Superantigens Modulate Bacterial Density during Staphylococcus aureus Nasal Colonization
Abstract
:1. Introduction
2. Results
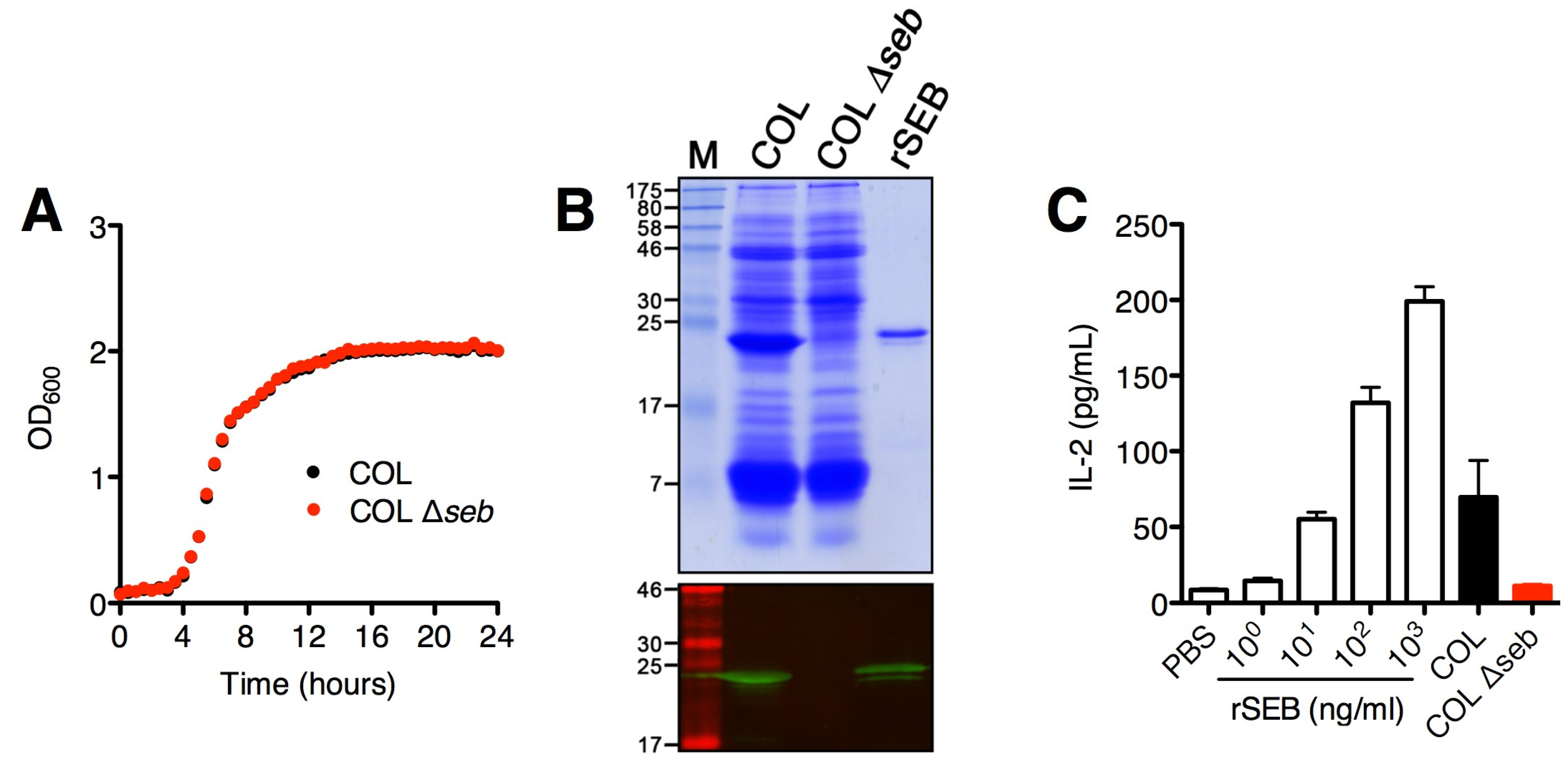
2.1. SAg Deletion Strains Have Reduced Superantigen Production and Activity in vitro
2.2. Lack of SEA Transiently Increases S. aureus Newman Δsea Nasal Colonization
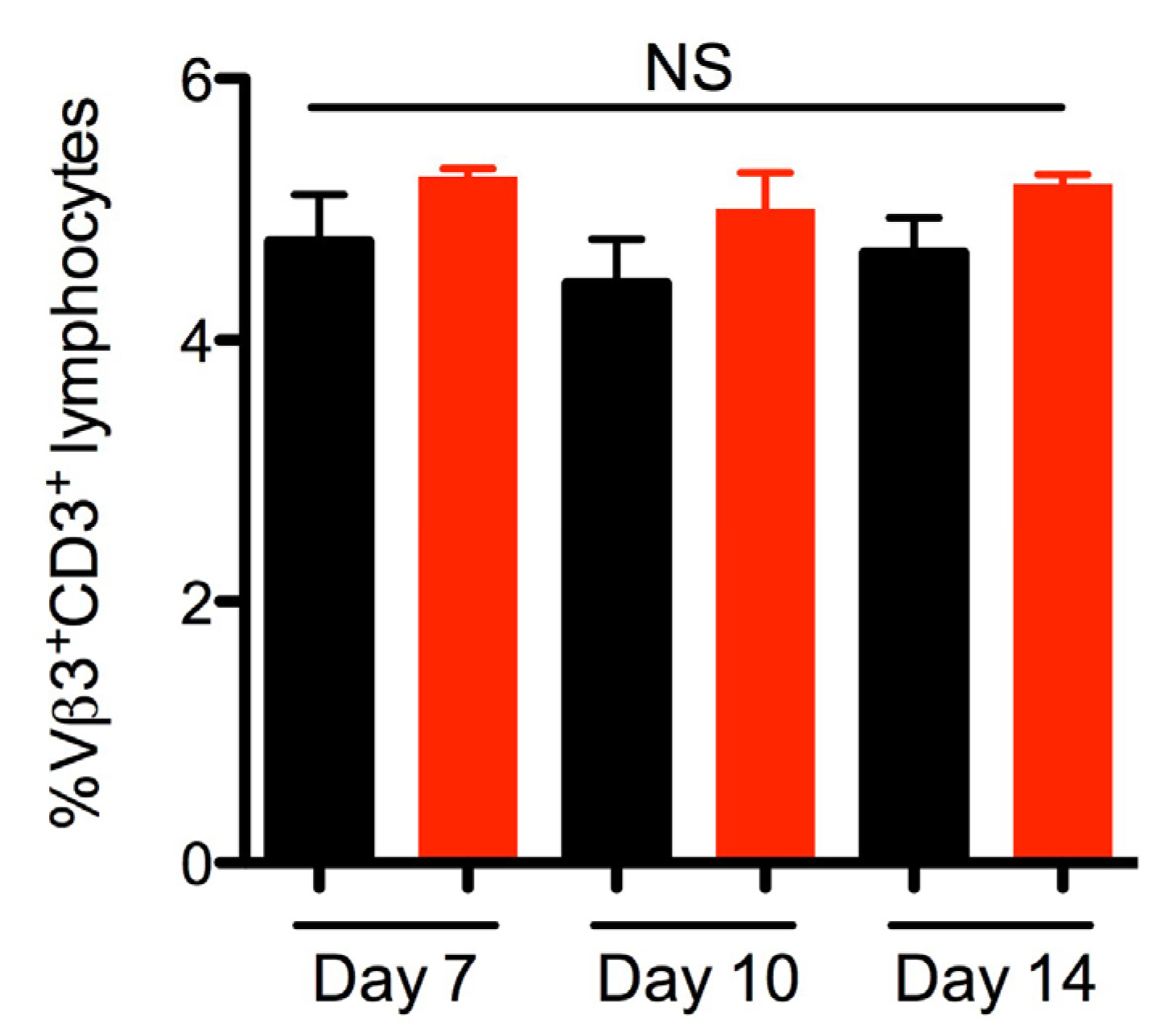
2.3. SEA Does Not Skew Vβ3 Subsets in vivo
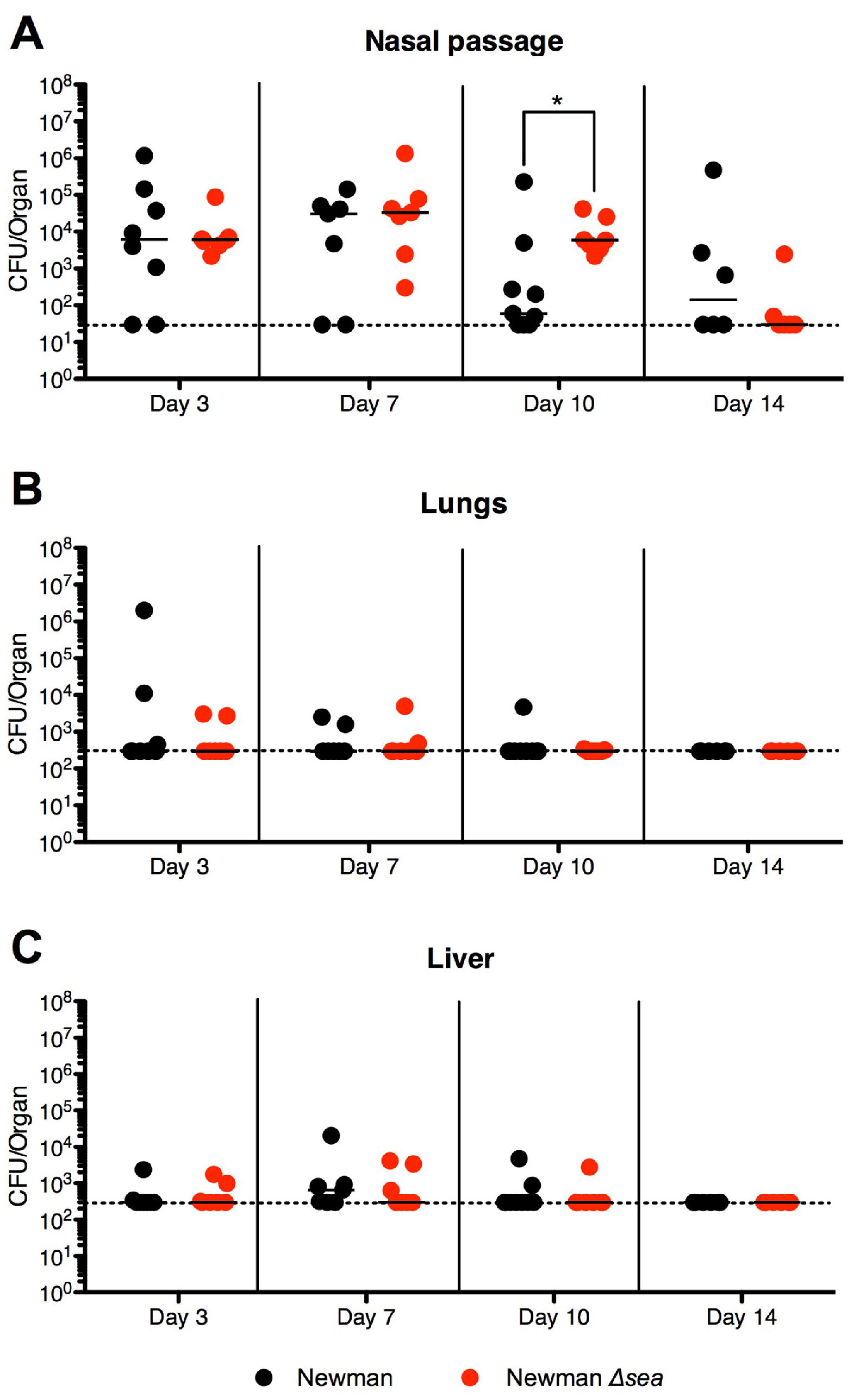
2.4. SEB Decreases Nasal Colonization

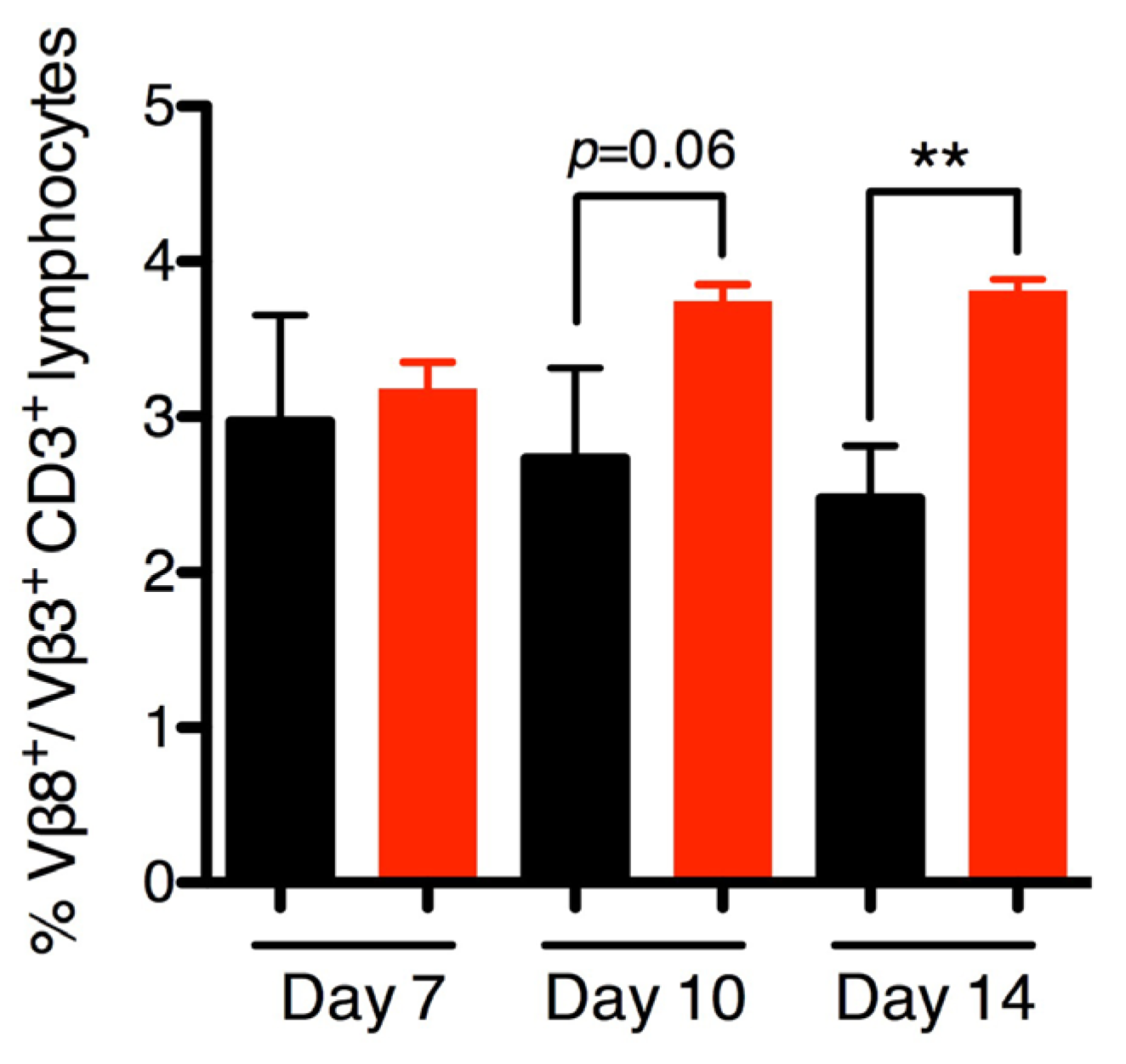
2.5. SEB Induces Late Vβ8 Skewing but not Anti-SEB IgG during Nasal Colonization
3. Discussion
4. Experimental Section
4.1. Mice
4.2. Bacterial Strains, Media and Growth Conditions
| Strain | Description | Source |
|---|---|---|
| S. aureus Newman | Early methicillin sensitive isolate from secondary infection in a patient with tubercular osteomyelitis (Sm sensitive) | [38] |
| S. aureus Newman SmR | S. aureus Newman resistant to Sm | This study |
| S. aureus Newman SmR Δsea | sea-null S. aureus Newman (with resistance to Sm) | [26] |
| S. aureus RN4220 | Restriction-deficient derivation of NCTC8325-4 | [39] |
| S. aureus COL | Early methicillin-resistant strain of S. aureus isolated in the 1960s | [40] |
| S. aureus COL Δseb | seb deletion strain of S. aureus COL | This study |
| E. coli DH5α | Cloning strain | Invitrogen |
4.3. Selection of a Streptomycin-Resistant S. aureus Strain
4.4. Construction of S. aureus COL Δseb
4.5. Detection of SAgs in Cultural Supernatants in vitro
4.6. Assessment of Superantigenic Activity of S. aureus COL Strains in vitro
4.7. Staphylococcus aureus Nasal Colonization Model
4.8. Determination of SAg Function in vivo
