Bee Venom Protects against Rotenone-Induced Cell Death in NSC34 Motor Neuron Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Preparation of Primary Cortical Neuronal Culture
2.4. Western Blot
2.5. Mitochondria Staining
2.6. Data Analysis
3. Results
3.1. Effect of Bee Venom on Rotenone-Induced Cell Death in NSC34 Cells
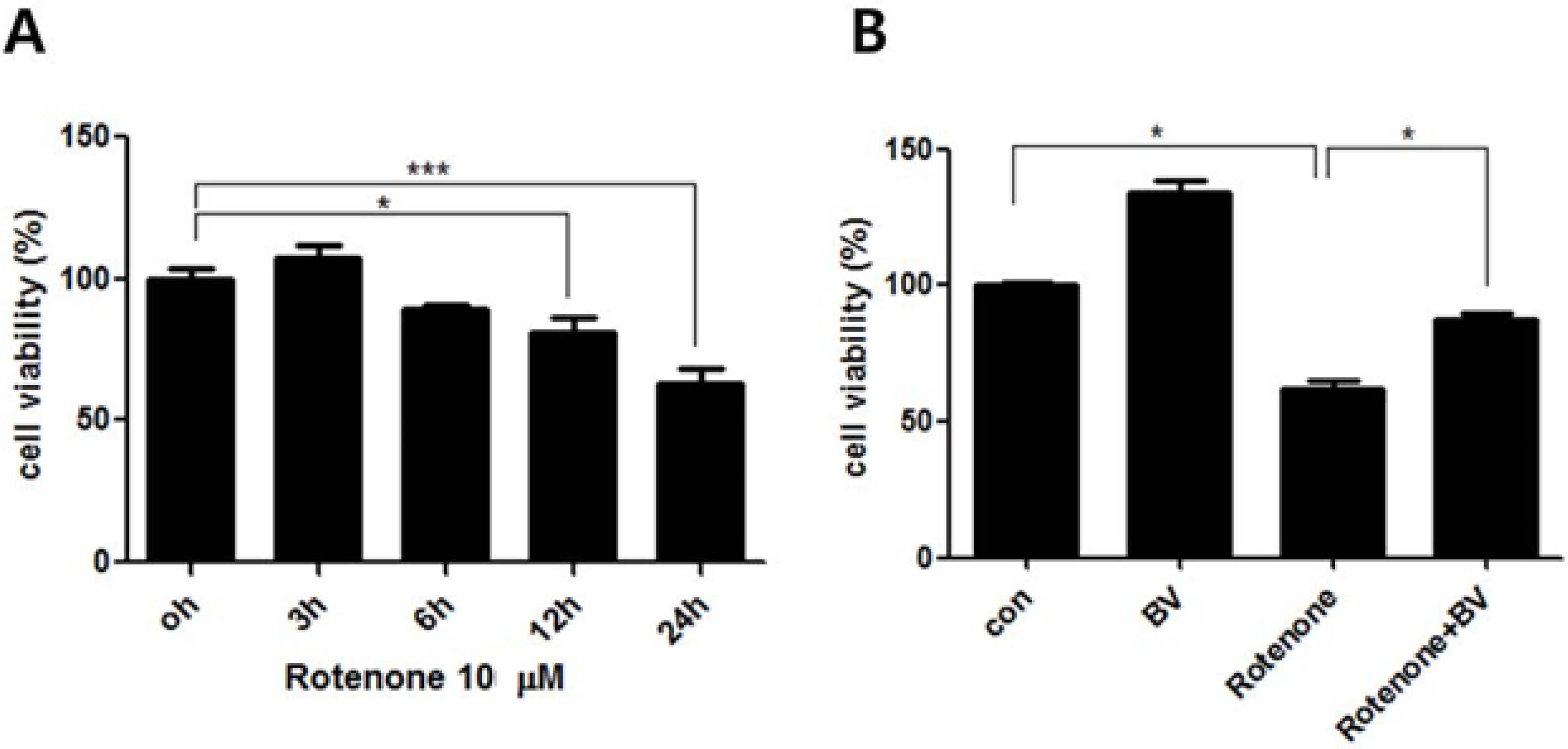
3.2. Bee Venom Affects ERK and JNK Phosphorylation
3.3. Bee Venom Attenuates Rotenone- Induced Caspase-3 Activation in NSC34 Neuronal Cells

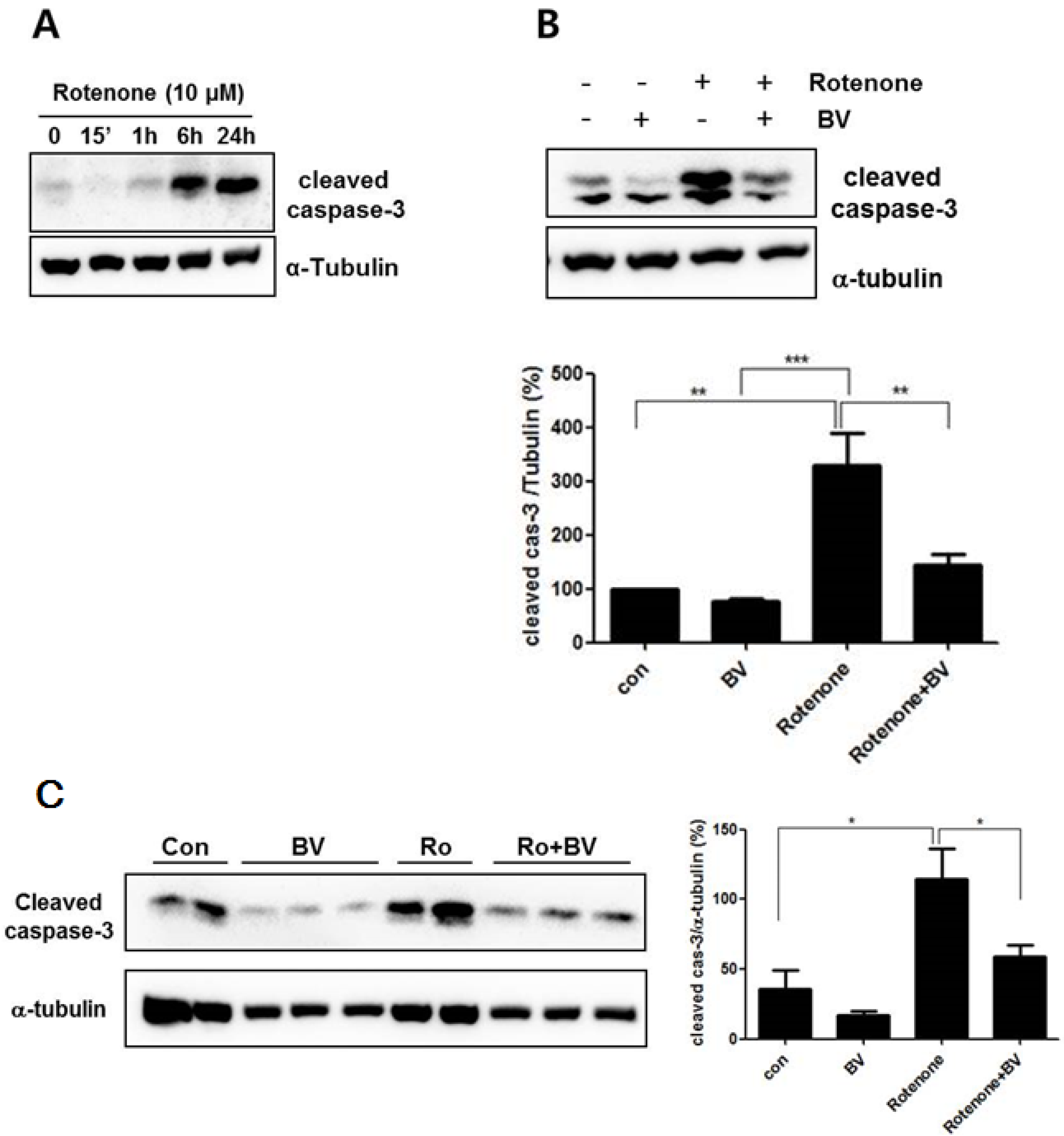
3.4. Bee Venom Suppresses Rotenone-Mediated Mitochondrial Impairment
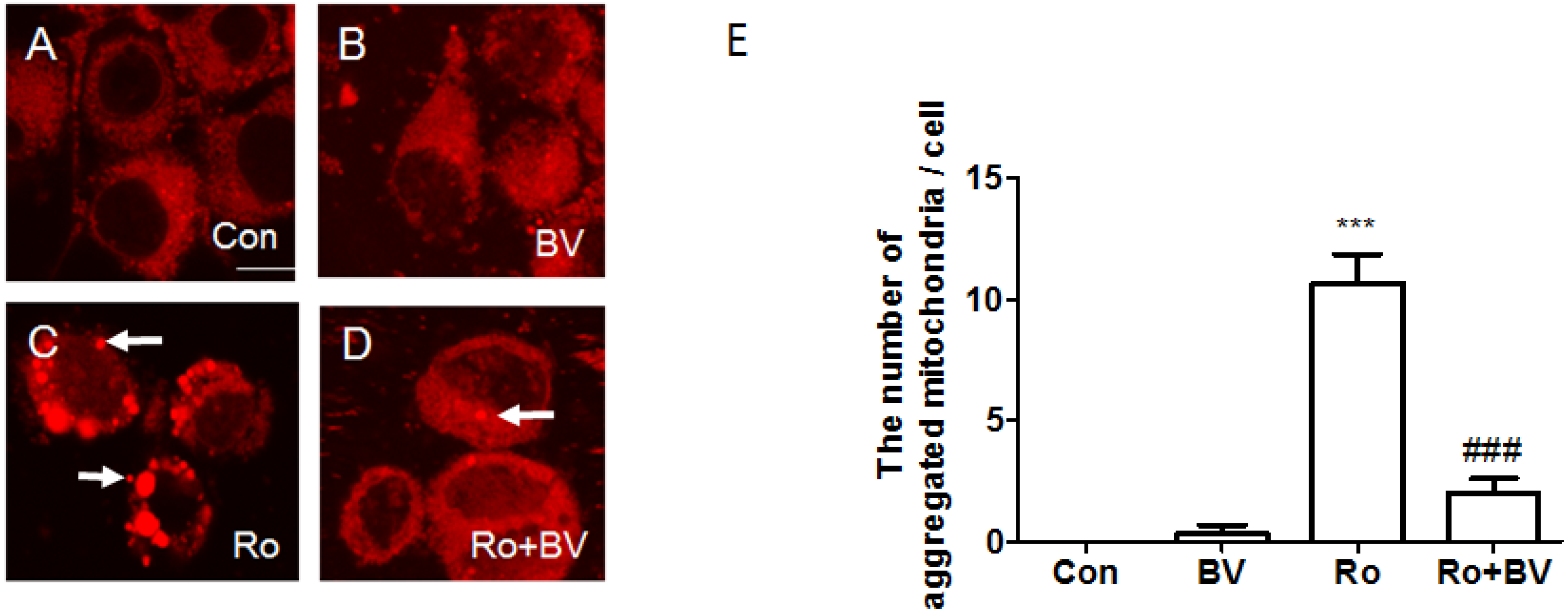
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A. Robinson Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Kim, Y.H.; Uddin, M.R.; Chae, S.; Lee, H.W.; Kim, Y.H.; Kim, Y.S.; Lee, M.Y. Betaine protects against rotenone-induced neurotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2013, 33, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 102741. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Knaryan, V.H.; Guyton, M.K.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats. Neuroscience 2007, 146, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Eiseman, J.L.; von Bredow, J.; Alvares, A.P. Effect of honeybee (Apis mellifera) venom on the course of adjuvant-induced arthritis and depression of drug metabolism in the rat. Biochem. Pharmacol. 1982, 31, 1139–1146. [Google Scholar] [CrossRef]
- Alvarez-Fischer, D.; Noelker, C.; Vulinovic, F.; Grunewald, A.; Chevarin, C.; Klein, C.; Oertel, W.H.; Hirsch, E.C.; Michel, P.P.; Hartmann, A. Bee Venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS ONE 2013, 8, e61700. [Google Scholar] [CrossRef] [PubMed]
