The Efficacy of Bamboo Charcoal in Comparison with Smectite to Reduce the Detrimental Effect of Aflatoxin B1 on In Vitro Rumen Fermentation of a Hay-Rich Feed Mixture
Abstract
:1. Introduction
2. Methods
2.1. Binders
| Binder | Density (kg/m3) | Surface area (m2/g) | Pore volume (cm3/g) |
|---|---|---|---|
| Smectite clay | 618 | 115 | 0.296 |
| Bamboo charcoal | 800 | 300 | 0.300 |
2.2. Adsorption Capacity and Adsorption Proportion of Two Binders for the Binding of Aflatoxin B1 (Experiment 1)
2.2.1. Experimental Design
2.2.2. In Vitro Incubation and Sampling Procedure
2.3. Animals and Rumen Fluid Collection
| Items | Value |
|---|---|
| Ingredients (g/kg DM) | |
| Corn silage | 250 |
| Chinese wildrye grass hay | 167 |
| Alfalfa hay | 83 |
| Corn meal | 267 |
| Soybean meal | 138 |
| Wheat bran | 69 |
| Limestone | 11 |
| Calcium phosphate | 6.1 |
| Salt | 4.4 |
| Premix † | 4.5 |
| Nutrients | |
| Net energy for lactation (MJ/kg DM) | 6.69 |
| Crude protein (g/kg DM) | 160 |
| Neutral detergent fiber (g/kg DM) | 382 |
| Acid detergent fiber (g/kg DM) | 225 |
2.4. Effect of BC and SC on In Vitro Rumen Fermentation of a Hay-Rich Feed in the Presence of AFB1 (Experiment 2)
2.4.1. Preparation of a Hay-Rich Feed
2.4.2. Experimental Design
2.4.3. In Vitro Ruminal Batch Cultures
2.4.4. Gas Production and Curve Fitting

2.4.5. Sampling Procedure and Digestibility Determination
2.5. Chemical Analysis and Calculations

2.6. Statistical Analysis
3. Results
3.1. Experiment 1
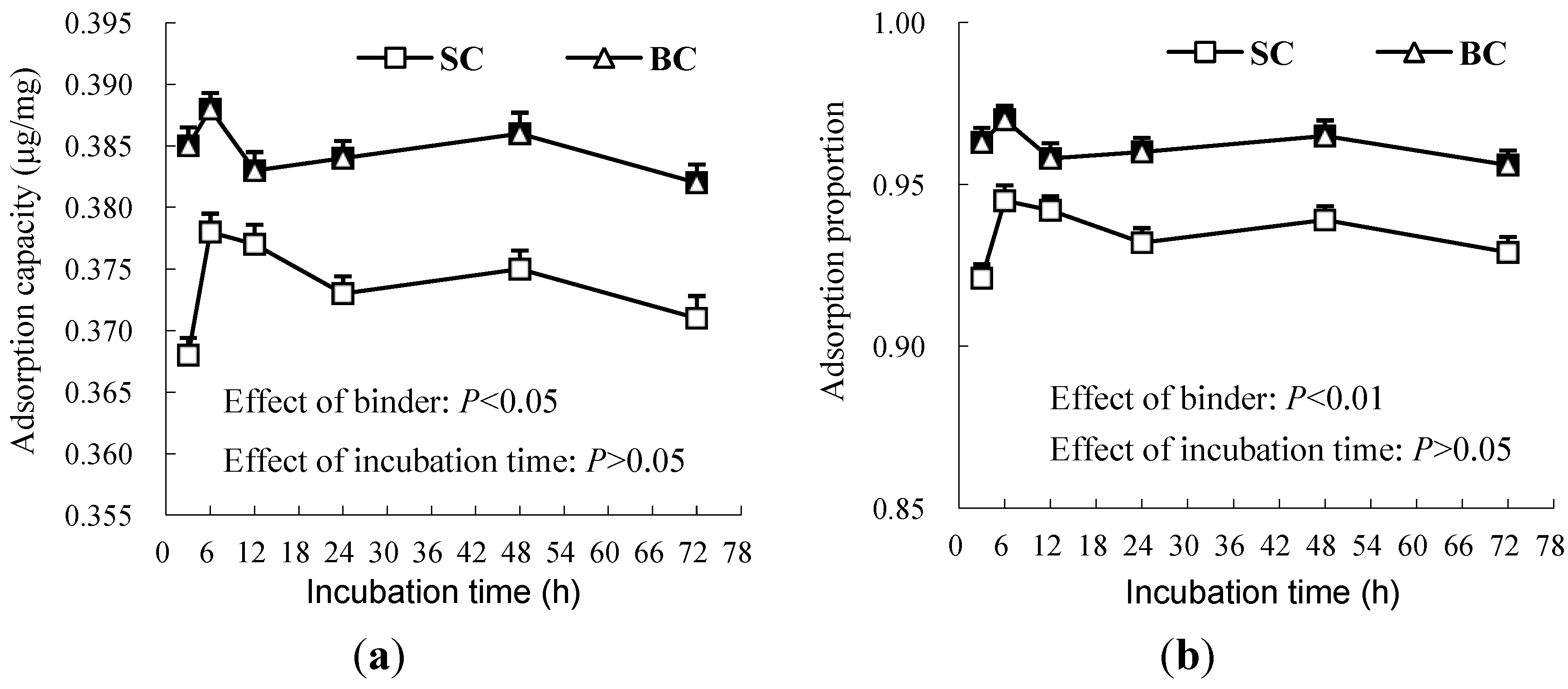
3.2. Experiment 2
| Items | NC * | Smectite clay | SEM ‡ | p-value | Bamboo charcoal | SEM ‡ | p-value | Contrast § | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control † | 0.1 g/L | 1 g/L | 10 g/L | Control † | 0.1 g/L | 1 g/L | 10 g/L | |||||||
| IVDMD (g/kg DM) | 604 | 550 b | 586 a,b | 593 a | 599 a | 11.9 | 0.048 | 550 b | 576 a,b | 589 a | 594 a | 7.8 | <0.0001 | 0.184 |
| GP at 72 h (mL/g DM) | 208.4 | 147.0 b | 151.0 b | 169.1 a,b | 183.2 a | 6.86 | 0.021 | 147.0 b | 148.0 b | 179.3 a | 193.2 a | 6.20 | 0.0014 | 0.366 |
| Fermentation kinetics # | ||||||||||||||
| GPmax (mL/g DM) | 209.1 | 186.7 c | 193.8 c | 240.4 b | 282.9 a | 6.60 | <0.0001 | 186.7 c | 182.5 c | 270.2 b | 315.9 a | 10.77 | <0.0001 | <0.0001 |
| c (/h) | 0.085 | 0.021 a | 0.020 a | 0.020 a | 0.012 b | 0.0010 | 0.0006 | 0.021 a | 0.020 a | 0.015 b | 0.007 c | 0.0009 | <0.0001 | 0.001 |
| Lag time (h) | 0.008 | 0.021 | 0.020 | 0.018 | 0.010 | 0.0007 | 0.0001 | 0.021 | 0.020 | 0.015 | 0.007 | 0.0010 | <0.0001 | 0.458 |