4.9. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Rehm, S.J.; Tice, A. Staphylococcus aureus: Methicillin-susceptible S. aureus to methicillin-resistant S. aureus and vancomycin-resistant S. aureus. Clin. Infect. Dis. 2010, 51, S176–S182. [Google Scholar]
- Boucher, H.; Miller, L.G.; Razonable, R.R. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2010, 51, S183–S197. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.A.J.W.; Wertheim, H.F.L. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 2005, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Ten Broeke-Smits, N.J.P.; Kummer, J.A.; Bleys, R.L.A.W.; Fluit, A.C.; Boel, C.H.E. Hair follicles as a niche of Staphylococcus aureus in the nose; is a more effective decolonisation strategy needed? J. Hosp. Infect. 2010, 76, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Bibel, D.J.; Aly, R.; Shinefield, H.R.; Maibach, H.I.; Strauss, W.G. Importance of the keratinized epithelial cell in bacterial adherence. J. Investig. Dermatol. 1982, 79, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, R.M.; Miajlovic, H.; Foster, T.J. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Vos, M.C.; Ott, A.; van Belkum, A.; Voss, A.; Kluytmans, J.A.; van Keulen, P.H.; Vandenbroucke-Grauls, C.M.; Meester, M.H.; Verbrugh, H.A. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004, 364, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Kolata, J.; Bode, L.G.M.; Holtfreter, S.; Steil, L.; Kusch, H.; Holtfreter, B.; Albrecht, D.; Hecker, M.; Engelmann, S.; van Belkum, A.; et al. Distinctive patterns in the human antibody response to Staphylococcus aureus bacteremia in carriers and non-carriers. Proteomics 2011, 11, 3914–3927. [Google Scholar]
