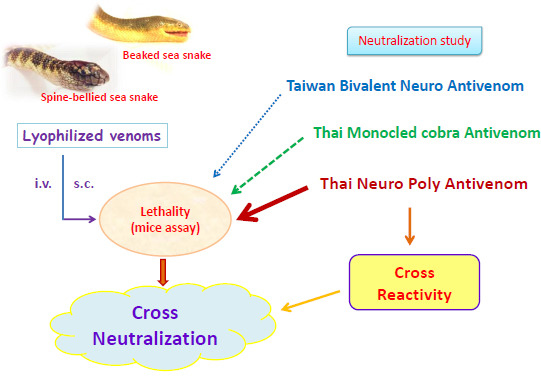
Antivenom Cross-Neutralization of the Venoms of Hydrophis schistosus and Hydrophis curtus, Two Common Sea Snakes in Malaysian Waters
Abstract
:1. Introduction
2. Experimental Section
2.1. Venoms and Antivenoms
2.2. Animals Use and Supply
2.3. Lethality Study
2.4. Neutralization Study
2.4.1. Preincubation Neutralization
2.4.2. Challenge-Rescue Neutralization
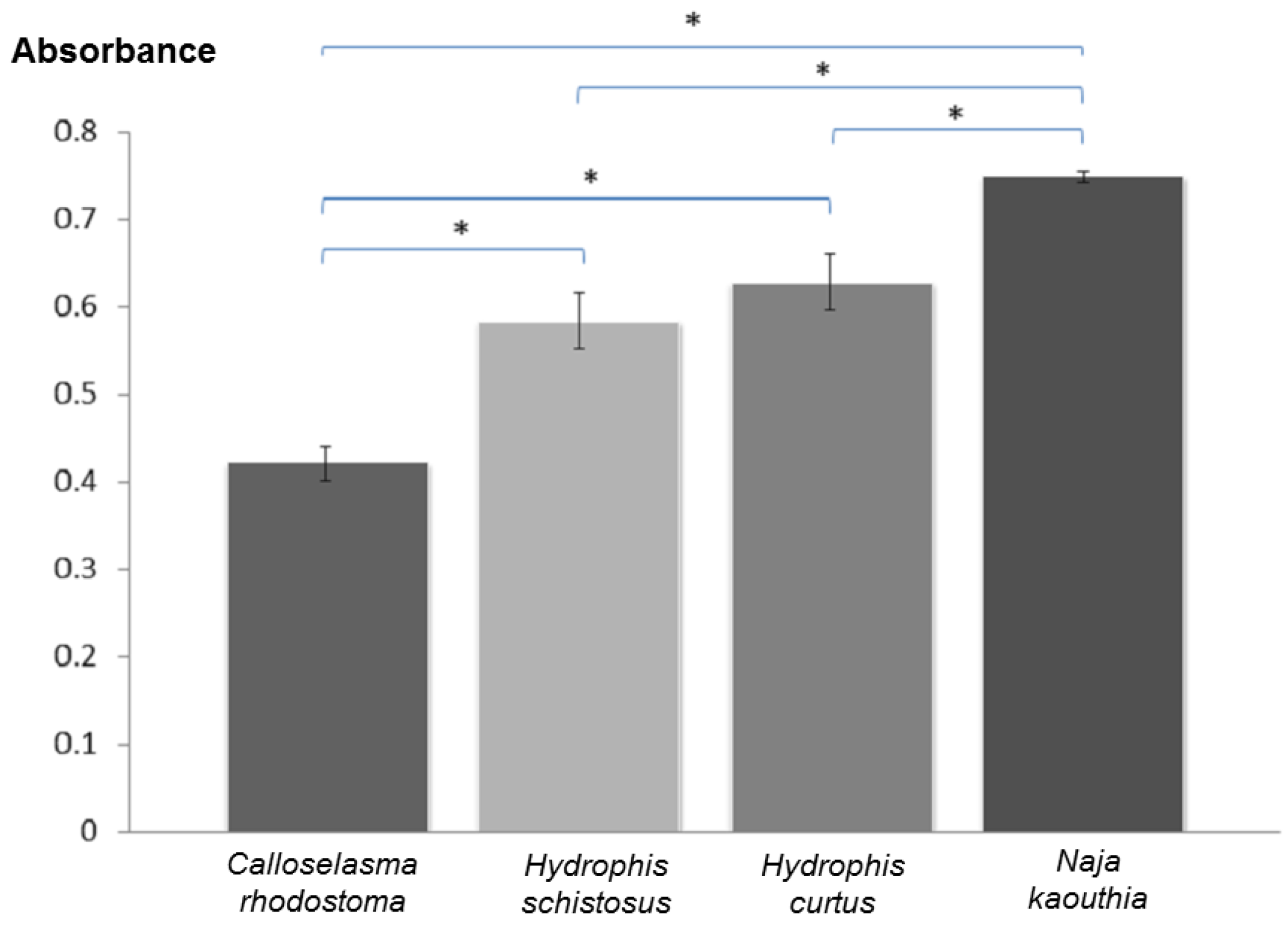
2.5. Immunological Cross-Reactivity Study
2.6. Statistical Analysis
3. Results and Discussion
| Venom | LD50 i.v. (µg/g) | LD50 i.m. (µg/g) | LD50 s.c. (µg/g) |
|---|---|---|---|
| Hydrophis schistosus (Malaysia) | 0.07 (0.05–0.09) | 0.10 (0.08–0.12) | 0.09 (0.06–0.14) |
| Hydrophis curtus (Malaysia) | 0.11 (0.07–0.17) | 0.18 (0.12–0.27) | 0.18 (0.17–0.18) |
| Laticauda laticaudata | 0.10 (0.08–0.12) | - | - |
| Laticauda semifasciata | 0.22 (0.15–0.34) | - | - |
| Venom | TBAV (preincubation) | NKMAV (preincubation) | NPAV (preincubation) | NPAV (challenge-rescue) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ED50 (µL) | ER50 (mg/mL) | P (mg/mL) | ED50 (µL) | ER50 (mg/mL) | P (mg/mL) | ED50 (µL) | ER50 (mg/mL) | P (mg/mL) | ED50 (µL) | ER50 (mg/mL) | P (mg/mL) | |
| Hydrophis schistosus | 141.36 | 0.027 (0.019–0.035) | 0.016 | 89.89 | 0.045 (0.032–0.058) | 0.027 | 100.00 | 0.074 (0.053–0.095) | 0.059 | 70.68 | 0.070 (0.047–0.109) | 0.042 |
| Hydrophis curtus | 200.00 | 0.032 (0.021–0.050) | 0.019 | 89.89 | 0.070 (0.045–0.109) | 0.042 | 125.00 | 0.092 (0.059–0.143) | 0.074 | 70.68 | 0.143 (0.135–0.143) | 0.086 |
| Laticauda laticaudata | - | - | - | - | - | - | 75.00 | 0.075 (0.060–0.090) | 0.045 | - | - | - |
| Laticauda semifasciata | - | - | - | - | - | - | 50.00 | 0.253 (0.173–0.391) | 0.15 | - | - | - |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jamaiah, I.; Rohela, M.; Ng, T.K.; Ch’ng, K.B.; Teh, Y.S.; Nurulhuda, A.L.; Suhaili, N. Retrospective prevalence of snakebites from Hospital Kuala Lumpur (HKL) (1999–2003). South. Asian J. Trop. Med. Public Health 2006, 37, 200–205. [Google Scholar]