| AGPR (mL/h) | 3.92 | 2.67 | 2.82 | 2.84 | 2.85 | 0.197 | 0.640 | 2.67 | 2.88 | 2.95 | 2.73 | 0.153 | 0.641 | 0.711 |
| Items | NC * | Smectite clay | SEM ‡ | p-value | Bamboo charcoal | SEM ‡ | p-value | Contrast § | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control † | 0.1 g/L | 1 g/L | 10 g/L | Control † | 0.1 g/L | 1 g/L | 10 g/L | |||||||
| AFB1 disappearance (µg/µg) | - | 0.836 c | 0.844 c | 0.901 b | 0.969 a | 0.0073 | <0.0001 | 0.836 b,c | 0.818c | 0.862 b | 0.962 a | 0.0092 | <0.0001 | <0.0001 |
| AFB1 recovery (µg/µg) | - | 0.062 | 0.059 | 0.042 | 0.026 | 0.0112 | 0.186 | 0.062 | 0.068 | 0.055 | 0.043 | 0.0099 | 0.197 | 0.035 |
| Final pH | 6.76 | 6.88 | 6.88 | 6.88 | 6.82 | 0.023 | 0.215 | 6.88 | 6.93 | 6.99 | 6.87 | 0.053 | 0.445 | 0.081 |
| Ammonia N (mM) | 15.7 | 13.6 | 13.6 | 13.1 | 13.2 | 0.47 | 0.816 | 13.6 | 14.1 | 13.7 | 13.2 | 0.31 | 0.332 | 0.257 |
| Total VFA # (mM) | 78.7 | 68.0 | 68.5 | 66.2 | 60.4 | 3.64 | 0.487 | 68.0 b | 81.3 a | 85.8 a | 87.1 a | 2.50 | 0.012 | <0.0001 |
| Acetate (mol/100 mol) | 71.8 | 70.2 a | 68.7 a,b | 68.0 b | 67.2 b | 0.59 | 0.025 | 70.2 | 70.3 | 69.0 | 68.8 | 0.57 | 0.184 | 0.001 |
| Propionate (mol/100 mol) | 18.5 | 20.0 c | 21.7 b | 22.1 a,b | 22.5 a | 0.19 | <0.0001 | 20.0 b | 20.4 b | 21.5 a | 22.1 a | 0.24 | 0.0004 | 0.0004 |
| Butyrate (mol/100 mol) | 4.09 | 4.19 | 4.31 | 4.36 | 4.40 | 0.13 | 0.700 | 4.19 | 4.03 | 4.24 | 3.98 | 0.10 | 0.322 | 0.002 |
| Iso-butyrate (mol/100 mol) | 0.74 | 0.71 | 0.78 | 0.73 | 0.82 | 0.026 | 0.227 | 0.71 | 0.75 | 0.76 | 0.76 | 0.020 | 0.974 | 0.263 |
| Valerate (mol/100 mol) | 1.32 | 1.49 | 1.51 | 1.64 | 1.57 | 0.056 | 0.403 | 1.49 | 1.47 | 1.46 | 1.40 | 0.049 | 0.709 | 0.007 |
| Iso-valerate (mol/100 mol) | 2.96 | 2.97 | 3.06 | 2.99 | 3.07 | 0.010 | 0.916 | 2.97 | 2.97 | 3.02 | 2.92 | 0.074 | 0.817 | 0.286 |
| NGR ζ | 3.97 | 3.76 a | 3.40 b | 3.29 b | 3.22 b | 0.039 | 0.003 | 3.76 a | 3.65 a,b | 3.44 b,c | 3.32 c | 0.055 | 0.006 | 0.001 |
4. Discussion
4.1. AFB1 Adsorption by BC in Comparison with SC
4.2. Disappearance of AFB1 in the Presence of BC in Comparison with SC
4.3. In Vitro Ruminal Fermentation Responses to BC in Comparison with SC
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diekman, M.A.; Green, M.L. Mycotoxins and reproduction in domestic livestock. J. Anim. Sci. 1992, 70, 1615–1627. [Google Scholar]
- Van Egmond, H.P. Aflatoxin M1: Occurrence, toxicity, regulation. In Mycotoxins in Dairy Products; Van Egmond, H.P., Ed.; Elsevier Applied Science: London, UK, 1989; pp. 11–55. [Google Scholar]
