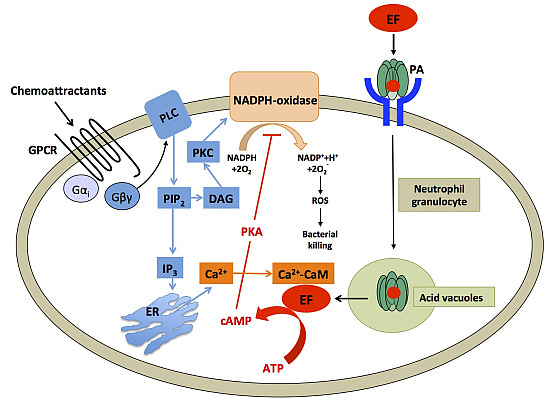
Different Roles of N-Terminal and C-Terminal Domains in Calmodulin for Activation of Bacillus anthracis Edema Factor
Abstract
:1. Introduction
| CaM | Position in CaM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 36 | 51 | 71 | 72 | 76 | 109 | 124 | 144 | 145 | |
| wt | M | M | M | M | M | M | M | M | M |
| L9 | L | L | L | L | L | L | L | L | L |
| M36/L8 | M | L | L | L | L | L | L | L | L |
| M51/L8 | L | M | L | L | L | L | L | L | L |
| M71/L8 | L | L | M | L | L | L | L | L | L |
| M72/L8 | L | L | L | M | L | L | L | L | L |
| M76/L8 | L | L | L | L | M | L | L | L | L |
| M144/L8 | L | L | L | L | L | L | L | M | L |
| M145/L8 | L | L | L | L | L | L | L | L | M |
| M36,M51/L7 | M | M | L | L | L | L | L | L | L |
| M71,M72,M76/L6 | L | L | M | M | M | L | L | L | L |
| M109,M124/L7 | L | L | L | L | L | M | M | L | L |
| M144,M145/L7 | L | L | L | L | L | L | L | M | M |
| L2 | M | M | M | M | M | M | M | L | L |
2. Results
2.1. Ca2+-Dependence of CaM Stimulation of EF
2.2. Regulation of EF by the Degree of Met Oxidation in CaM
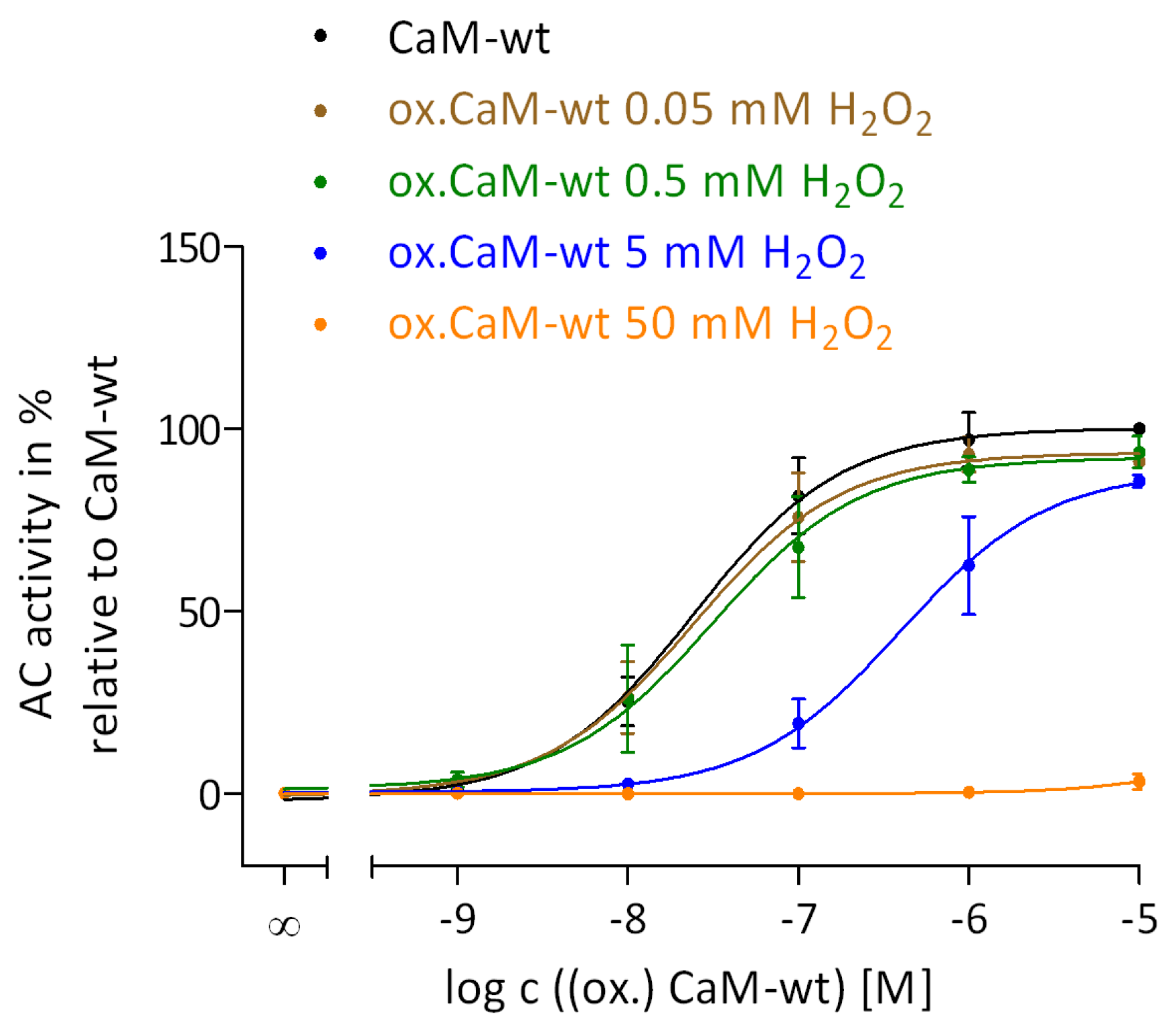
2.3. Regulation of EF Activation by Native CaM-mut with Met to Leu Substitutions

2.4. Regulation of EF Activation by Site-Specific N- and C-Terminal Met Oxidation
| CaM | AC Activity with 10 μM CaM-wt/CaM-mut [%] | |
|---|---|---|
| Oxidized | Oxidized | |
| wt | 3.3 ± 2.1 *** | 86.3 ± 6.9 *** |
| L9 | 100.3 ± 15.9 | 100.6 ± 5.2 |
| M36/L8 | 95.7 ± 7.5 | 97.7 ± 5.0 |
| M51/L8 | 85.9 ± 15.7 | 99.1 ± 10.0 |
| M71/L8 | 91.2 ± 3.0 | 99.1 ± 4.7 |
| M72/L8 | 94.9 ± 7.8 | 101.3 ± 5.2 |
| M76/L8 | 84.7 ± 12.8 | 102.7 ± 10.4 |
| M144/L8 | 101.5 ± 15.4 | 95.7 ± 2.4 |
| M145/L8 | 104.9 ± 16.6 | 100.8 ± 16.7 |
| M36,M51/L7 | 90.9 ± 9.7 | 108.4 ± 7.2 |
| M71,M72,M76/L6 | 86.4 ± 12.5 | 111.0 ± 7.1 |
| M109,M124/L7 | 82.4 ± 11.4 | 106.9 ± 6.9 |
| M144,M145/L7 | 96.2 ± 6.7 | 96.3 ± 3.9 |
| L2 | 74.8 ± 5.8 ** | 91.3 ± 7.0 |
| CaM | pEC50 | ||
|---|---|---|---|
| Native | Oxidized | MsrA-treated | |
| wt | 7.61 ± 0.05 | - | 6.35 ± 0.09 *** |
| L9 | 7.40 ± 0.10 | 7.31 ± 0.13 ** | 6.96 ± 0.05 *** |
| M36/L8 | 6.81 ± 0.06 | 6.50 ± 0.06 *** | 6.29 ± 0.04 *** |
| M51/L8 | 6.96 ± 0.09 | 6.83 ± 0.14 ** | 6.50 ± 0.07 *** |
| M71/L8 | 6.83 ± 0.08 | 6.35 ± 0.05 *** | 6.35 ± 0.05 *** |
| M72/L8 | 7.05 ± 0.07 | 6.75 ± 0.07 *** | 6.57 ± 0.05 *** |
| M76/L8 | 6.81 ± 0.10 | 6.47 ± 0.10 *** | 6.37 ± 0.06 *** |
| M144/L8 | 7.54 ± 0.11 | 7.08 ± 0.10 *** | 6.86 ± 0.04 *** |
| M145/L8 | 7.73 ± 0.05 | 7.14 ± 0.10 *** | 6.97 ± 0.07 *** |
| M36,M51/L7 | 6.88 ± 0.07 | 6.19 ± 0.06 *** | 6.29 ± 0.04 *** |
