Malaysian C. purpureomaculatus venom. PLB: phospholipase B, vPDE: venom phosphodiesterase, SVSP: snake venom serine protease, QPCT: glutaminyl-peptide cyclotransferase, BCNP: bradykinin-potentiating and C-type natriuretic peptide, CRVP: cysteine-rich venom protein, PLA2: phospholipase A2, LAAO: l-amino acid oxidase, SNACLEC: snake venom C-type lectin, SVMP: snake venom metalloproteinase.
2.2.1. Enzymatic Protein Families of T. wagleri and C. purpureomaculatus Venom
SVMP was identified to be 7% of the total proteins detected in T. wagleri (Table 5). In contrast, it is the major venom protein family representing 35% of the total proteins in C. purpureomaculatus, 5 times more compared to T. wagleri (Table 5). The high abundance of SVMP in C. purpureomaculatus is consistent with the biochemical studies on the venom [19,20]. SVMPs are one of the most important components of the viperid venoms, causing hemorrhage by affecting blood coagulation and/or integrity of extracellular matrix components such as collagen, laminin, and fibronectin [21,22,23]. SVMPs are classified into three classes, P-I to P-III, based on their domain structure [24]. The majority of SVMPs that were detected in both T. wagleri and C. purpureomaculatus belong to P-II and P-III classes (Table 1 and Table 2). P-II class contains metalloproteinase and disintegrin domains while P-III class contains metalloproteinase, disintegrin-like, and cysteine-rich domains [24]. Biological activities of P-II SVMPs include proteolytic induction [25], platelet aggregation inhibition [26], and hemorrhagic [27]. Most P-III SVMPs were identified as hemorrhagic and have the most potent biological activity among the three SVMP classes [24]. Additionally, some P-III SVMPs can induce apoptosis in vascular endothelial cells [28,29,30]. The detection of SVMP in T. wagleri is intriguing, as several earlier reports have shown the absence of hemorrhage activity from the venom [4,31]. Higher sensitivity and resolution of LC–MS/MS compared to biochemical methods could be the reason for the detection of SVMPs in the T. wagleri venom. SVMPs from T. wagleri have never been isolated and studied, therefore there is a need to study these SVMPs and elucidate their biological activity and function. It is possible that intraspecific snake venom variations and regional variations could contribute toward the presence of SVMPs in the venom [32,33]. More extensive study using samples from various regions where T. wagleri is endemic is required to confirm this possibility.

Table 5.
Differences in the composition of venom protein families in Malaysian T. wagleri and C. purpureomaculatus venoms.
LAAO and PLA2 were other major components in the venoms (Table 5). LAAO and PLA2 activities of T. wagleri and C. purpureomaculatus venoms have been described in other studies [4,19]. LAAO is a ubiquitous component of snake venom, but its abundance varies between species [34]. This was demonstrated by our present findings where the percentage of LAAO was found to be lower in T. wagleri (8%), compared to C. purpureomaculatus (10%) (Table 5). The LAAOs that were detected in this study showed sequence similarity with LAAO from several genera including Calloselasma, Gloydius, Viridovipera, Bothrops, Daboia, and Cerastes (Table 1 and Table 2). LAAOs in T. wagleri and C. purpureomaculatus could be partially or synergistically responsible for various biological effects upon envenomation, including hemorrhage [35], edema [36], and platelet aggregation [37,38]. Venom PLA2 may interrupt normal physiological processes, causing various pharmacological effects such as neurotoxicity, myotoxicity, and cardiotoxicity [39,40,41]. The PLA2 isoforms that were detected in T. wagleri and C. purpureomaculatus venom showed sequence similarity with PLA2 that were described in the other viper species venoms including Bothrops, Trimeresurus, and Ovophis venoms (Table 1 and Table 2). PLA2 detected from both species were identified as basic and acidic isoforms of PLA2 (Table 1 and Table 2). The presence of PLA2 isoforms in T. wagleri was consistent with the discovery of acidic and basic variants from Sulawesi and Sumatran origin Tropidolaemus genus [42].
