Engineering Venom’s Toxin-Neutralizing Antibody Fragments and Its Therapeutic Potential
Abstract
:1. Introduction
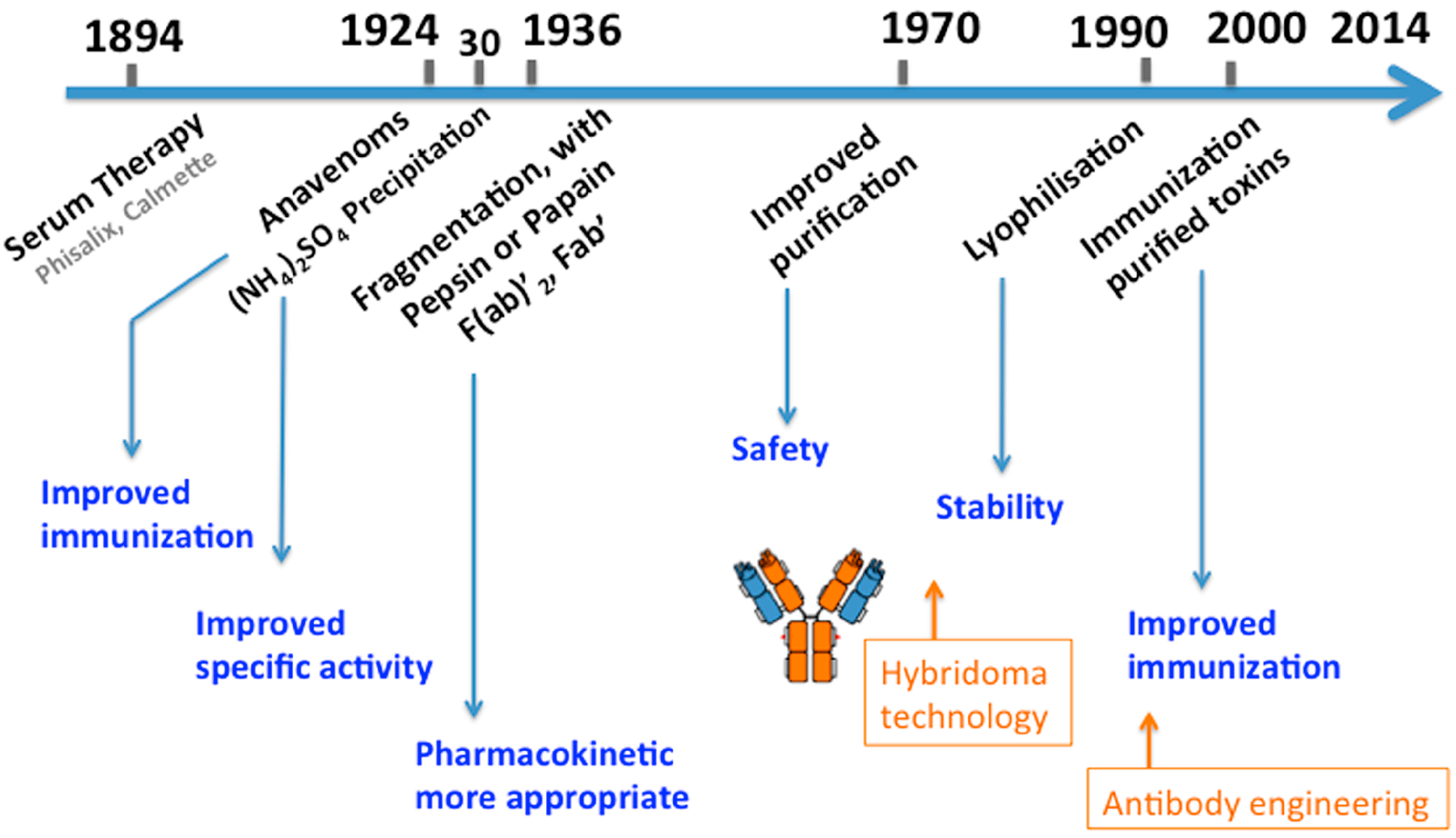
2. Historical Development of Serum Therapy
3. Conventional Anti-Venoms: Strengths and Weaknesses
3.1. Main Points
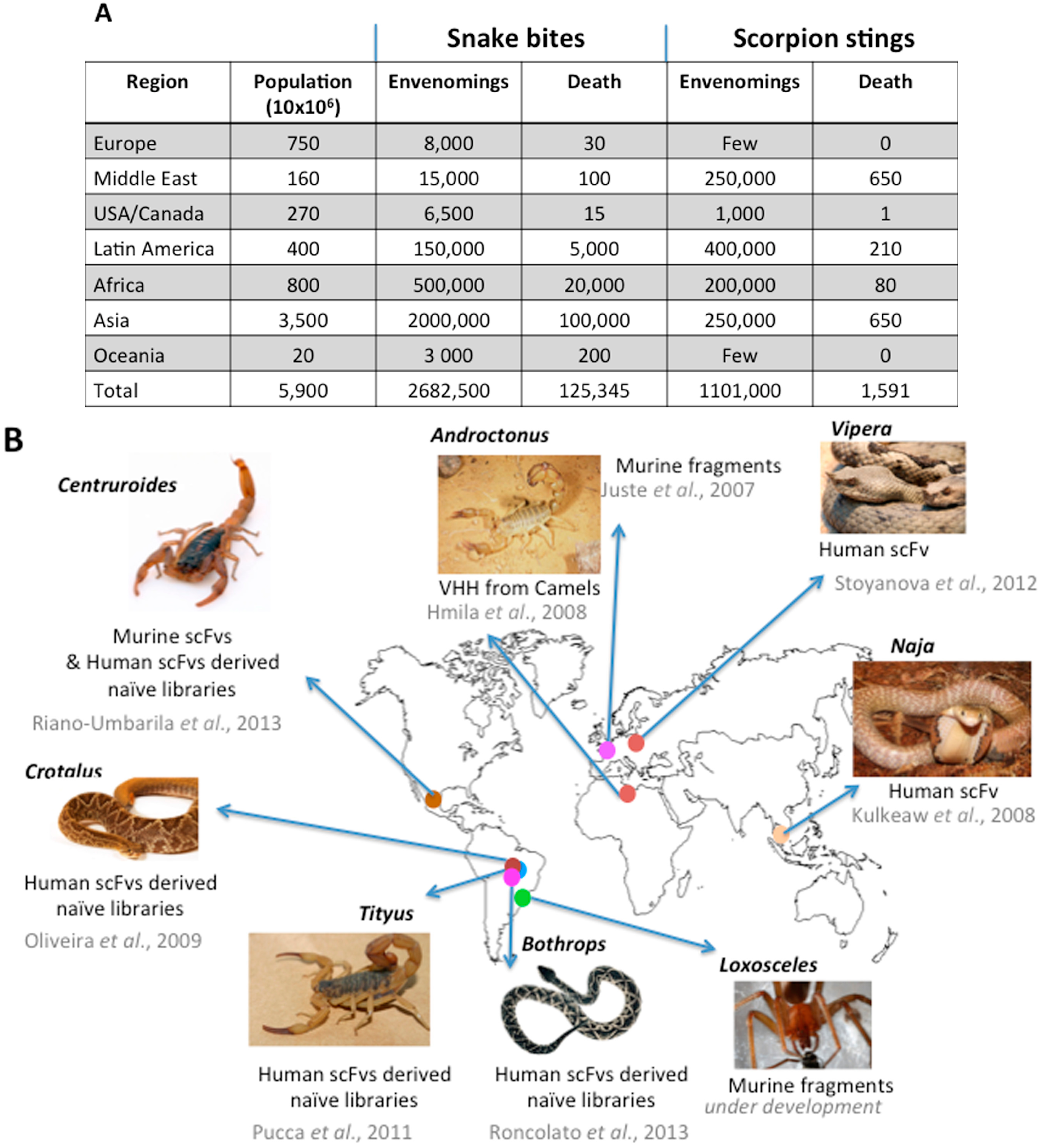
3.2. Immunogens and Host Animals

3.3. Fragmentation and Purification Process
4. Monoclonal Antibodies: Precursor of Therapeutic Antibodies
5. Recombinant Antibodies and Antibody Fragments: New Opportunities
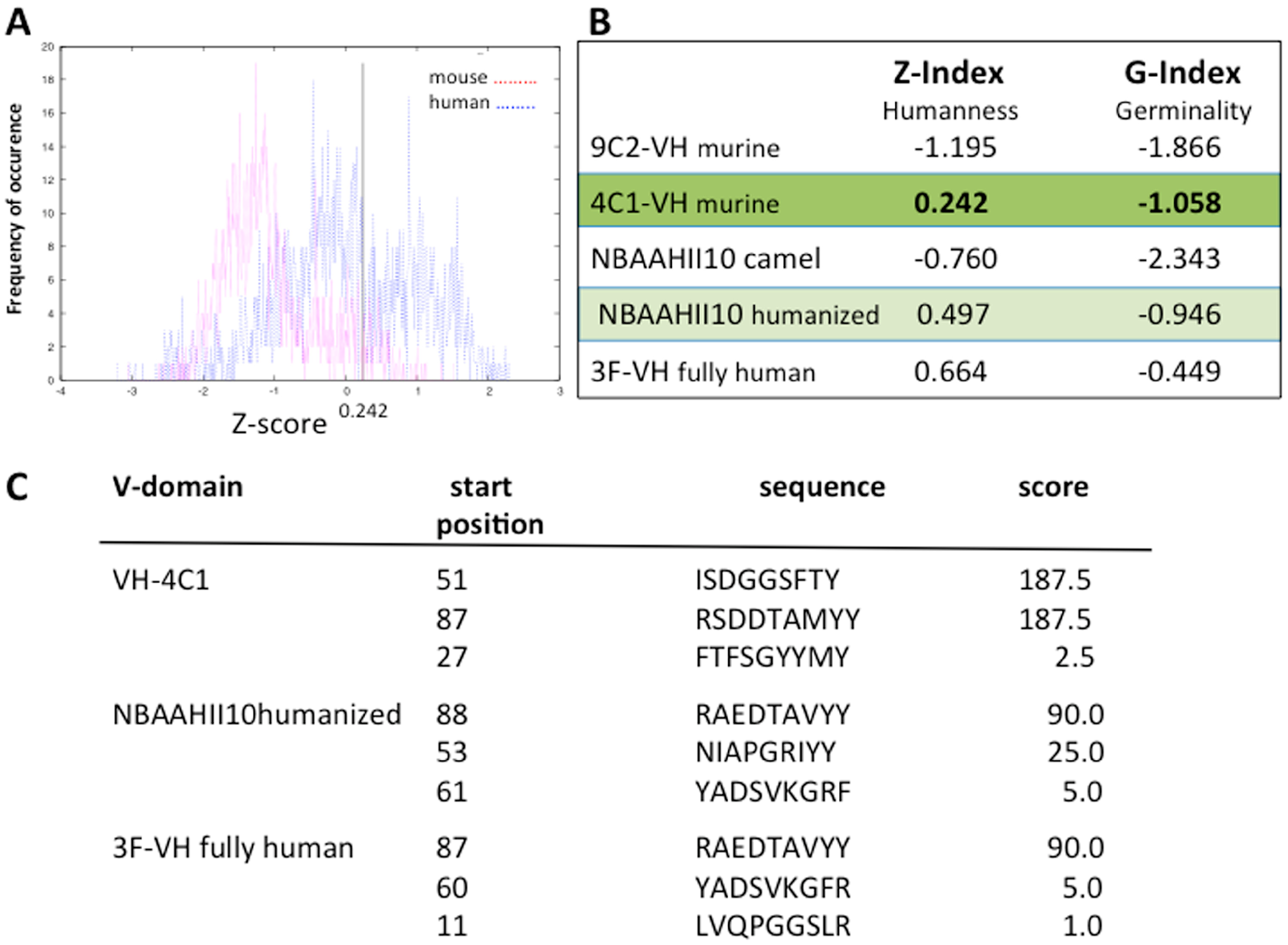
5.1. Making Monoclonal Antibodies More Human
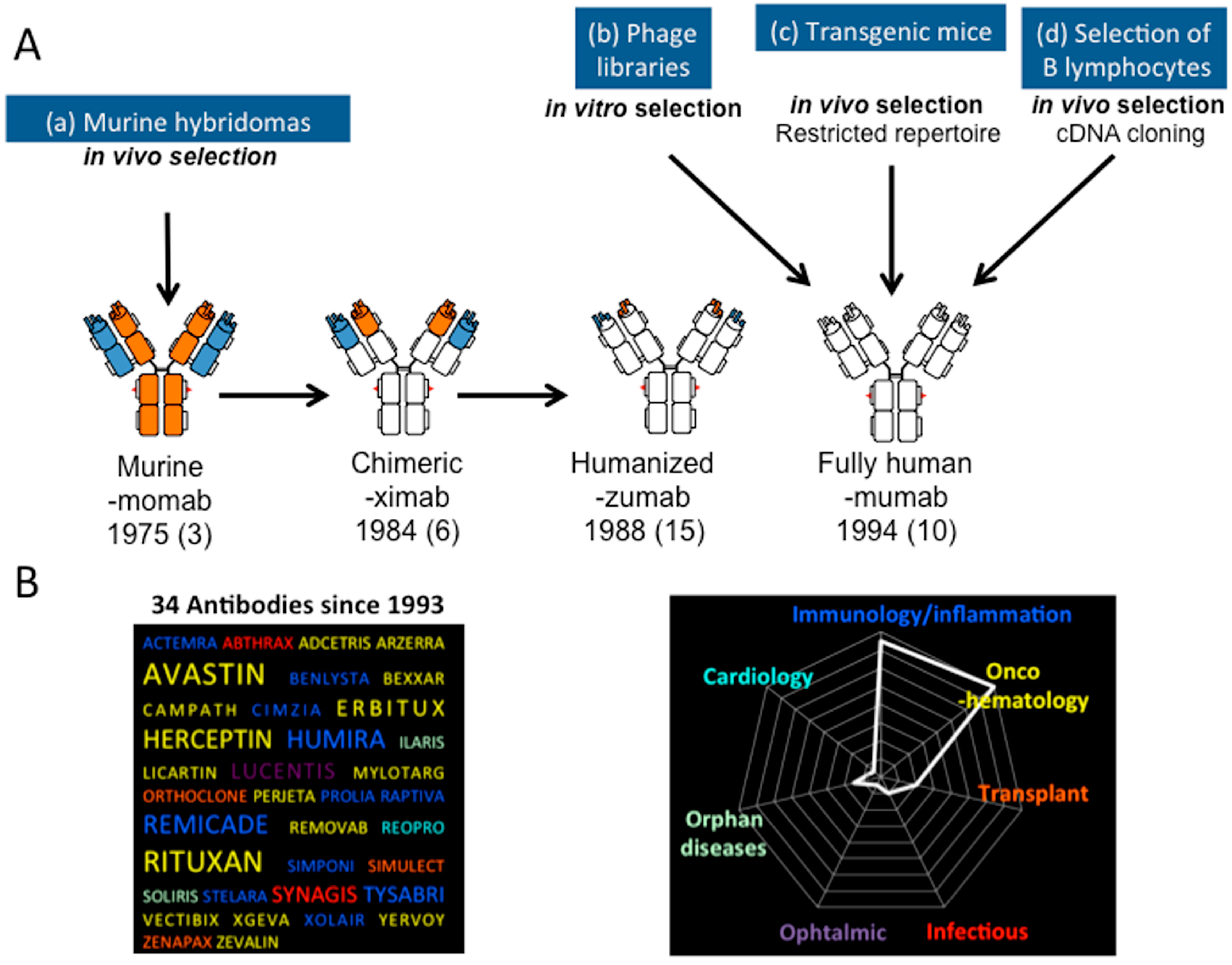
5.2. Enlarging the Diversity of Antibody Fragment Formats

6. Preclinical Evaluation of Recombinant Antibodies for the Treatment of Envenomings
6.1. Snake Venoms
6.2. Arachnid Venoms
6.2.1. Tityus
6.2.2. Centruroides
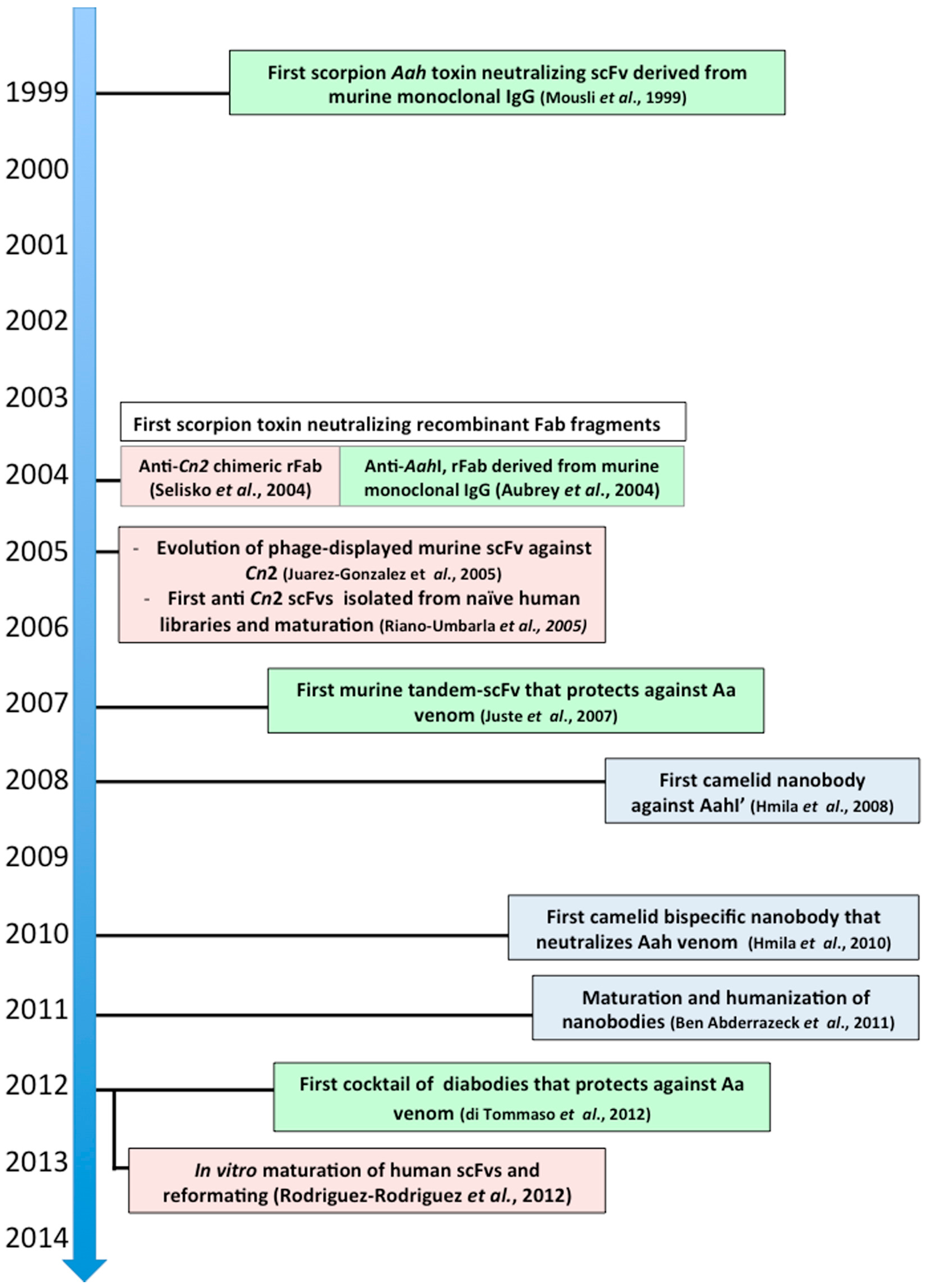
6.2.3. Androctonus australis
7. Prospects and Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fry, B.G.; Roelants, K.; Norman, J.A. Tentacles of venom: Toxic protein convergence in the Kingdom Animalia. J. Mol. Evol. 2009, 68, 311–321. [Google Scholar] [CrossRef]
- Goyffon, M.; Landon, C. Scorpion toxins and defensins. C. R. Séances Soc. Biol. Fil. 1998, 192, 445–462. [Google Scholar]
- Behring, E.; Kitasato, S. Ueber das zustandekommen der diphterie-immunitaet und der tetanus-immunitatet bei thieren. Deutsch. Med. Wochenschr. 1890, 16, 1113–1114. [Google Scholar] [CrossRef]
- Goyffon, M. Passive immunotherapy today: Brief history. Biol. Aujourdhui 2010, 204, 51–54. [Google Scholar] [CrossRef]
- Eibl, M.M. History of immunoglobulin replacement. Immunol. Allergy Clin. N. Am. 2008, 28, 737–764. [Google Scholar] [CrossRef]
- Casadeval, A.; Scharff, M.D. Return to the past: The case for antibody-based therapies in infectious diseases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1995, 21, 150–161. [Google Scholar]
- Cross, A.S. Intravenous immunoglobins (IVIGs) to prevent and treat infectious diseases. Adv. Exp. Med. Biol. 1995, 383, 123–130. [Google Scholar] [CrossRef]
- Nelson, A.L.; Reichert, J.M. Development trends for therapeutic antibody fragments. Nat. Biotechnol. 2009, 27, 331–337. [Google Scholar] [CrossRef]
- Ducancel, F.; Muller, B.H. Molecular engineering of antibodies for therapeutic and diagnostic purposes. mAbs 2012, 4, 445–457. [Google Scholar] [CrossRef]
- Reichert, J.M. Antibodies to watch in 2014. mAbs 2014, 6, 5–14. [Google Scholar]
- Anonymous. What are biosimilars and are they important? Drug Ther. Bull. 2013, 51, 57–60. [Google Scholar] [CrossRef]
- Lawrie, R. First clinical use of penicillin. Br. Med. J. Clin. Res. Ed. 1985, 290, 397. [Google Scholar] [CrossRef]
- Casadevall, A.; Dadachova, E.; Pirofski, L. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004, 2, 695–703. [Google Scholar] [CrossRef]