| M71,M72,M76/L6 | 6.94 ± 0.06 | 6.30 ± 0.07 *** | 6.27 ± 0.04 *** |
| M109,M124/L7 | 6.95 ± 0.05 | 5.90 ± 0.06 *** | 6.35 ± 0.04 *** |
| M144,M145/L7 | 7.56 ± 0.09 | 6.52 ± 0.06 *** | 6.92 ± 0.05 *** |
| L2 | 7.40 ± 0.10 | 5.33 ± 0.07 *** | 6.62 ± 0.05 *** |
2.5. Restoration of CaM Stimulation of EF by Msr Catalyzed Reduction of MetSO
3. Discussion
3.1. Impact of Intact C-Terminal Structure of CaM on EF Activation
3.2. Comparison of CaM-EF Interaction to Other CaM-Target Interactions
| M a | Membranous AC1 b | Bacillus anthracis AC toxin EF | Bordetella pertussis AC Toxin CyaA c |
|---|---|---|---|
| 36 | High relevance: Single Met in the N-terminal region of CaM enhances AC1 activation and MetSO impairs AC1 activation | Moderate relevance: Important for CaM-EF binding, oxidation decreases CaM-EF affinity; structure: No contact to CaM within the CaM-EF complex | No relevance: Met is accessible to oxidation within the CaM-CyaA complex; oxidation does not prevent binding to CyaA |
| 51 | High relevance: MetSO impairs AC1 activation | Moderate relevance: Like M36 | No relevance: Like M36 |
| 71 | Only relevant in combination of oxidized M72, M72 and M76: AC1 activity decreases significantly | Moderate relevance: Like M36 | No relevance: Like M36 |
| 72 | Like M71 | Moderate relevance: Like M36 | No relevance: Like M36 |
| 76 | Like M71 | Moderate relevance: Like M36 | No relevance: Like M36 |
| 109 | High relevance in combination of oxidized M109 and M124: AC1 activity decreases significantly | Low relevance: Oxidation of M109 and M124 does not affect EF activation; structure: Met is in contact with CaM within the CaM-EF complex | High relevance: Crystallographic studies reveal important hydrophobic interactions with CyaA; preservation of this Met from oxidation within the CaM-CyaA complex and prevention of binding to CyaA in oxidized state suggest an involvement in the formation of CaM-CyaA complex |
| 124 | High relevance: Like M109 | Low relevance: Like M109 | High relevance: Like M109 |
| 144 | High relevance: Met enhances AC1 activation and MetSO impairs AC1 activation; no altered AC1 activation in combination of two Met or MetSO at position 144 and 145 | High relevance: An intact C-terminal region next to M144 and M145 is sufficient for effective EF activation also upon oxidation of all other Met, important for the high affinity of EF to CaM; molecular modeling: Met is in contact with CaM within the CaM-EF complex | Moderate relevance: Crystallographic studies reveal important hydrophobic interactions with CyaA; Met is accessible to oxidation within the CaM-CyaA complex; site-specific oxidation does not prevent binding to CyaA but in combination with other C-terminal Met oxidation prevents binding to CyaA |