Other families of enzymatic proteins detected in T. wagleri and C. purpureomaculatus include snake venom 5′-nucleotidase, venom phosphodiesterase (vPDE), and PLB. Each of these proteins was detected at 8% in T. wagleri and 2% in C. purpureomaculatus (Table 5). Snake venom serine protease (SVSP) was determined at 8% in T. wagleri and 12% in C. purpureomaculatus (Table 5). Glutaminyl-peptide cyclotransferase (QPCT) was demonstrated in C. purpureomaculatus at 2%, but was not found in T. wagleri (Table 5). Snake venom QPCT has been suggested to have an indirect contribution to venom toxicity via posttranslational modification of venom proteins [43,44]. QPCT is important in the N-terminal glutamine cyclization that induces toxin maturation, protects from exopeptidase degradation, and/or assists proper protein conformation [44].
The presence and activity of snake venom 5′-nucleotidase and vPDE in T. wagleri and C. purpureomaculatus have been demonstrated by biochemical assays, which agrees with our findings [4,19]. LC–MS/MS data showed that these proteins shared sequence similarity to proteins found in Gloydius brevicaudus and Crotalus adamanteus (Table 1 and Table 2). These proteins were thought to inhibit platelet aggregation through the liberation of purines using endogenous source from their envenomed victims [45]. We have also detected the presence of PLB in T. wagleri and C. purpureomaculatus crude venoms, similar to those found in Crotalus adamanteus (Table 1 and Table 2). The role of PLB in snake venom was not well understood, but its hemolytic activity has been demonstrated in vitro [46]. SVSP has several biological activities such as platelet aggregation, coagulation, and fibrinolysis [47,48]. In this study, different classes of SVSP were detected from T. wagleri and C. purpureomaculatus including TLE, fibrinogenase, and vPA (Table 1 and Table 2). TLE plays a significant role in coagulation process [49]. TLE has been purified and characterized from C. purpureomaculatus, but not from T. wagleri [50]. The antibody developed from purpurase, a TLE isolated from C. purpureomaculatus, was found to strongly react with Trimeresurus complex venom, suggesting sequence homology of TLEs within the complex [50]. This finding is consistent with our LC–MS/MS data that detected TLEs that shared similar sequences with TLEs found in Cryptelytrops albolabris, and Viridovipera stejnegeri (Table 2). Fibrinogenase affects the blood clotting mechanism through fibrinolytic action [51,52]. vPA is another class of serine protease that activates plasminogen to plasmin, promoting fibrinolysis upon envenomation of its victim [53].
2.2.2. Nonenzymatic Protein Families of T. wagleri and C. purpureomaculatus
Snake venom C-type lectin (SNACLEC) was found to be the largest nonenzymatic protein family and accounted for 23% and 19% of T. wagleri and C. purpureomaculatus crude venom, respectively (Table 5). The SNACLECs in T. wagleri venom shared similarity with SNACLEC found in Ophiophagus hannah, Viridovipera stejnegeri, and Cryptelytrops albolabris venoms (Table 1). The SNACLECs found in C. purpureomaculatus shared sequence similarity with SNACLEC found in genus Trimeresurus, Deinagkistrodon acutus, and Echis multisquamatus (Table 2). SNACLEC from both T. wagleri (trowaglerix) and C. purpureomaculatus (purpureotin) have been characterized previously [54,55]. These proteins were known to either inhibit or activate specific platelet receptors, such as integrins, which affect thrombosis or hemostasis processes [56,57]. Trowaglerix induces platelet aggregation through specific binding to glycoprotein IV (GPIV) receptor [54], while purpureotin binds to glycoprotein Ib (GPIb) receptor [57].