- Stock, R.P.; Massougbodji, A.; Alagón, A.; Chippaux, J.-P. Bringing antivenoms to Sub-Saharan Africa. Nat. Biotechnol. 2007, 25, 173–177. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Emerging options for the management of scorpion stings. Drug Des. Dev. Ther. 2012, 6, 165–173. [Google Scholar] [CrossRef]
- Chippaux, J.-P.; Goyffon, M. Epidemiology of scorpionism: A global appraisa. Acta Trop. 2008, 107, 71–79. [Google Scholar] [CrossRef]
- Hogan, C.J.; Barbaro, K.C.; Winkel, K. Loxoscelism: Old obstacles, new directions. Ann. Emerg. Med. 2004, 44, 608–624. [Google Scholar] [CrossRef]
- Currie, B.J. Marine antivenoms. J. Toxicol. Clin. Toxicol. 2003, 41, 301–308. [Google Scholar] [CrossRef]
- Boyer, L.; Degan, J.; Ruha, A.-M.; Mallie, J.; Mangin, E.; Alagón, A. Safety of intravenous equine F(ab')2: Insights following clinical trials involving 1534 recipients of scorpion antivenom. Toxicon Off. J. Int. Soc. Toxinol. 2013, 76, 386–393. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Guidelines for the production, control and regulation of snake antivenom immunoglobulins. Biol. Aujourdhui 2010, 204, 87–91. [Google Scholar] [CrossRef]
- Fernandes, I.; Takehara, H.A.; Mota, I. Isolation of IgGT from hyperimmune horse anti-snake venom serum: Its protective ability. Toxicon Off. J. Int. Soc. Toxinol. 1991, 29, 1373–1379. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Warrell, D.A.; Griffiths, E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon Off. J. Int. Soc. Toxinol. 2003, 41, 541–557. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Burnouf, T. Antivenoms for the treatment of snakebite envenomings: The road ahead. Biol. J. Int. Assoc. Biol. Stand. 2011, 39, 129–142. [Google Scholar]
- Chippaux, J.P.; Goyffon, M. Venoms, antivenoms and immunotherapy. Toxicon Off. J. Int. Soc. Toxinol. 1998, 36, 823–846. [Google Scholar] [CrossRef]
- Nguyen, L. Production of highly purified therapeutic immunoglobulins (HPTI): Analysis of a purification process. Biol. Aujourdhui 2010, 204, 55–59. [Google Scholar] [CrossRef]
- Dart, R.C.; McNally, J. Efficacy, safety, and use of snake antivenoms in the United States. Ann. Emerg. Med. 2001, 37, 181–188. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Theakston, R.D.G. Snake antivenoms. J. Toxicol. Clin. Toxicol. 2003, 41, 277–290;317–327. [Google Scholar] [CrossRef]
- Mouthon, L. Intravenous immunoglobulin therapy. Rev. Prat. 2005, 55, 1049–1056. [Google Scholar]
- Nissim, A.; Chernajovsky, Y. Historical development of monoclonal antibody therapeutics. Handb. Exp. Pharmacol. 2008, 181, 3–18. [Google Scholar] [CrossRef]
- Brodsky, F.M. Monoclonal antibodies as magic bullets. Pharm. Res. 1988, 5, 1–9. [Google Scholar] [CrossRef]
- Sun, M. A million dollars for the magic bullet. Science 1981, 214, 1326–1327. [Google Scholar]
- Waldmann, H. A personal history of the CAMPATH-1H antibody. Med. Oncol. Northwood Lond. Engl. 2002, 19 (Suppl.), S3–S9. [Google Scholar] [CrossRef]