- Brown, A.F.; Leech, J.M.; Rogers, T.R.; McLoughlin, R.M. Staphylococcus aureus colonization: Modulation of host immune response and impact on human vaccine design. Front. Immun. 2013, 4. [Google Scholar] [CrossRef]
- Wertheim, H.F.; Walsh, E.; Choudhurry, R.; Melles, D.C.; Boelens, H.A.; Miajlovic, H.; Verbrugh, H.A.; Foster, T.; van Belkum, A. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 2008, 5, e17. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Kokai-Kun, J.F.; Kristian, S.A.; Chanturiya, T.; Kalbacher, H.; Gross, M.; Nicholson, G.; Neumeister, B.; Mond, J.J.; Peschel, A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 2004, 10, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Roche, F.M.; Meehan, M.; Foster, T.J. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiol. 2003, 149, 2759–2767. [Google Scholar] [CrossRef]
- Clarke, S.R.; Andre, G.; Walsh, E.J.; Dufrêne, Y.F.; Foster, T.J.; Foster, S.J. Iron-regulated surface determinant protein A mediates adhesion of Staphylococcus aureus to human corneocyte envelope proteins. Infect. Immun. 2009, 77, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.X.; McCormick, J.K. Staphylococcal superantigens in colonization and disease. Front. Cell Infect. Microbiol. 2012, 2. [Google Scholar] [CrossRef]
- Salgado-Pabón, W.; Breshears, L.; Spaulding, A.R.; Merriman, J.A.; Stach, C.S.; Horswill, A.R.; Peterson, M.L.; Schlievert, P.M. Superantigens are critical for Staphylococcus aureus infective endocarditis, sepsis, and acute kidney injury. mBio. 2013, 4. [Google Scholar] [CrossRef]