- Veldman, A.; Meijst, J.A.C.; Borggreve, G.J.; Heeres-van Tol, J.J. Carry-over of aflatoxin from cow’s food to milk. Anim. Prod. 1992, 55, 163–168. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 172–180. [Google Scholar] [CrossRef]
- Samarajeewa, U.; Sen, A.C.; Cohen, M.D.; Wei, C.I. Detoxification of aflatoxins in foods and feeds by physical and chemical methods. J. Food Prot. 1990, 53, 489–501. [Google Scholar]
- Huwig, A.; Freimund, S.; Kappeli, O.; Dutler, H. Mycotoxin detoxification of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Diaz, D.E.; Hagler, W.M., Jr.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin Binder II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef]
- Kittinaovarat, S.; Suthamnoi, W. Physical properties of polyolefin/bamboo charcoal composites. J. Met. Mater. Miner. 2009, 19, 9–15. [Google Scholar]
- Lemke, S.L.; Ottinger, S.E.; Mayura, K.; Ake, C.L.; Pimpukdee, K.; Wang, N.; Phillips, T.D. Development of a multi-tiered approach to the in vitro prescreening of clay-based enterosorbent. Anim. Feed Sci. Technol. 2001, 93, 17–29. [Google Scholar] [CrossRef]
- Spotti, M.; Fracchiolla, M.L.; Arioli, F.; Caloni, F.; Pompa, G. Aflatoxin B1 binding to sorbents in bovine ruminal fluid. Vet. Res. Commun. 2005, 29, 507–515. [Google Scholar]
- Jiang, Y.H.; Yang, H.J.; Lund, P. Effect of aflatoxin B1 on in vitro ruminal fermentation of ratio high in alfalfa hay or ryegrass hay. Anim. Feed Sci. Technol. 2012, 175, 85–89. [Google Scholar] [CrossRef]
- Ramos, A.J.; Fink-Gremmels, J.; Hernandez, E. Prevention of toxic effects of mycotoxins by means of non-nutritive adsorbent compounds. J. Food Prot. 1996, 59, 631–641. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Vekiru, E.; Fruhauf, S.; Sahin, M.; Ottner, F.; Schatzmayr, G.; Krska, R. Investigation of various adsorbents for their ability to bind Aflatoxin B1. Mycotoxin Res. 2007, 23, 27–33. [Google Scholar] [CrossRef]
- Jaynes, W.F.; Zartman, R.E.; Hudnall, W.H. Aflatoxin B1 adsorption by clays from water and corn meal. Appl. Clay Sci. 2007, 36, 197–205. [Google Scholar]
- Zhang, D.F.; Yang, H.J. In vitro ruminal methanogenesis of a hay-rich substrate in response to different combination supplements of nitrocompounds, pyromellitic diimideand, 2-bromoethanesulphonate. Anim. Feed Sci. Technol. 2011, 163, 20–32. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; López, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds in vivo from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef]