Waglerin was found exclusively in T. wagleri and it is the second largest protein family, which constitutes 15% of the crude venom (Figure 1, Table 5). Waglerin has been well characterized and considered as T. wagleri’s most unique and most lethal protein [5,7,58,59]. To date, four types of waglerins have been described [5,58]. The peptides waglerins-1 and -2 differ by one amino acid at position 10 (waglerin-1: histidine, waglerin-2; tyrosine). Waglerins-3 and -4 are almost homologous to waglerins-1 and -2, respectively, except for two additional amino acids (serine and leucine) at their N-terminal [5,58]. Waglerins identified in this study were noted as waglerins-3 and -4 because the database used in this study indicated that waglerins-1 and -2 corresponded with waglerins-3 and -4, respectively (Table 1). Waglerin exerts its toxicity through competitive antagonism with the nicotinic acetylcholine receptor (nAChR) at nanomolar concentrations [7,58]. Earlier studies found that waglerin-1 selectivity binds to the α-ε nAChR interface with 2000-fold higher affinity compared to other binding sites [6]. The finding of high abundance of waglerin in our study (15%) agrees with several reports that identified the protein as the major toxic component of the venom [4,5,7]. In addition, the percentage of peptide coverage identified by MS spectra correlates with the protein abundance [60]. This data is supported by LC–MS/MS, which showed 100% waglerin peptide detection coverage (Table 1, Supplementary File 1).
Cysteine-rich venom protein (CRVP), also known as snake venom cysteine-rich secretory protein (CRISP) and BCNP were found specifically in C. purpureomaculatus at 2% of the total venom (Table 5). The amino acid sequence of CRVP from various snake species has been well characterized, however, the biological activity has not been fully understood [61]. Nonetheless, CRVP isolated from several viper species may exhibit neurotoxicity through the blocking of several ion channels [62,63]. Three different CRVPs were detected from C. purpureomaculatus venom using LC–MS/MS. These CRVPs shared sequence similarity with CRVP from Viridovipera stejnegeri, Protobothrops flavoviridis and a CRVP from C. purpureomaculatus, tripurin (Table 2). BCNP is a unique snake venom protein, and its cDNA encodes both bradykinin-potentiating peptide (BPP) and C-type natriuretic peptides (CNP), thereby integrating two different vasodilating molecules into one [64]. Biological effects of BCNP upon envenomation include hypotension and loss of consciousness [65,66]. In this study, BCNP was found specifically in C. purpureomaculatus at 2% of the total venom (Table 5). BPPs can inhibit angiotensin-converting enzyme activity and, along with CNP, have been investigated for the treatment of human hypertension and several other cardiovascular diseases, including congestive heart failure [65,66,67]. We believe this is the first study to identify the presence of BCNP in C. purpureomaculatus venom.
3. Conclusions
We have successfully characterized and compared the venom proteome of Malaysian T. wagleri and C. purpureomaculatus by using shotgun-proteomics, LC–MS/MS, and protein de novo sequencing. The present data have revealed the complex composition of the crude venom from both species. The venom proteome of both snakes consists of enzymatic and nonenzymatic protein families. The proteins detected in both T. wagleri and C. purpureomaculatus were SVMP, SVSP, LAAO, PLA2, vPDE, snake venom 5′-nucleotidase, and SNACLEC. Neurotoxin waglerin was unique in T. wagleri venom, whereas BCNP and QPCT were unique in C. purpureomaculatus venom. This information may be useful to predict the clinical prognosis after envenoming and provide better guidance for the production of effective antivenom. Moreover, proteins detected in T. wagleri and C. purpureomaculatus venoms could be selectively investigated for therapeutic potentials.
4. Materials and Methods
4.1. Materials
Snake Venom
Crude T. wagleri and C. purpureomaculatus venoms were obtained from Mr. Zainuddin Ismail, a private snake enthusiast from Perlis in Peninsula Malaysia. All snakes originated from the west of Peninsular Malaysia. Snake venoms were collected in a sterile container covered with parafilm. The venoms were transported back to Monash Malaysia campus on ice and frozen at −80 °C before subjecting them to the freeze-drying process. Freeze dried venom was weighed, labeled, and stored at −20 °C until use. Venom samples from three different sampling sessions were used for the experiment.