| 145 | High relevance: Like M144 | High relevance: Like M144 | High relevance: Like M109, additionally: Important for CyaA activation |
4. Experimental Section
4.1. Materials
4.2. Expression and Purification of EF
4.3. Cloning, Expression, and Purification of CaM-wt and CaM-mut
4.4. Oxidation of Met and Reduction of MetSO
4.5. AC Activity Assay
4.6. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klinman, D.M.; Yamamoto, M.; Tross, D.; Tomaru, K. Anthrax prevention and treatment: Utility of therapy combining antibiotic plus vaccine. Expert Opin. Biol. Ther. 2009, 9, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Doganay, M.; Metan, G.; Alp, E. A Review of cutaneous anthrax and its outcome. J. Infect. Public Health 2010, 3, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Sweeney, D.A.; Cui, X.; Li, Y.; Eichacker, P.Q. An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Med. 2012, 38, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Inglesby, T.V.; O’Toole, T.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Friedlander, A.M.; Gerberding, J.; Hauer, J.; Hughes, J.; et al. Anthrax as a biological weapon, 2002: Updated recommendations for management. JAMA 2002, 287, 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, N.; Kumar, P.; Bhatnagar, R. The adenylate cyclase toxins. Crit. Rev. Microbiol. 2004, 30, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Guo, Q. The adenylyl cyclase activity of anthrax edema factor. Mol. Aspects Med. 2009, 30, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Cui, X.; Sweeney, D.A.; Li, Y.; Barochia, A.; Eichacker, P.Q. The potential contributions of lethal and edema toxins to the pathogenesis of anthrax associated shock. Toxins 2011, 3, 1185–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, X.; Solomon, S.B.; Remy, K.; Fitz, Y.; Eichacker, P.Q.B. Anthracis edema toxin increases cAMP levels and inhibits phenylephrine-stimulated contraction in a rat aortic ring model. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H238–H250. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Dove, S. Inhibitors of Bacillus anthracis edema factor. Pharmacol. Ther. 2013, 140, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Drum, C.L.; Yan, S.Z.; Bard, J.; Shen, Y.Q.; Lu, D.; Soelaiman, S.; Grabarek, Z.; Bohm, A.; Tang, W.J. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 2002, 415, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Aylott, C.V.; Bourdeau, R.W.; Bokoch, G.M. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J. Immunol. 2006, 176, 7557–7565. [Google Scholar] [CrossRef] [PubMed]