- García-Martínez, R.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Effects of disodium fumarate on in vitro rumen microbial growth, methane production and fermentation of diets differing in their forage: Concentrate ratio. Br. J. Nutr. 2005, 94, 71–77. [Google Scholar] [CrossRef]
- Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1999.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Sung, H.G.; Lee, C.H.; Lee, S.Y.; Kim, S.W.; Cho, K.J.; Ha, J.K. Comparative study on the aflatoxin B1 degradation ability of rumen fluid from Holstein Steers and Korean native goats. J. Vet. Sci. 2009, 10, 29–34. [Google Scholar] [CrossRef]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar]
- rskov, E.R. Manipulation of rumen fermentation for maximum food utilization. World Rev. Nutr. Diet. 1975, 22, 152–182. [Google Scholar]
- Dixon, J.B.; Kannewischer, I.; Arvide, M.G.T.; Velazquez, A.L.B. Aflatoxin sequestration in animal feeds by quality-labeled smectite clays: An introductory plan. Appl. Clay Sci. 2008, 40, 201–208. [Google Scholar] [CrossRef]
- Harvey, R.B.; Phillips, T.D.; Ellis, J.A.; Kubena, L.F.; Huff, W.E.; Petersen, D.V. Effects of aflatoxin M1 residues in milk by addition of hydrated sodium calcium aluminosilicate to aflatoxin-contaminated diets of dairy cows. Am. J. Vet. Res. 1991, 52, 1556–1559. [Google Scholar]
- Ramos, A.J.; Hernandez, E. In vitro aflatoxin adsorption by means of a montmorillonite silicate. A study of adsorption isotherms. Anim. Feed Sci. Technol. 1996, 62, 263–269. [Google Scholar] [CrossRef]
- Dvorak, M. Ability of bentonite and natural zeolite to adsorb aflatoxin from liquid media. Vet. Med. Praha 1989, 34, 733–741. [Google Scholar]
- Arvide, M.G.T.; Mulder, I.; Velazquez, A.L.B.; Dixon, J.B. Smectite clay adsorption of aflatoxin versus octahedral composition, as indicated by FTIR. Clays Clay Miner. 2008, 56, 571–578. [Google Scholar] [CrossRef]
- Jaynes, W.F.; Zartman, R.E. Influence of soluble feed proteins and clay additive charge density on aflatoxin binding in ingested feeds. In Aflatoxins—Biochemistry and Molecular Biology; Guevara-Gonzalez, R.G., Ed.; InTech.: Rijeka, Croatia, 2011; ISBN: 978-953-307-395-8; Available online: http://www.intechopen.com/books/aflatoxins-biochemistryand-molecular-biology/ influence-of-soluble-feedproteins-and-clay-additive-chargedensity-on-aflatoxin-binding-in-ingested (accessed on 5 October 2011).