- Gatineau, E.; Lee, C.Y.; Fromageot, P.; Menez, A. Reversal of snake neurotoxin binding to mammalian acetylcholine receptor by specific antiserum. Eur. J. Biochem. FEBS 1988, 171, 535–539. [Google Scholar]
- Bahraoui, E.; Pichon, J.; Muller, J.M.; Darbon, H.; Elayeb, M.; Granier, C.; Marvaldi, J.; Rochat, H. Monoclonal antibodies to scorpion toxins. Characterization and molecular mechanisms of neutralization. J. Immunol. 1988, 141, 214–220. [Google Scholar]
- Alvarenga, L.M.; Diniz, C.R.; Granier, C.; Chávez-Olórtegui, C. Induction of neutralizing antibodies against Tityus serrulatus scorpion toxins by immunization with a mixture of defined synthetic epitopes. Toxicon Off. J. Int. Soc. Toxinol. 2002, 40, 89–95. [Google Scholar] [CrossRef]
- Laffly, E.; Sodoyer, R. Monoclonal and recombinant antibodies, 30 years after …. Hum. Antibodies 2005, 14, 33–55. [Google Scholar]
- Lonberg, N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005, 23, 1117–1125. [Google Scholar] [CrossRef]
- Almagro, J.C.; Fransson, J. Humanization of antibodies. Front. Biosci. J. Virtual Libr. 2008, 13, 1619–1633. [Google Scholar]
- Hoogenboom, H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 2005, 23, 1105–1116. [Google Scholar] [CrossRef]
- Eisenhardt, S.U.; Schwarz, M.; Bassler, N.; Peter, K. Subtractive single-chain antibody (scFv) phage-display: Tailoring phage-display for high specificity against function-specific conformations of cell membrane molecules. Nat. Protoc. 2007, 2, 3063–3073. [Google Scholar] [CrossRef]
- Pansri, P.; Jaruseranee, N.; Rangnoi, K.; Kristensen, P.; Yamabhai, M. A compact phage display human scFv library for selection of antibodies to a wide variety of antigens. BMC Biotechnol. 2009, 9, 6. [Google Scholar] [CrossRef]
- Jensen, K.B.; Larsen, M.; Pedersen, J.S.; Christensen, P.A.; Alvarez-Vallina, L.; Goletz, S.; Clark, B.F.C.; Kristensen, P. Functional improvement of antibody fragments using a novel phage coat protein III fusion system. Biochem. Biophys. Res. Commun. 2002, 298, 566–573. [Google Scholar] [CrossRef]
- Huang, J.; Doria-Rose, N.A.; Longo, N.S.; Laub, L.; Lin, C.-L.; Turk, E.; Kang, B.H.; Migueles, S.A.; Bailer, R.T.; Mascola, J.R.; et al. Isolation of human monoclonal antibodies from peripheral blood B cells. Nat. Protoc. 2013, 8, 1907–1915. [Google Scholar] [CrossRef]
- Fields, C.; O’Connell, D.; Xiao, S.; Lee, G.U.; Billiald, P.; Muzard, J. Creation of recombinant antigen-binding molecules derived from hybridomas secreting specific antibodies. Nat. Protoc. 2013, 8, 1125–1148. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of recombinant antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Huston, J.S.; Levinson, D.; Mudgett-Hunter, M.; Tai, M.S.; Novotný, J.; Margolies, M.N.; Ridge, R.J.; Bruccoleri, R.E.; Haber, E.; Crea, R. Protein engineering of antibody binding sites: Recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 1988, 85, 5879–5883. [Google Scholar] [CrossRef]