- Jarraud, S.; Peyrat, M.A.; Lim, A.; Tristan, A.; Bes, M.; Mougel, C.; Etienne, J.; Vandenesch, F.; Bonneville, M.; Lina, G.; et al. A highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 2001, 166, 669–677. [Google Scholar]
- Holtfreter, S.; Roschack, K.; Eichler, P.; Eske, K.; Holtfreter, B.; Kohler, C.; Engelmann, S.; Hecker, M.; Greinacher, A.; Bröker, B.M. Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: A potential explanation for their improved prognosis in severe sepsis. J. Infect. Dis. 2006, 193, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, N.J.; de Vogel, C.P.; Boelens, H.A.; Grumann, D.; Hoogenboezem, T.; Vink, C.; Hooijkaas, H.; Foster, T.J.; Verbrugh, H.A.; van Belkum, A.; et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 2009, 199, 625–632. [Google Scholar]
- Holtfreter, S.; Grumann, D.; Schmudde, M.; Nguyen, H.T.T.; Eichler, P.; Strommenger, B.; Kopron, K.; Kolata, J.; Giedrys-Kalemba, S.; Steinmetz, I.; et al. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J. Clin. Microbiol. 2007, 45, 2669–2680. [Google Scholar]
- Ferry, T.; Thomas, D.; Genestier, A.L.; Bes, M.; Lina, G.; Vandenesch, F.; Etienne, J. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin. Infect. Dis. 2005, 41, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Burian, M.; Grumann, D.; Holtfreter, S.; Wolz, C.; Goerke, C.; Bröker, B.M. Expression of staphylococcal superantigens during nasal colonization is not sufficient to induce a systemic neutralizing antibody response in humans. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Hu, D.L.; Tsuji, T.; Nakane, A. Intranasal immunization of mutant toxic shock syndrome toxin 1 elicits systemic and mucosal immune response against Staphylococcus aureus infection. FEMS Immunol. Med. Microbiol. 