- Chew, K.S.; Khor, H.W.; Ahmad, R.; Rahman, N.H. A five-year retrospective review of snakebite patients admitted to a tertiary university hospital in Malaysia. Int. J. Emerg. Med. 2011, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.L.; Lee, M.S.Y.; Mumpuni; Bertozzi, T.; Rasmussen, A.R. Multilocus phylogeny and recent rapid radiation of the viviparous sea snakes (Elapidae: Hydrophiinae). Mol. Phylogenet. Evol. 2013, 66, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.A. Symptomology, pathology and treatment of the bites of sea snakes. In Handbook of Experimental Pharmacology; Lee, C.Y., Ed.; Springer-Verlag: Berlin, Germany, 1979; Volume 52, pp. 922–955. [Google Scholar]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteomics 2011, 74, 1735–1767. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.M.; Isbister, G.K.; Hodgson, W.C. Alpha neurotoxins. Toxicon 2013, 66, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, R.; Isbister, G.K.; O’Leary, M.A.; Mirtschin, P.; Dunstan, N.; Hodgson, W.C. Cross-neutralisation of the neurotoxic effects of Egyptian cobra venom with commercial tiger snake antivenom. Basic Clin. Pharmacol. Toxicol. 2013, 112, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of common Southeast Asian viperid venoms by a Thai polyvalent snake antivenom (Hemato Polyvalent Snake Antivenom). Acta Trop. 2014, 132, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Leong, P.K.; Fung, S.Y.; Sim, S.M.; Ponnudurai, G.; Ariaratnam, C.; Khomvilai, S.; Sitprija, V.; Tan, N.H. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop. 2011, 117, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Minton, S.A., Jr. Paraspecific protection by elapid and sea snake antivenins. Toxicon 1967, 5, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Khow, O.; Chanhome, L.; Omori-Satoh, T.; Sitprija, V. Effectiveness of Thai cobra (Naja kaouthia) antivenom against sea snake (Lapemis hardwickii) venom: Verification by affinity purified F(AB')2 fragments. J. Nat. Toxins 2001, 10, 249–253. [Google Scholar] [PubMed]
- Khomvilai, S. New improvement in the production technique of polyvalent snake antivenom imunoglobulins. In Proceedings of the Inaugural Conference on Global Issues in Clinical Toxinology, Melbourne, Australia, 23–28 November 2008.