4.2. Methods
4.2.1. In-Solution Tryptic Digestion
Approximately 0.5 mg of crude venom was added into 1.5 mL tube in triplicates and mixed with 25 μL of 100 mM ammonium bicarbonate (ABC), 25 μL of trifluoroethanol, and 1 μL of 200 mM 1,4-dithiothreitol (DTT). The mixture was then briefly vortexed, and incubated at 60 °C for 1 h. Next, the protein in the tube was alkylated by adding 4.0 μL of 200 mM iodoacetamide, briefly vortexed, and incubated at room temperature in the dark (covered with aluminum foil) for 1 h. Subsequently, 1 μL of 200 mM DTT was added to the tube and incubated at room temperature in the dark for another 1 h. Double-distilled water and 100 mM ABC were then added to the sample mixture to dilute the protein denaturant and raised the pH to 7–9. Trypsin solution was added to the tubes at the weight ratio of 1:50, briefly vortexed and incubated overnight at 37 °C. On the next day, 1 μL of formic acid was added to stop the trypsin digestion, briefly vortexed, and left in a vacuum concentrator overnight to concentrate the digested proteins. Samples were kept at −20 °C prior to LC–MS/MS analysis.
4.2.2. Nanoflow Liquid Chromatography Electrospray-Ionization Coupled with Tandem Mass Spectrometry (Nanoflow–ESI–LC–MS/MS)
The digested peptides were loaded into an Agilent C18 300 Å Large Capacity Chip (Agilent, Santa Clara, CA, USA) column that was equilibrated with 0.1% formic acid in water (solution A). The peptides were eluted from the column with 90% acetonitrile in 0.1% formic acid in water (solution B) using the following gradient; 3%–50% solution B over 0–30 min, 50%–95% solution B over 2 min, 95% solution B for 7 min, and 95%–3% solution B over 39–47 min. Quadrupole-time of flight (Q-TOF) polarity was set at positive with capillary and fragmenter voltage being set at 2050 V and 300 V, respectively, and 5 L/min of gas flow with a temperature of 300 °C. The peptide spectrum was analyzed in auto MS mode ranging from 110–3000 m/z for MS scan and 50–3000 m/z for MS/MS scan. The spectrum was then analyzed with Agilent MassHunter (Agilent Technologies, Santa Clara, CA, USA) data acquisition software and then PEAKS 7.0 software (Bioinformatics Solutions Inc., Waterloo, ON, Canada).
4.2.3. Venom Protein Identification by Automated de Novo Sequencing (PEAKS Studio 7.0)
Protein identification by automated de novo sequencing was performed with PEAKS Studio 7.0 (Bioinformatics Solution Inc., Waterloo, ON, Canada). SwissProt.Serpentes (May 2015) database was used for protein identification and homology search by comparing the de novo sequence tag. Carbamidomethylation was set as fixed modification with maximum mixed cleavages at 3. Parent mass and fragment mass error tolerance were both set 0.1 Da with monoisotopic as the precursor mass search type. Trypsin was selected as the enzyme used for digestion. False discovery rate (FDR) of 1% and unique peptide ≥2 were used for filtering out inaccurate proteins. A −10lgP score of greater than 20 indicates detected proteins are relatively high in confidence as it targets very few decoy matches above that threshold [68]. The percentage of the venom protein family in the crude venom was calculated using the following formula:
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/8/10/299/s1, Supplementary Files 1 and 2.