2008, 52, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Krismer, B.; Peschel, A. Does Staphylococcus aureus nasal colonization involve biofilm formation? Future Microbiol. 2011, 6, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.X.; Gilmore, K.J.; Szabo, P.A.; Zeppa, J.J.; Baroja, M.L.; Haeryfar, S.M.M.; McCormick, J.K. Superantigens subvert the neutrophil response to promote abscess formation and enhance Staphylococcus aureus survival in vivo. Infect. Immun. 2014, 82, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, M.E.; Khan, S.A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J. Biol. Chem. 1988, 263, 6276–6280. [Google Scholar] [PubMed]
- Janeway, C.A., Jr. Selective elements for the V beta region of the T cell receptor: Mls and the bacterial toxic mitogens. Adv. Immunol. 1991, 50, 1–53. [Google Scholar] [PubMed]
- Bohach, G.; Schlievert, P.M. Staphylococcal and streptococcal superantigens: An update. In Superantigens: Molecular Basis for the Role in Human Diseases; Fraser, J.D., Kotb, M., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 21–36. [Google Scholar]
- Holtfreter, S.; Nguyen, T.T.H.; Wertheim, H.; Steil, L.; Kusch, H.; Truong, Q.P.; Engelmann, S.; Hecker, M.; Völker, U.; van Belkum, A.; et al. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin. Vaccine Immunol. 2009, 16, 1607–1614. [Google Scholar]
- Spaulding, A.R.; Lin, Y.C.; Merriman, J.A.; Brosnahan, A.J.; Peterson, M.L.; Schlievert, P.M. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine 2012, 30, 5099–5109. [Google Scholar] [CrossRef] [PubMed]
