Indole Alkaloids from Fischerella Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cyanobacterial Material
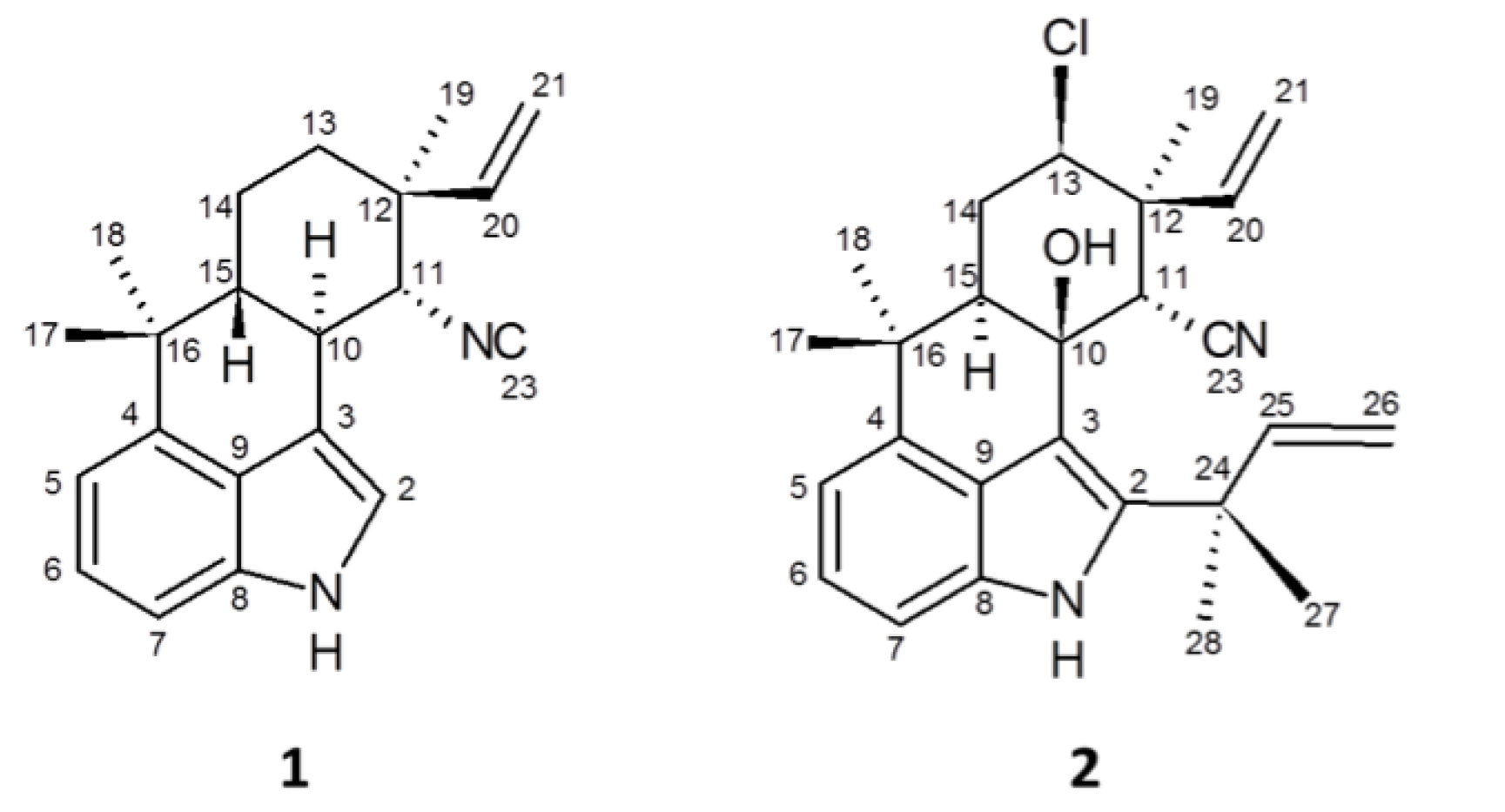