Acknowledgments
The authors would like to acknowledge Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia for research facilities and support. This study was financially supported by Fundamental Research Grant Scheme (FRGS) from Ministry of Education, Malaysia (FRGS/2/2014/SKK01/MUSM/01/1). We would also like to thank Nurziana Sharmilla and Adillah Akhasan from Jeffrey Cheah School of Medicine and Health Sciences, LC-MS laboratory for assisting us operating the LC-MS system. Syafiq Asnawi Zainal Abidin is supported by MyBrain15 (MyPhD) scholarship under Ministry of Education Malaysia.
Author Contributions
Syafiq Asnawi Zainal Abidin was responsible for the experimental design, conducted the experiments, analyzed the data and wrote the manuscript. Pathmanathan Rajadurai, Md Ezharul Hoque Chowdhurry, and Muhamad Rusdi Ahmad Rusmili, provided critical feedbacks and approved the manuscript. Iekhsan Othman analyzed the data and provided critical feedbacks. Rakesh Naidu contributed in the experimental design, analyzed the data, and provided critical feedbacks for the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, I.; Ahmed, N.; Liat, L.B. Venomous terrestrial snakes of Malaysia: Their identity and biology. In Clinical Toxinology; Gopalakrishnakone, P., Faiz, S.M.A., Gnanathasan, A.C., Habib, G.A., Fernando, R., Yang, C.-C., Eds.; Malaysian Academic Library Institutional Repository: Kuala Lumpur, Malaysia, 2013; pp. 1–15. [Google Scholar]
- Vogel, G.; David, P.; Lutz, M.; Van Rooijen, J.; Vidal, N. Revision of the Tropidolaemus wagleri-complex (serpentes: Viperidae: Crotalinae). I. Definition of included taxa and redescription of Tropidolaemus wagleri (Boie, 1827). Zootaxa 2007, 1644, 1–40. [Google Scholar]
- Castoe, T.A.; Parkinson, C.L. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes). Mol. Phylogenet. Evol. 2006, 39, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Tan, C.S. The enzymatic activities and lethal toxins of Trimeresurus wagleri (speckled pit viper) venom. Toxicon 1989, 27, 349–357. [Google Scholar] [PubMed]
- Weinstein, S.A.; Schmidt, J.J.; Bernheimer, A.W.; Smith, L.A. Characterization and amino acid sequences of two lethal peptides isolated from venom of wagler’s pit viper, Trimeresurus wagleri. Toxicon 1991, 29, 227–236. [Google Scholar] [CrossRef]
- Molles, B.E.; Tsigelny, I.; Nguyen, P.D.; Gao, S.X.; Sine, S.M.; Taylor, P. Residues in the epsilon subunit of the nicotinic acetylcholine receptor interact to confer selectivity of waglerin-1 for the alpha-epsilon subunit interface site. Biochemistry 2002, 41, 7895–7906. [Google Scholar] [CrossRef] [PubMed]
- Molles, B.E.; Rezai, P.; Kline, E.F.; McArdle, J.J.; Sine, S.M.; Taylor, P. Identification of residues at the alpha and epsilon subunit interfaces mediating species selectivity of waglerin-1 for nicotinic acetylcholine receptors. J. Biol. Chem. 2002, 277, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom—Milestones of discovery. Toxicon 2013, 62, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Das, I. A Naturalist’s Guide to the Snakes of Southeast Asia; John Beaufoy Publishing: Oxford, UK, 2012. [Google Scholar]
- David, P.; Vogel, G. Snakes of Sumatra: Annotated Checklist and Key with Natural History Notes; Bucher Kreth: Frankfurt, Germany, 1996. [Google Scholar]
- Mong, R.; Tan, H.H. Snakebite by the shore pit viper (Trimeresurus purpureomaculatus) treated with polyvalent antivenom. Wilderness Environ. Med. 2016, 27, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Armugam, A.; Tan, C.S. A comparative study of the enzymatic and toxic properties of venoms of the Asian lance-headed pit viper (genus Trimeresurus). Comp. Biochem. Phys. Part B 1989, 93, 757–762. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Lomonte, B.; Leon, G.; Alape-Giron, A.; Flores-Diaz, M.; Sanz, L.; Angulo, Y.; Calvete, J.J. Snake venomics and antivenomics: Proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J. Proteom. 2009, 72, 165–182. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: http://www.uniprot.org (accessed on 13 October 2016).