- Holliger, P.; Prospero, T.; Winter, G. “Diabodies”: Small bivalent and bispecific antibody fragments. Proc. Natl. Acad. Sci. USA 1993, 90, 6444–6448. [Google Scholar] [CrossRef]
- Turki, I.; Hammami, A.; Kharmachi, H.; Mousli, M. Engineering of a recombinant trivalent single-chain variable fragment antibody directed against rabies virus glycoprotein G with improved neutralizing potency. Mol. Immunol. 2014, 57, 66–73. [Google Scholar] [CrossRef]
- De Marco, A. Biotechnological applications of recombinant single-domain antibody fragments. Microb. Cell Factories 2011, 10, 44. [Google Scholar] [CrossRef]
- Ward, E.S.; Güssow, D.; Griffiths, A.D.; Jones, P.T.; Winter, G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 1989, 341, 544–546. [Google Scholar]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Nuttall, S.D. Overview and discovery of IgNARs and generation of VNARs. Methods Mol. Biol. (Clifton, NJ, USA) 2012, 911, 27–36. [Google Scholar]
- Funayama, J.C.; Pucca, M.B.; Roncolato, E.C.; Bertolini, T.B.; Campos, L.B.; Barbosa, J.E. Production of human antibody fragments binding to melittin and phospholipase A2 in Africanised bee venom: Minimising venom toxicity. Basic Clin. Pharmacol. Toxicol. 2012, 110, 290–297. [Google Scholar]
- Warrell, D.A.; Gutiérrez, J.M.; Calvete, J.J.; Williams, D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013, 138, 38–59. [Google Scholar]
- Harrison, R.A.; Cook, D.A.; Renjifo, C.; Casewell, N.R.; Currier, R.B.; Wagstaff, S.C. Research strategies to improve snakebite treatment: Challenges and progress. J. Proteomics 2011, 74, 1768–1780. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef]
- Tanjoni, I.; Butera, D.; Bento, L.; Della-Casa, M.S.; Marques-Porto, R.; Takehara, H.A.; Gutiérrez, J.M.; Fernandes, I.; Moura-da-Silva, A.M. Snake venom metalloproteinases: Structure/function relationships studies using monoclonal antibodies. Toxicon Off. J. Int. Soc. Toxinol. 2003, 42, 801–808. [Google Scholar] [CrossRef]
- Lomonte, B.; Gutiérrez, J.M.; Ramírez, M.; Díaz, C. Neutralization of myotoxic phospholipases A2 from the venom of the snake Bothrops asper by monoclonal antibodies. Toxicon Off. J. Int. Soc. Toxinol. 1992, 30, 239–245. [Google Scholar] [CrossRef]
- Boulain, J.C.; Ménez, A. Neurotoxin-specific immunoglobulins accelerate dissociation of the neurotoxin-acetylcholine receptor complex. Science 1982, 217, 732–733. [Google Scholar]
- Gutiérrez, J.M.; Lomonte, B.; Sanz, L.; Calvete, J.J.; Pla, D. Immunological profile of antivenoms: Preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J. Proteomics 2014, 105, 340–350. [Google Scholar]
- Harrison, R.A.; Hasson, S.S.; Harmsen, M.; Laing, G.D.; Conrath, K.; Theakston, R.D.G. Neutralisation of venom-induced haemorrhage by IgG from camels and llamas immunised with viper venom and also by endogenous, non-IgG components in camelid sera. Toxicon Off. J. Int. Soc. Toxinol. 2006, 47, 364–368. [Google Scholar] [CrossRef]