- Lussow, A.R.; MacDonald, H.R. Differential effects of superantigen-induced “anergy” on priming and effector stages of a T cell-dependent antibody response. Eur. J. Immunol. 1994, 24, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lappin, E.; Ferguson, A.J. Gram-positive toxic shock syndromes. Lancet Infect. Dis. 2009, 9, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.F.; Newell, K.; Duke, R.C.; Schlievert, P.M.; Freed, J.H.; Leung, D.Y. Differential effects of staphylococcal toxic shock syndrome toxin-1 on B cell apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 5425–5430. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.; Cohen, J. Superantigens: Microbial agents that corrupt immunity. Lancet Infect. Dis. 2002, 2, 156–162. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic shock syndrome and bacterial superantigens: An update. Annu. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Bian, H.J.; Molina, M.; Han, J.; Magram, J.; Saar, E.; Belunis, C.; Bolin, D.R.; Arceo, R.; Campbell, R.; et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996, 183, 2635–2644. [Google Scholar]
- Duthie, E.; Lorenz, L.L. Staphylococcal coagulase: Mode of action and antigenicity. Microbiology 1952, 6, 95–107. [Google Scholar]
- Novick, R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 1967, 33, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Fouts, D.E.; Archer, G.L.; Mongodin, E.F.; DeBoy, R.T.; Ravel, J.; Paulsen, I.T.; Kolonay, J.F.; Brinkac, L.; Beanan, M.; et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 2005, 187, 2426–2438. [Google Scholar]
- Kiser, K.B.; Cantey-Kiser, J.M.; Lee, J.C. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect. Immun. 1999, 67, 5001–5006. [Google Scholar] [PubMed]