- Howard-Jones, N.A. A CIOMS ethical code for animal experimentation. WHO Chron. 1995, 39, 51–56. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, England, UK, 1952. [Google Scholar]
- Tan, C.H.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Gnanathasan, C.A. Immunological properties of Hypnale hypnale (hump-nosed pit viper) venom: Antibody production with diagnostic and therapeutic potentials. Acta Trop. 2012, 122, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Morais, V.; Ifran, S.; Berasain, P.; Massaldi, H. Antivenoms: Potency or median effective dose, which to use? J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 191–193. [Google Scholar] [CrossRef]
- Tan, N.H. The Biochemistry of venoms of some venomous snakes of Malaysia—A review. Trop. Biomed. 1991, 8, 91–103. [Google Scholar]
- Yap, M.K.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Tan, C.H. Pharmacokinetics of Naja sumatrana (Equatorial Spitting Cobra) venom and its major toxins in experimentally envenomed rabbits. PLoS Negl. Trop. 2014, 6, e2890. [Google Scholar] [CrossRef]
- Tan, C.H.; Sim, S.M.; Gnanathasan, C.A.; Fung, S.Y.; Tan, N.H. Pharmacokinetics of the Sri Lankan hump-nosed pit viper (Hypnale hypnale) venom following intravenous and intramuscular injections of the venom into rabbits. Toxicon 2014, 79, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C. Cobrotoxin: Structure and function. J. Nat. Toxins 1999, 8, 221–233. [Google Scholar] [PubMed]
- Karlsson, E.; Eaker, D.; Fryklund, L.; Kadin, S. Chromatographic separation of Enhydrina schistosa (common sea snake) venom and the characterization of two principal neurotoxins. Biochemistry 1972, 11, 4628–4633. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, N. Erabutoxins a, b and c in sea snake Laticauda semifasciata venom. Toxicon 1973, 11, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Chetty, N.; Du, A.; Hodgson, W.C.; Winkel, K.; Fry, B.G. The in vitro neuromuscular activity of Indo-Pacific sea-snake venoms: Efficacy of two commercially available antivenoms. Toxicon 2004, 44, 193–200. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Management of Snake-Bites; WHO Press: New Delhi, India, 2010. [Google Scholar]
- Geh, S.L.; Toh, H.T. Ultrastructural changes in skeletal muscle caused by a phospholipase A2 fraction isolated from the venom of a sea snake, Enhydrina schistosa. Toxicon 1978, 16, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnakone, P.; Ponraj, D.; Thwinn, M.M. Myotoxic phospholipases from snake venoms: General myoglobinuric and local myonecrotic toxins. In Venom Phospholipases A2 Enzymes: Structure, Function and Mechanism; Kini, R.N., Ed.; John Wiley and Sons: New York, NY, USA, 1997; pp. 287–320. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.H.; Tan, N.H.; Tan, K.Y.; Kwong, K.O. Antivenom Cross-Neutralization of the Venoms of Hydrophis schistosus and Hydrophis curtus, Two Common Sea Snakes in Malaysian Waters. Toxins 2015, 7, 572-581. https://doi.org/10.3390/toxins7020572
Tan CH, Tan NH, Tan KY, Kwong KO. Antivenom Cross-Neutralization of the Venoms of Hydrophis schistosus and Hydrophis curtus, Two Common Sea Snakes in Malaysian Waters. Toxins. 2015; 7(2):572-581. https://doi.org/10.3390/toxins7020572
Chicago/Turabian StyleTan, Choo Hock, Nget Hong Tan, Kae Yi Tan, and Kok Onn Kwong. 2015. "Antivenom Cross-Neutralization of the Venoms of Hydrophis schistosus and Hydrophis curtus, Two Common Sea Snakes in Malaysian Waters" Toxins 7, no. 2: 572-581. https://doi.org/10.3390/toxins7020572
APA StyleTan, C. H., Tan, N. H., Tan, K. Y., & Kwong, K. O. (2015). Antivenom Cross-Neutralization of the Venoms of Hydrophis schistosus and Hydrophis curtus, Two Common Sea Snakes in Malaysian Waters. Toxins, 7(2), 572-581. https://doi.org/10.3390/toxins7020572