- Armugam, A.; Cher, C.D.; Lim, K.; Koh, D.C.; Howells, D.W.; Jeyaseelan, K. A secretory phospholipase A2-mediated neuroprotection and anti-apoptosis. BMC Neurosci. 2009, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, S.H.; Yang, S.C.; Lee, S.M.; Choi, S.M. Melittin restores proteasome function in an animal model of ALS. J. Neuroinflammation 2011, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Koburova, K.L.; Michailova, S.G.; Shkenderov, S.V. Further investigation on the antiinflammatory properties of adolapin—Bee venom polypeptide. Acta Physiol. Pharmacol. Bulg. 1985, 11, 50–55. [Google Scholar] [PubMed]
- Kwon, Y.B.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Yoon, O.B.; Beitz, A.J.; Lee, J.H. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002, 71, 191–204. [Google Scholar] [CrossRef]
- Yang, E.J.; Jiang, J.H.; Lee, S.M.; Yang, S.C.; Hwang, H.S.; Lee, M.S.; Choi, S.M. Bee venom attenuates neuroinflammatory events and extends survival in amyotrophic lateral sclerosis models. J. Neuroinflammation 2010, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Putz, T.; Ramoner, R.; Gander, H.; Rahm, A.; Bartsch, G.; Thurnher, M. Antitumor action and immune activation through cooperation of bee venom secretory phospholipase A2 and phosphatidylinositol-(3,4)-bisphosphate. Cancer Immunol. Immunother. 2006, 55, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Kim, H.; Lee, G.; Park, S.; Kim, H.; Bae, H. Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson’s disease: Role of regulatory T cells. Brain Behav. Immun. 2012, 26, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.T.; Cheng, H.H.; Huang, C.J.; Chang, H.C.; Chi, C.C.; Su, H.H.; Hsu, S.S.; Wang, J.L.; Chen, I.S.; Liu, S.I.; et al. Phospholipase A2-independent Ca2+ entry and subsequent apoptosis induced by melittin in human MG63 osteosarcoma cells. Life Sci. 2007, 80, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Liao, S.S.; Lin, S.Y.; Lin, J.P.; Yang, J.S.; Lin, M.L.; Chen, G.W.; Lu, H.F.; Lin, M.W.; Han, S.M.; et al. The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. In Vivo 2008, 22, 237–245. [Google Scholar] [PubMed]