- Fenner, L.; Widmer, A.F.; Dangel, M.; Frei, R. Distribution of spa types among meticillin-resistant Staphylococcus aureus isolates during a 6 year period at a low-prevalence university hospital. J. Med. Microbiol. 2008, 57, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, J.M.; McCormick, J.K.; Paustian, M.L.; Orwin, P.M.; Kapur, V.; Schlievert, P.M. Characterization and expression analysis of Staphylococcus aureus pathogenicity island 3. Implications for the evolution of staphylococcal pathogenicity islands. J. Biol. Chem. 2002, 277, 13138–13147. [Google Scholar]
- Arnaud, M.; Chastanet, A.; Debarbouille, M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 2004, 70, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Arsic, B.; Zhu, Y.; Heinrichs, D.E.; McGavin, M.J. Induction of the staphylococcal proteolytic cascade by antimicrobial fatty acids in community acquired methicillin resistant Staphylococcus aureus. PLoS One 2012, 7, e45952. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.X.; Kasper, K.J.; Zeppa, J.J.; McCormick, J.K. Superantigens Modulate Bacterial Density during Staphylococcus aureus Nasal Colonization. Toxins 2015, 7, 1821-1836. https://doi.org/10.3390/toxins7051821
Xu SX, Kasper KJ, Zeppa JJ, McCormick JK. Superantigens Modulate Bacterial Density during Staphylococcus aureus Nasal Colonization. Toxins. 2015; 7(5):1821-1836. https://doi.org/10.3390/toxins7051821
Chicago/Turabian StyleXu, Stacey X., Katherine J. Kasper, Joseph J. Zeppa, and John K. McCormick. 2015. "Superantigens Modulate Bacterial Density during Staphylococcus aureus Nasal Colonization" Toxins 7, no. 5: 1821-1836. https://doi.org/10.3390/toxins7051821