- Marcotte, E.M. How do shotgun proteomics algorithms identify proteins? Nat. Biotechnol. 2007, 25, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; MacCoss, M.J. Shotgun proteomics: Tools for the analysis of complex biological systems. Curr. Opin. Mol. Ther. 2002, 4, 242–250. [Google Scholar] [PubMed]
- Rusmili, M.R.; Yee, T.T.; Mustafa, M.R.; Hodgson, W.C.; Othman, I. Proteomic characterization and comparison of malaysian Bungarus candidus and Bungarus fasciatus venoms. J. Proteom. 2014, 110, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Malih, I.; Ahmad Rusmili, M.R.; Tee, T.Y.; Saile, R.; Ghalim, N.; Othman, I. Proteomic analysis of moroccan cobra Naja haje legionis venom using tandem mass spectrometry. J. Proteom. 2014, 96, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Tan, C.S. Biological properties of Trimeresurus purpureomaculatus (shore pit viper) venom and its fractions. Toxicon 1988, 26, 989–996. [Google Scholar] [CrossRef]
- Khow, O.; Chanhome, L.; Omori-Satoh, T.; Puempunpanich, S.; Sitprija, V. A hemorrhagin as a metalloprotease in the venom of Trimeresurus purpureomaculatus: Purification and characterization. Toxicon 2002, 40, 455–461. [Google Scholar] [CrossRef]
- Kamiguti, A.S.; Hay, C.R.; Theakston, R.D.; Zuzel, M. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon 1996, 34, 627–642. [Google Scholar] [CrossRef]
- Estevao-Costa, M.I.; Diniz, C.R.; Magalhaes, A.; Markland, F.S.; Sanchez, E.F. Action of metalloproteinases mutalysin I and II on several components of the hemostatic and fibrinolytic systems. Thromb. Res. 2000, 99, 363–376. [Google Scholar] [CrossRef]
- Loria, G.D.; Rucavado, A.; Kamiguti, A.S.; Theakston, R.D.; Fox, J.W.; Alape, A.; Gutierrez, J.M. Characterization of ‘basparin A’, a prothrombin-activating metalloproteinase, from the venom of the snake Bothrops asper that inhibits platelet aggregation and induces defibrination and thrombosis. Arch. Biochem. Biophys. 2003, 418, 13–24. [Google Scholar] [CrossRef]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. BBA Proteins Proteom. 2012, 1824, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.-H.; Kim, D.-S. Molecular cloning and functional characterization of a snake venom metalloprotease. Eur. J. Biochem. 1999, 263, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Q.; Jin, Y.; Wu, J.B.; Zhou, X.D.; Lu, Q.M.; Wang, W.Y.; Xiong, Y.L. A new protein structure of P-II class snake venom metalloproteinases: It comprises metalloproteinase and disintegrin domains. Biochem. Biophys. Res. Commun. 2003, 310, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nikai, T.; Taniguchi, K.; Komori, Y.; Masuda, K.; Fox, J.W.; Sugihara, H. Primary structure and functional characterization of bilitoxin-1, a novel dimeric P-II snake venom metalloproteinase from Agkistrodon bilineatus venom. Arch. Biochem. Biophys. 2000, 378, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Hayashi, H.; Araki, S. Two vascular apoptosis-inducing proteins from snake venom are members of the metalloprotease/disintegrin family. Eur. J. Biochem. 1998, 253, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Ohta, T.; Kaji, K.; Fox, J.W.; Hayashi, H.; Araki, S. cDNA cloning and characterization of vascular apoptosis-inducing protein 1. Biochem. Biophys. Res. Commun. 2000, 278, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Hayashi, H.; Atoda, H.; Morita, T.; Araki, S. Purification, cDNA cloning and characterization of the vascular apoptosis-inducing protein, HV1, from Trimeresurus flavoviridis. Eur. J. Biochem. 