- Jang, M.H.; Shin, M.C.; Lim, S.; Han, S.M.; Park, H.J.; Shin, I.; Lee, J.S.; Kim, K.A.; Kim, E.H.; Kim, C.J. Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. J. Pharmacol. Sci. 2003, 91, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Doo, A.R.; Kim, S.N.; Kim, S.T.; Park, J.Y.; Chung, S.H.; Choe, B.Y.; Chae, Y.; Lee, H.; Yin, C.S.; Park, H.J. Bee venom protects SH-SY5Y human neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death. Brain Res. 2012, 1429, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.M. Karin Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.M.M. Mouradian Apoptotic signaling in dopamine-induced cell death: The role of oxidative stress, p38 mitogen-activated protein kinase, cytochrome c and caspases. J. Neurochem. 2001, 78, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Lee, S.M.; Yang, E.J.; Choi, S.M.; Kim, S.H.; Baek, M.G.; Jiang, J.H. Effects of bee venom on glutamate-induced toxicity in neuronal and glial cells. Evid. Based Complement. Altern. Med. 2012, 2012, 368196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.R. Lapadat mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, H.; Qiao, B.; Luo, L.; Ma, H.; Li, H.; Jiang, J.; Niu, D.; Yin, Z. Opposing effects of ERK and p38 MAP kinases on Hela cell apoptosis induced by dipyrithione. Mol. Cells 2007, 23, 30–38. [Google Scholar] [PubMed]
- Minden, A.M. Karin Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta 1997, 1333, F85–F104. [Google Scholar] [PubMed]
- Kim, J.I.; Yang, E.J.; Lee, M.S.; Kim, Y.S.; Huh, Y.; Cho, I.H.; Kang, S.; Koh, H.K. Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. Int. J. Neurosci. 2011, 121, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Graos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Nadra, I.; Mason, J.C.; Philippidis, P.; Florey, O.; Smythe, C.D.; McCarthy, G.M.; Landis, R.C.; Haskard, D.O. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ. Res. 2005, 96, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Clerk, A.P.H. Sugden Untangling the Web: Specific signaling from PKC isoforms to MAPK cascades. Circ. Res. 2001, 89, 847–849. [Google Scholar] [PubMed]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [PubMed]