- Leppla, S.H. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic amp concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1982, 79, 3162–3166. [Google Scholar] [CrossRef] [PubMed]
- Squier, T.C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001, 36, 1539–1550. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.S.; Ferrington, D.A.; Squier, T.C.; Schöneich, C. Diastereoselective reduction of protein-bound methionine sulfoxide by methionine sulfoxide reductase. FEBS Lett. 1999, 455, 247–250. [Google Scholar] [CrossRef]
- Bigelow, D.J.; Squier, T.C. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim. Biophys. Acta 2005, 1703, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shen, Y.; Lee, Y.S.; Gibbs, C.S.; Mrksich, M.; Tang, W.J. Structural basis for the interaction of bordetella pertussis adenylyl cyclase toxin with calmodulin. EMBO J. 2005, 24, 3190–3201. [Google Scholar] [CrossRef] [PubMed]
- Vougier, S.; Mary, J.; Dautin, N.; Vinh, J.; Friguet, B.; Ladant, D. Essential Role of Methionine Residues in Calmodulin Binding to Bordetella pertussis Adenylate Cyclase, as Probed by Selective Oxidation and Repair by the Peptide Methionine Sulfoxide Reductases. J. Biol. Chem. 2004, 279, 30210–30218. [Google Scholar] [CrossRef] [PubMed]
- Lübker, C.; Urbauer, R.J.; Moskovitz, J.; Dove, S.; Weisemann, J.; Fedorova, M.; Urbauer, J.L.; Seifert, R. Membranous adenylyl cyclase 1 activation is regulated by oxidation of N- and C-terminal methionine residues in calmodulin. Biochem. Pharmacol. 2015, 93, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.S.; Richardson, D.C. Principles and patterns of protein conformation. In Prediction of Protein Structure and the Principles of Protein Conformation; Fasman, G.D., Ed.; Springer: Boston, MA, USA, 1989; pp. 1–98. [Google Scholar]
- Moskovitz, J.; Poston, J.M.; Berlett, B.S.; Nosworthy, N.J.; Szczepanowski, R.; Stadtman, E.R. Identification and characterization of a putative active site for peptide methionine sulfoxide reductase (MsrA) and its substrate stereospecificity. J. Biol. Chem. 2000, 275, 14167–14172. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.S.; Schöneich, C. Diastereoselective protein methionine oxidation by reactive oxygen species and diastereoselective repair by methionine sulfoxide reductase. Free Radic. Biol. Med. 2000, 29, 986–994. [Google Scholar] [CrossRef]