- Cook, D.A.N.; Samarasekara, C.L.; Wagstaff, S.C.; Kinne, J.; Wernery, U.; Harrison, R.A. Analysis of camelid IgG for antivenom development: Immunoreactivity and preclinical neutralisation of venom-induced pathology by IgG subclasses, and the effect of heat treatment. Toxicon Off. J. Int. Soc. Toxinol. 2010, 56, 596–603. [Google Scholar] [CrossRef]
- Oliveira, J.G.; Soares, S.G.; Soares, A.M.; Giglio, J.R.; Teixeira, J.E.; Barbosa, J.E. Expression of human recombinant antibody fragments capable of partially inhibiting the phospholypase activity of Crotalus durissus terrificus venom. Basic Clin. Pharmacol. Toxicol. 2009, 105, 84–91. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Sakolvaree, Y.; Srimanote, P.; Tongtawe, P.; Maneewatch, S.; Sookrung, N.; Tungtrongchitr, A.; Tapchaisri, P.; Kurazono, H.; Chaicumpa, W. Human monoclonal ScFv neutralize lethal Thai cobra, Naja kaouthia, neurotoxin. J. Proteomics 2009, 72, 270–282. [Google Scholar] [CrossRef]
- Stoyanova, V.; Aleksandrov, R.; Lukarska, M.; Duhalov, D.; Atanasov, V.; Petrova, S. Recognition of Vipera ammodytes meridionalis neurotoxin vipoxin and its components using phage-displayed scFv and polyclonal antivenom sera. Toxicon Off. J. Int. Soc. Toxinol. 2012, 60, 802–809. [Google Scholar] [CrossRef]
- Roncolato, E.C.; Pucca, M.B.; Funayama, J.C.; Bertolini, T.B.; Campos, L.B.; Barbosa, J.E. Human antibody fragments specific for Bothrops jararacussu venom reduce the toxicity of other Bothrops sp. venoms. J. Immunotoxicol. 2013, 10, 160–168. [Google Scholar]
- Chavanayarn, C.; Thanongsaksrikul, J.; Thueng-In, K.; Bangphoomi, K.; Sookrung, N.; Chaicumpa, W. Humanized-single domain antibodies (VH/VHH) that bound specifically to Naja kaouthia phospholipase A2 and neutralized the enzymatic activity. Toxins 2012, 4, 554–567. [Google Scholar] [CrossRef]
- Devaux, C.; Jouirou, B.; Naceur Krifi, M.; Clot-Faybesse, O.; El Ayeb, M.; Rochat, H. Quantitative variability in the biodistribution and in toxinokinetic studies of the three main alpha toxins from the Androctonus australis hector scorpion venom. Toxicon Off. J. Int. Soc. Toxinol. 2004, 43, 661–669. [Google Scholar] [CrossRef]
- Amaro, I.; Riaño-Umbarila, L.; Becerril, B.; Possani, L.D. Isolation and characterization of a human antibody fragment specific for Ts1 toxin from Tityus serrulatus scorpion. Immunol. Lett. 2011, 139, 73–79. [Google Scholar] [CrossRef]
- Pucca, M.B.; Zoccal, K.F.; Roncolato, E.C.; Bertolini, T.B.; Campos, L.B.; Cologna, C.T.; Faccioli, L.H.; Arantes, E.C.; Barbosa, J.E. Serrumab: A human monoclonal antibody that counters the biochemical and immunological effects of Tityus serrulatus venom. J. Immunotoxicol. 2012, 9, 173–183. [Google Scholar]