- Martins, J.B.; Bastos Mde, L.; Carvalho, F.; Capela, J.P. Differential Effects of Methyl-4-Phenylpyridinium Ion, Rotenone, and Paraquat on Differentiated SH-SY5Y Cells. J. Toxicol. 2013, 2013, 347312. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Langston Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.K.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V. Mitochondria as decision-makers in cell death. Environ. Mol. Mutagen. 2010, 51, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.S. Shimizu Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Mena, N.P.; Urrutia, P.J.; Lourido, F.; Carrasco, C.M.; Nunez, M.T. Mitochondrial iron homeostasis and its dysfunctions in neurodegenerative disorders. Mitochondrion 2015, 21, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Rossi, S.; Mirra, A.; Carri, M.T. Mitochondrial dynamism and the pathogenesis of Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2015, 9, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.Y.; Lee, K.-W.; Choi, S.-M.; Yang, E.J. Bee Venom Protects against Rotenone-Induced Cell Death in NSC34 Motor Neuron Cells. Toxins 2015, 7, 3715-3726. https://doi.org/10.3390/toxins7093715
Jung SY, Lee K-W, Choi S-M, Yang EJ. Bee Venom Protects against Rotenone-Induced Cell Death in NSC34 Motor Neuron Cells. Toxins. 2015; 7(9):3715-3726. https://doi.org/10.3390/toxins7093715
Chicago/Turabian StyleJung, So Young, Kang-Woo Lee, Sun-Mi Choi, and Eun Jin Yang. 2015. "Bee Venom Protects against Rotenone-Induced Cell Death in NSC34 Motor Neuron Cells" Toxins 7, no. 9: 3715-3726. https://doi.org/10.3390/toxins7093715
APA StyleJung, S. Y., Lee, K.-W., Choi, S.-M., & Yang, E. J. (2015). Bee Venom Protects against Rotenone-Induced Cell Death in NSC34 Motor Neuron Cells. Toxins, 7(9), 3715-3726. https://doi.org/10.3390/toxins7093715