- Sun, H.; Gao, J.; Ferrington, D.A.; Biesiada, H.; Williams, T.D.; Squier, T.C. Repair of oxidized calmodulin by methionine sulfoxide reductase restores ability to activate the plasma membrane Ca-ATPase. Biochemistry 1999, 38, 105–112. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, K.T.; de Grado, W.F. How calmodulin binds its targets: Sequence independent recognition of amphiphilic α-Helices. Trends Biochem. Sci. 1990, 15, 59–64. [Google Scholar] [CrossRef]
- Shen, Y.; Lee, Y.S.; Soelaiman, S.; Bergson, P.; Lu, D.; Chen, A.; Beckingham, K.; Grabarek, Z.; Mrksich, M.; Tang, W.J. Physiological calcium concentrations regulate calmodulin binding and catalysis of adenylyl cyclase exotoxins. EMBO J. 2002, 21, 6721–6732. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhukovskaya, N.L.; Guo, Q.; Florian, J.; Tang, W.J. Calcium-independent calmodulin binding and two-metal-ion catalytic mechanism of anthrax edema factor. EMBO J. 2005, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Squier, T.C.; Sacksteder, C.A. An altered mode of calcium coordination in methionine-oxidized calmodulin. Biophys. J. 2008, 95, 5268–5280. [Google Scholar] [CrossRef] [PubMed]
- Laine, E.; Martinez, L.; Blondel, A.; Malliavin, T.E. Activation of the edema factor of Bacillus anthracis by calmodulin: Evidence of an interplay between the EF-calmodulin interaction and calcium binding. Biophys. J. 2010, 99, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Schuler, D.; Lübker, C.; Lushington, G.H.; Tang, W.J.; Shen, Y.; Richter, M.; Seifert, R. Interactions of Bordetella pertussis adenylyl cyclase toxin CyaA with calmodulin mutants and calmodulin antagonists: Comparison with membranous adenylyl cyclase I. Biochem. Pharmacol. 2012, 83, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, M.; Wang, J.H.; Vogel, H.J. The effect of met → Leu mutations on calmodulin’s ability to activate cyclic nucleotide phosphodiesterase. J. Biol. Chem. 1994, 269, 15546–15552. [Google Scholar] [PubMed]
- Bartlett, R.K.; Bieber Urbauer, R.J.; Anbanandam, A.; Smallwood, H.S.; Urbauer, J.L.; Squier, T.C. Oxidation of Met144 and Met145 in calmodulin blocks calmodulin dependent activation of the plasma membrane Ca-ATPase. Biochemistry 2003, 42, 3231–3238. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, H.J.; Bartlett, R.; Perdicakis, B.; Jervis, E.; Squier, T.C.; Guillemette, J.G. Activation of constitutive nitric oxide synthases by oxidized calmodulin mutants. Biochemistry 2003, 42, 7759–7768. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, D.H.; Yao, Y.; Sun, H.; Qin, Z.; Schöneich, C.; Williams, T.D.; Squier, T.C. Loss of conformational stability in calmodulin upon methionine oxidation. Biophys. J. 1998, 74, 1115–1134. [Google Scholar] [CrossRef]