- Pucca, M.B.; Cerni, F.A.; Peigneur, S.; Arantes, E.C.; Tytgat, J.; Barbosa, J.E. Serrumab: A novel human single chain-fragment antibody with multiple scorpion toxin-neutralizing capacities. J. Immunotoxicol. 2014, 11, 133–140. [Google Scholar] [CrossRef]
- Espino-Solis, G.P.; Riaño-Umbarila, L.; Becerril, B.; Possani, L.D. Antidotes against venomous animals: State of the art and prospectives. J. Proteomics 2009, 72, 183–199. [Google Scholar] [CrossRef]
- Riaño-Umbarila, L.; Juárez-González, V.R.; Olamendi-Portugal, T.; Ortíz-León, M; Possani, L.D.; Becerril, B. A strategy for the generation of specific human antibodies by directed evolution and phage display. An example of a single-chain antibody fragment that neutralizes a major component of scorpion venom. FEBS J. 2005, 272, 2591–2601. [Google Scholar] [CrossRef]
- Riaño-Umbarila, L.; Contreras-Ferrat, G.; Olamendi-Portugal, T.; Morelos-Juárez, C.; Corzo, G.; Possani, L.D.; Becerril, B. Exploiting cross-reactivity to neutralize two different scorpion venoms with one single chain antibody fragment. J. Biol. Chem. 2011, 286, 6143–6151. [Google Scholar] [CrossRef]
- Hugo, N.; Lafont, V.; Beukes, M.; Altschuh, D. Functional aspects of co-variant surface charges in an antibody fragment. Protein Sci. Publ. Protein Soc. 2002, 11, 2697–2705. [Google Scholar]
- Rodríguez-Rodríguez, E.R.; Ledezma-Candanoza, L.M.; Contreras-Ferrat, L.G.; Olamendi-Portugal, T.; Possani, L.D.; Becerril, B.; Riaño-Umbarila, L. A single mutation in framework 2 of the heavy variable domain improves the properties of a diabody and a related single-chain antibody. J. Mol. Biol. 2012, 423, 337–350. [Google Scholar] [CrossRef]
- Colcher, D.; Bird, R.; Roselli, M.; Hardman, K.D.; Johnson, S.; Pope, S.; Dodd, S.W.; Pantoliano, M.W.; Milenic, D.E.; Schlom, J. In vivo tumor targeting of a recombinant single-chain antigen-binding protein. J. Natl. Cancer Inst. 1990, 82, 1191–1197. [Google Scholar]
- Quintero-Hernández, V.; del Pozo-Yauner, L.; Pedraza-Escalona, M.; Juárez-González, V.R.; Alcántara-Recillas, I.; Possani, L.D.; Becerril, B. Evaluation of three different formats of a neutralizing single chain human antibody against toxin Cn2: Neutralization capacity versus thermodynamic stability. Immunol. Lett. 2012, 143, 152–160. [Google Scholar] [CrossRef]
- Mousli, M.; Devaux, C.; Rochat, H.; Goyffon, M.; Billiald, P. A recombinant single-chain antibody fragment that neutralizes toxin II from the venom of the scorpion Androctonus australis hector. FEBS Lett. 1999, 442, 183–188. [Google Scholar]
- Devaux, C.; Moreau, E.; Goyffon, M.; Rochat, H.; Billiald, P. Construction and functional evaluation of a single-chain antibody fragment that neutralizes toxin AahI from the venom of the scorpion Androctonus australis hector. Eur. J. Biochem. FEBS 2001, 268, 694–702. [Google Scholar] [CrossRef]