- Carraro, A.; De Giacomo, A.; Giannossi, M.L.; Medici, L.; Muscarella, M.; Palazzo, L.; Quaranta, V.; Summa, V.; Tateo, F. Clay minerals as adsorbents of aflatoxin M1 from contaminated milk and effects on milk quality. Appl. Clay Sci. 2013, 88–89, 92–99. [Google Scholar]
- Duarte, E.D.; Winston, M.H.J.; Brinton, A.H.; Lon, W.W. Aflatoxin binders I: In vitro binding assay for aflatoxin B1 by several potential sequestering agents. Mycopathologia 2002, 156, 223–226. [Google Scholar]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Fusconi, G.; Galvano, M.; Piva, A.; Piva, G. Reduction of carryover of aflatoxin from cow feed to milk by addition of activated carbons. J. Food Prot. 1996, 59, 551–554. [Google Scholar]
- Kim, B.C.; Kim, Y.H.; Yamamoto, T. Adsorption characteristics of bamboo activated carbon. Korean J. Chem. Eng. 2008, 25, 1140–1144. [Google Scholar] [CrossRef]
- Li, J.J.; Suo, D.C.; Su, X.O. Binding capacity for aflatoxin B1 by different adsorbents. Agric. Sci. Chin. 2010, 9, 449–456. [Google Scholar] [CrossRef]
- Engel, V.G.; Hagemeister, H. Untersuchungenueber denverblieb von aflatoxin B1 im Verdaaundtarkt von Kuehen. In Biological Detoxification of Fungal Toxin and its use in Plant Breeding, Feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Westlake, K.; Mackie, R.I.; Dutton, M.F. In vitro metabolism of mycotoxins by bacterial, protozoal and ovine ruminal fluid preparations. Anim. Feed Sci. Technol. 1989, 25, 169–178. [Google Scholar] [CrossRef]
- Kiessling, K.H.; Pettersson, H.; Sandholm, K.; Olsen, M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984, 47, 1070–1073. [Google Scholar]
- Reynal, S.M.; Ipharraguerre, I.R.; Lin˜eiro, M.; Brito, A.F.; Broderick, G.A.; Clark, J.H. Omasal flow of soluble proteins, peptides and free amino acids in dairy cows fed diets supplemented with proteins of varying ruminal degradabilities. J. Dairy Sci. 2007, 90, 1887–1903. [Google Scholar] [CrossRef]
- Cook, W.O.; Richard, J.L.; Osweiller, G.D.; Trampel, D.W. Clinical and pathologic changes in acute bovine aflatoxicosis: Rumen motility and tissue and fluid concentrations of aflatoxins B1 and M1. Am. J. Vet. Res. 1986, 47, 1817–1825. [Google Scholar]
- Bergman, E.N. Glucose metabolism in ruminants as related to hypoglycemia and ketosis. Am. J. Physiol. 1973, 215, 865–873. [Google Scholar]
- Edrington, T.S.; Harvey, R.B.; Kubena, L.F. Effect of aflatoxin in growing lambs fed ruminally degradable or escape protein sources. J. Anim. Sci. 1994, 72, 1274–1281. [Google Scholar]
- Helferich, W.G.; Garrett, W.N.; Hsieh, D.P.H.; Baldwin, R.L. Feedlot performance and tissue residues of cattle consuming diets containing aflatoxins. J. Anim. Sci. 1986, 62, 691–696. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, Y.-H.; Wang, P.; Yang, H.-J.; Chen, Y. The Efficacy of Bamboo Charcoal in Comparison with Smectite to Reduce the Detrimental Effect of Aflatoxin B1 on In Vitro Rumen Fermentation of a Hay-Rich Feed Mixture. Toxins 2014, 6, 2008-2023. https://doi.org/10.3390/toxins6072008
Jiang Y-H, Wang P, Yang H-J, Chen Y. The Efficacy of Bamboo Charcoal in Comparison with Smectite to Reduce the Detrimental Effect of Aflatoxin B1 on In Vitro Rumen Fermentation of a Hay-Rich Feed Mixture. Toxins. 2014; 6(7):2008-2023. https://doi.org/10.3390/toxins6072008
Chicago/Turabian StyleJiang, Ya-Hui, Ping Wang, Hong-Jian Yang, and Ying Chen. 2014. "The Efficacy of Bamboo Charcoal in Comparison with Smectite to Reduce the Detrimental Effect of Aflatoxin B1 on In Vitro Rumen Fermentation of a Hay-Rich Feed Mixture" Toxins 6, no. 7: 2008-2023. https://doi.org/10.3390/toxins6072008