- Anbanandam, A.; Bieber Urbauer, R.J.; Bartlett, R.K.; Smallwood, H.S.; Squier, T.C.; Urbauer, J.L. Mediating molecular recognition by methionine oxidation: Conformational switching by oxidation of methionine in the carboxyl-terminal domain of calmodulin. Biochemistry 2005, 44, 9486–9496. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.M.; Schmidt, J.; Göttle, M.; Suryanarayana, S.; Shen, Y.; Tang, W.J.; Gille, A.; Geduhn, J.; König, B.; Dove, S.; et al. Molecular analysis of the interaction of anthrax adenylyl cyclase toxin, edema factor, with 2′(3′)-O-(N-(methyl)anthraniloyl)-substituted purine and pyrimidine nucleotides. Mol. Pharmacol. 2009, 75, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shen, Y.; Zhukovskaya, N.L.; Florian, J.; Tang, W.J. Structural and kinetic analyses of the interaction of anthrax adenylyl cyclase toxin with reaction products cAMP and pyrophosphate. J. Biol. Chem. 2004, 279, 29427–29435. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Moskovitz, J.; Berlett, B.S.; Poston, J.M.; Stadtman, E.R. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc. Natl. Acad. Sci. USA 1997, 94, 9585–9589. [Google Scholar] [CrossRef] [PubMed]
- Bar-Noy, S.; Moskovitz, J. Mouse methionine sulfoxide reductase B: Effect of selenocysteine incorporation on its activity and expression of the seleno-containing enzyme in bacterial and mammalian cells. Biochem. Biophys. Res. Commun. 2002, 297, 956–961. [Google Scholar] [CrossRef]
- Kim, H.Y.; Gladyshev, V.N. Methionine sulfoxide reduction in mammals: Characterization of methionine-R-sulfoxide reductases. Mol. Biol. Cell 2004, 15, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lübker, C.; Dove, S.; Tang, W.-J.; Urbauer, R.J.B.; Moskovitz, J.; Urbauer, J.L.; Seifert, R. Different Roles of N-Terminal and C-Terminal Domains in Calmodulin for Activation of Bacillus anthracis Edema Factor. Toxins 2015, 7, 2598-2614. https://doi.org/10.3390/toxins7072598
Lübker C, Dove S, Tang W-J, Urbauer RJB, Moskovitz J, Urbauer JL, Seifert R. Different Roles of N-Terminal and C-Terminal Domains in Calmodulin for Activation of Bacillus anthracis Edema Factor. Toxins. 2015; 7(7):2598-2614. https://doi.org/10.3390/toxins7072598
Chicago/Turabian StyleLübker, Carolin, Stefan Dove, Wei-Jen Tang, Ramona J. Bieber Urbauer, Jackob Moskovitz, Jeffrey L. Urbauer, and Roland Seifert. 2015. "Different Roles of N-Terminal and C-Terminal Domains in Calmodulin for Activation of Bacillus anthracis Edema Factor" Toxins 7, no. 7: 2598-2614. https://doi.org/10.3390/toxins7072598