- Aubrey, N.; Devaux, C.; Sizaret, P.Y.; Rochat, H.; Goyffon, M.; Billiald, P. Design and evaluation of a diabody to improve protection against a potent scorpion neurotoxin. Cell. Mol. Life Sci. 2003, 60, 617–628. [Google Scholar]
- Aubrey, N.; Muzard, J.; Christophe Peter, J.; Rochat, H.; Goyffon, M.; Devaux, C.; Billiald, P. Engineering of a recombinant Fab from a neutralizing IgG directed against scorpion neurotoxin AahI, and functional evaluation versus other antibody fragments. Toxicon Off. J. Int. Soc. Toxinol. 2004, 43, 233–241. [Google Scholar]
- Hmila, I.; Saerens, D.; Ben Abderrazek, R.; Vincke, C.; Abidi, N.; Benlasfar, Z.; Govaert, J.; el Ayeb, M.; Bouhaouala-Zahar, B.; Muyldermans, S. A bispecific nanobody to provide full protection against lethal scorpion envenoming. FASEB J. 2010, 24, 3479–3489. [Google Scholar] [CrossRef]
- Di Tommaso, A.; Juste, M.O.; Martin-Eauclaire, M.-F.; Dimier-Poisson, I.; Billiald, P.; Aubrey, N. Diabody mixture providing full protection against experimental scorpion envenoming with crude Androctonus australis venom. J. Biol. Chem. 2012, 287, 14149–14156. [Google Scholar]
- Muyldermans, S. Single domain camel antibodies: Current status. J. Biotechnol. 2001, 74, 277–302. [Google Scholar]
- Meddeb-Mouelhi, F.; Bouhaouala-Zahar, B.; Benlasfar, Z.; Hammadi, M.; Mejri, T.; Moslah, M.; Karoui, H.; Khorchani, T.; el Ayeb, M. Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon Off. J. Int. Soc. Toxinol. 2003, 42, 785–791. [Google Scholar] [CrossRef]
- Conrath, K.E.; Lauwereys, M.; Galleni, M.; Matagne, A.; Frère, J.M.; Kinne, J.; Wyns, L.; Muyldermans, S. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrob. Agents Chemother. 2001, 45, 2807–2812. [Google Scholar] [CrossRef]
- Hmila, I.; Abdallah, R.B.A.-B.; Saerens, D.; Benlasfar, Z.; Conrath, K.; Ayeb, M.E.; Muyldermans, S.; Bouhaouala-Zahar, B. VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI. Mol. Immunol. 2008, 45, 3847–3856. [Google Scholar] [CrossRef]
- Abderrazek, R.B.; Hmila, I.; Vincke, C.; Benlasfar, Z.; Pellis, M.; Dabbek, H.; Saerens, D.; el Ayeb, M.; Muyldermans, S.; Bouhaouala-Zahar, B. Identification of potent nanobodies to neutralize the most poisonous polypeptide from scorpion venom. Biochem. J. 2009, 424, 263–272. [Google Scholar] [CrossRef]
- Ben Abderrazek, R.; Vincke, C.; Hmila, I.; Saerens, D.; Abidi, N.; el Ayeb, M.; Muyldermans, S.; Bouhaouala-Zahar, B. Development of Cys38 knock-out and humanized version of NbAahII10 nanobody with improved neutralization of AahII scorpion toxin. Protein Eng. Des. Sel. PEDS 2011, 24, 727–735. [Google Scholar] [CrossRef]
- Hmila, I.; Cosyns, B.; Tounsi, H.; Roosens, B.; Caveliers, V.; Abderrazek, R.B.; Boubaker, S.; Muyldermans, S.; el Ayeb, M.; Bouhaouala-Zahar, B.; et al. Pre-clinical studies of toxin-specific nanobodies: Evidence of in vivo efficacy to prevent fatal disturbances provoked by scorpion envenoming. Toxicol. Appl. Pharmacol. 2012, 264, 222–231. [Google Scholar]