2001, 268, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Choy, S.K.; Chin, K.M.; Ponnudurai, G. Cross-reactivity of monovalent and polyvalent Trimeresurus antivenoms with venoms from various species of Trimeresurus (lance-headed pit viper) snake. Toxicon 1994, 32, 849–853. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Sanz, L.; Flores-Diaz, M.; Figueroa, L.; Madrigal, M.; Herrera, M.; Villalta, M.; Leon, G.; Estrada, R.; Borges, A.; et al. Impact of regional variation in Bothrops asper snake venom on the design of antivenoms: Integrating antivenomics and neutralization approaches. J. Proteome Res. 2010, 9, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Ponnudurai, G.; Chung, M.C.; Tan, N.H. Purification and properties of the L-amino acid oxidase from malayan pit viper (Calloselasma rhodostoma) venom. Arch. Biochem. Biophys. 1994, 313, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Stabeli, R.G.; Marcussi, S.; Carlos, G.B.; Pietro, R.C.; Selistre-de-Araujo, H.S.; Giglio, J.R.; Oliveira, E.B.; Soares, A.M. Platelet aggregation and antibacterial effects of an L-amino acid oxidase purified from Bothrops alternatus snake venom. Bioorg. Med. Chem. 2004, 12, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Stoeva, S.; Abbasi, A.; Alam, J.M.; Kayed, R.; Faigle, M.; Neumeister, B.; Voelter, W. Isolation, structural, and functional characterization of an apoptosis-inducing L-amino acid oxidase from leaf-nosed viper (Eristocophis macmahoni) snake venom. Arch. Biochem. Biophys. 2000, 384, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.R.; Jin, Y.; Wu, J.B.; Jia, Y.H.; Xu, G.L.; Wang, G.C.; Xiong, Y.L.; Lu, Q.M. Purification and characterization of a new L-amino acid oxidase from Daboia russellii siamensis venom. Toxicon 2009, 54, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; da Silva, J.F.; Boldrini Franca, J.; Fonseca, F.P.; Otaviano, A.R.; Henrique Silva, F.; Hamaguchi, A.; Magro, A.J.; Braz, A.S.; dos Santos, J.I.; et al. Structural and functional properties of BP-LAAO, a new L-amino acid oxidase isolated from Bothrops pauloensis snake venom. Biochimie 2009, 91, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P. The relationship between enzymatic activity and pharmacological properties of phospholipases in natural poisons. In Nature Toxins; Oxford University Press: Oxford, UK, 1986; pp. 129–174. [Google Scholar]
- Krizaj, I.; Siigur, J.; Samel, M.; Cotic, V.; Gubensek, F. Isolation, partial characterization and complete amino acid sequence of the toxic phospholipase A2 from the venom of the common viper, Vipera berus berus. Biochim. Biophys. Acta 1993, 1157, 81–85. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.H.; Chang, H.C.; Chen, J.M.; Cheng, A.C.; Khoo, K.H. Glycan structures and intrageneric variations of venom acidic phospholipases A2 from Tropidolaemus pitvipers. FEBS J. 2012, 279, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Huang, K.-F.; Tsai, I.-H. Snake venom glutaminyl cyclases: Purification, cloning, kinetic study, recombinant expression, and comparison with the human enzyme. Toxicon 2014, 86, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Manjunatha Kini, R. Snake venom glutaminyl cyclase. Toxicon 2006, 48, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Linder, R.; Weinstein, S.A.; Kim, K.S. Isolation and characterization of a phospholipase B from venom of collett’s snake, Pseudechis colletti. Toxicon 1987, 25, 547–554. [Google Scholar] [CrossRef]
- Longo, E.; Stamato, F.M.; Ferreira, R.; Tapia, O. The catalytic mechanism of serine proteases II: The effect of the protein environment in the alpha-chymotrypsin proton relay system. J. Theor. Biol. 1985, 112, 783–798. [Google Scholar] [CrossRef]
- Braud, S.; Bon, C.; Wisner, A. Snake venom proteins acting on hemostasis. Biochimie 2000, 82, 851–859. [Google Scholar] [CrossRef]
- Castro, H.C.; Zingali, R.B.; Albuquerque, M.G.; Pujol-Luz, M.; Rodrigues, C.R. Snake venom thrombin-like enzymes: From reptilase to now. Cell. Mol. Life Sci. 2004, 61, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H. Isolation and characterization of the thrombin-like enzyme from Cryptelytrops purpureomaculatus venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Huang, T.F. Alpha and beta-fibrinogenases from Trimeresurus gramineus snake venom. Biochim. Biophys. Acta 1979, 571, 270–283. [Google Scholar] [CrossRef]
- Ouyang, C.; Hwang, L.J.; Huang, T.F. Alpha-fibrinogenase from Agkistrodon rhodostoma (malayan pit viper) snake venom. Toxicon 1983, 21, 25–33. [Google Scholar] [CrossRef]
- Le Bonniec, B.F.; Libraire, J. Plasminogen activators from snake venoms. In Toxins and Hemostasis: From Bench to Bedside; Kini, M.R., Clemetson, J.K., Markland, S.F., McLane, A.M., Morita, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 371–392. [Google Scholar]
- Chang, C.H.; Chung, C.H.; Kuo, H.L.; Hsu, C.C.; Huang, T.F. The highly specific platelet glycoprotein (GP) VI agonist trowaglerix impaired collagen-induced platelet aggregation ex vivo through matrix metalloproteinase-dependent GPVI shedding. J. Thromb. Haemost. 2008, 6, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, L.; Kong, C.; Kolatkar, P.R.; Chung, M.C. Purpureotin: A novel di-dimeric c-type lectin-like protein from Trimeresurus purpureomaculatus venom is stabilized by noncovalent interactions. Arch. Biochem. Biophys. 2004, 424, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.J.; Weinstein, S.A.; Smith, L.A. Molecular properties and structure-function relationships of lethal peptides from venom of Wagler’s pit viper, Trimeresurus wagleri. Toxicon 1992, 30, 1027–1036. [Google Scholar] [CrossRef]
- Molles, B.E.; Taylor, P. Structure and function of the waglerins, peptide toxins from the venom of Wagler’s pit viper, Tropidolaemus wagleri. J. Toxicol. Toxin Rev. 2002, 21, 273–292. [Google Scholar] [CrossRef]
- Mallick, P.; Kuster, B. Proteomics: A pragmatic perspective. Nat. Biotechnol. 2010, 28, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Koike, H.; Sugiyama, Y.; Motoyoshi, K.; Wada, T.; Hishinuma, S.; Mita, M.; Morita, T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur. J. Biochem. 2002, 269, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef]
- Higuchi, S.; Murayama, N.; Saguchi, K.; Ohi, H.; Fujita, Y.; Camargo, A.C.M.; Ogawa, T.; Deshimaru, M.; Ohno, M. Bradykinin-potentiating peptides and C-type natriuretic peptides from snake venom. Immunopharmacology 1999, 44, 129–135. [Google Scholar] [CrossRef]
- Lameu, C.; Neiva, M.; Hayashi, M.A.F. Venom bradykinin-related peptides (BRPS) and its multiple biological roles. In An Integrated View of the Molecular Recognition and Toxinology—From Analytical Procedures to Biomedical Applications; Radis-Baptista, G., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Vink, S.; Jin, A.H.; Poth, K.J.; Head, G.A.; Alewood, P.F. Natriuretic peptide drug leads from snake venom. Toxicon 2012, 59, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. Peaks db: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).