- Garcia-Rodriguez, C.; Geren, I.N.; Lou, J.; Conrad, F.; Forsyth, C.; Wen, W.; Chakraborti, S.; Zao, H.; Manzanarez, G.; Smith, T.J.; et al. Neutralizing human monoclonal antibodies binding multiple serotypes of botulinum neurotoxin. Protein Eng. Des. Sel. PEDS 2011, 24, 321–331. [Google Scholar] [CrossRef]
- Skartved, N.J.O.; Jacobsen, H.J.; Pedersen, M.W.; Jensen, P.F.; Sen, J.W.; Jørgensen, T.K.; Hey, A.; Kragh, M. Preclinical pharmacokinetics and safety of Sym004: A synergistic antibody mixture directed against epidermal growth factor receptor. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 5962–5972. [Google Scholar]
- Juárez-González, V.R.; Riaño-Umbarila, L.; Quintero-Hernández, V.; Olamendi-Portugal, T.; Ortiz-León, M.; Ortíz, E.; Possani, L.D.; Becerril, B. Directed evolution, phage display and combination of evolved mutants: A strategy to recover the neutralization properties of the scFv version of BCF2 a neutralizing monoclonal antibody specific to scorpion toxin Cn2. J. Mol. Biol. 2005, 346, 1287–1297. [Google Scholar] [CrossRef]
- Dery, J.-P.; Braden, G.A.; Lincoff, A.M.; Kereiakes, D.J.; Browne, K.; Little, T.; George, B.S.; Sane, D.C.; Cines, D.B.; Effron, M.B.; et al. Final results of the ReoPro readministration registry. Am. J. Cardiol. 2004, 93, 979–984. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Abhinandan, K.R.; Martin, A.C.R. Analyzing the “degree of humanness” of antibody sequences. J. Mol. Biol. 2007, 369, 852–862. [Google Scholar]
- Parker, K.C.; Bednarek, M.A.; Coligan, J.E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. (Baltimore, MD, USA) 1950 1994, 152, 163–175. [Google Scholar]
- Isbister, G.K. Antivenom efficacy or effectiveness: The Australian experience. Toxicology 2010, 268, 148–154. [Google Scholar] [CrossRef]
- Hammoudi-Triki, D.; Ferquel, E.; Robbe-Vincent, A.; Bon, C.; Choumet, V.; Laraba-Djebari, F. Epidemiological data, clinical admission gradation and biological quantification by ELISA of scorpion envenomations in Algeria: Effect of immunotherapy. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 240–250. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarenga, L.M.; Zahid, M.; Tommaso, A.D.; Juste, M.O.; Aubrey, N.; Billiald, P.; Muzard, J. Engineering Venom’s Toxin-Neutralizing Antibody Fragments and Its Therapeutic Potential. Toxins 2014, 6, 2541-2567. https://doi.org/10.3390/toxins6082541
Alvarenga LM, Zahid M, Tommaso AD, Juste MO, Aubrey N, Billiald P, Muzard J. Engineering Venom’s Toxin-Neutralizing Antibody Fragments and Its Therapeutic Potential. Toxins. 2014; 6(8):2541-2567. https://doi.org/10.3390/toxins6082541
Chicago/Turabian StyleAlvarenga, Larissa M., Muhammad Zahid, Anne Di Tommaso, Matthieu O. Juste, Nicolas Aubrey, Philippe Billiald, and Julien Muzard. 2014. "Engineering Venom’s Toxin-Neutralizing Antibody Fragments and Its Therapeutic Potential" Toxins 6, no. 8: 2541-2567. https://doi.org/10.3390/toxins6082541