2.2. Teratogenicity and Identification of Indole Alkaloids from Fischerella 52-1
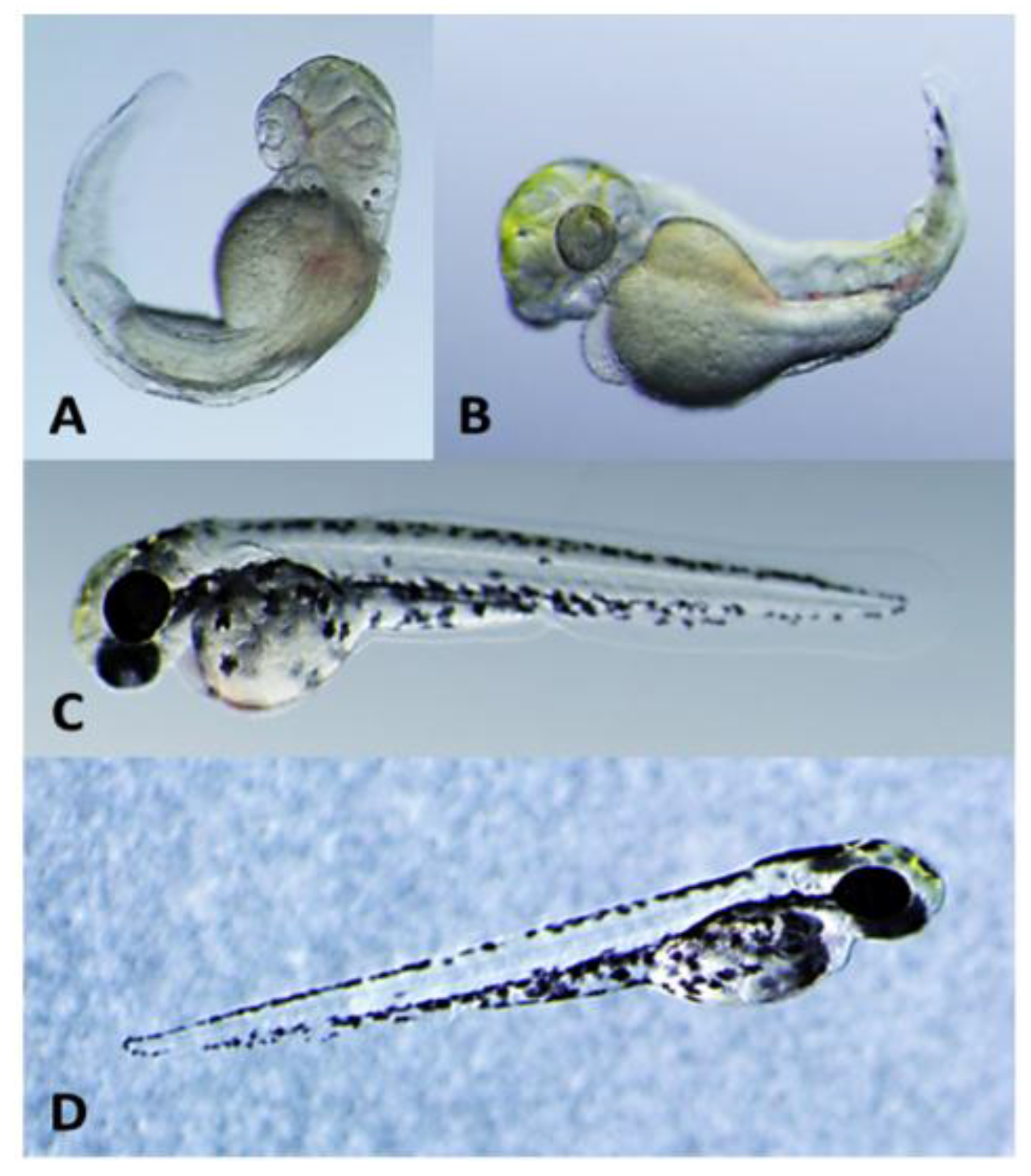
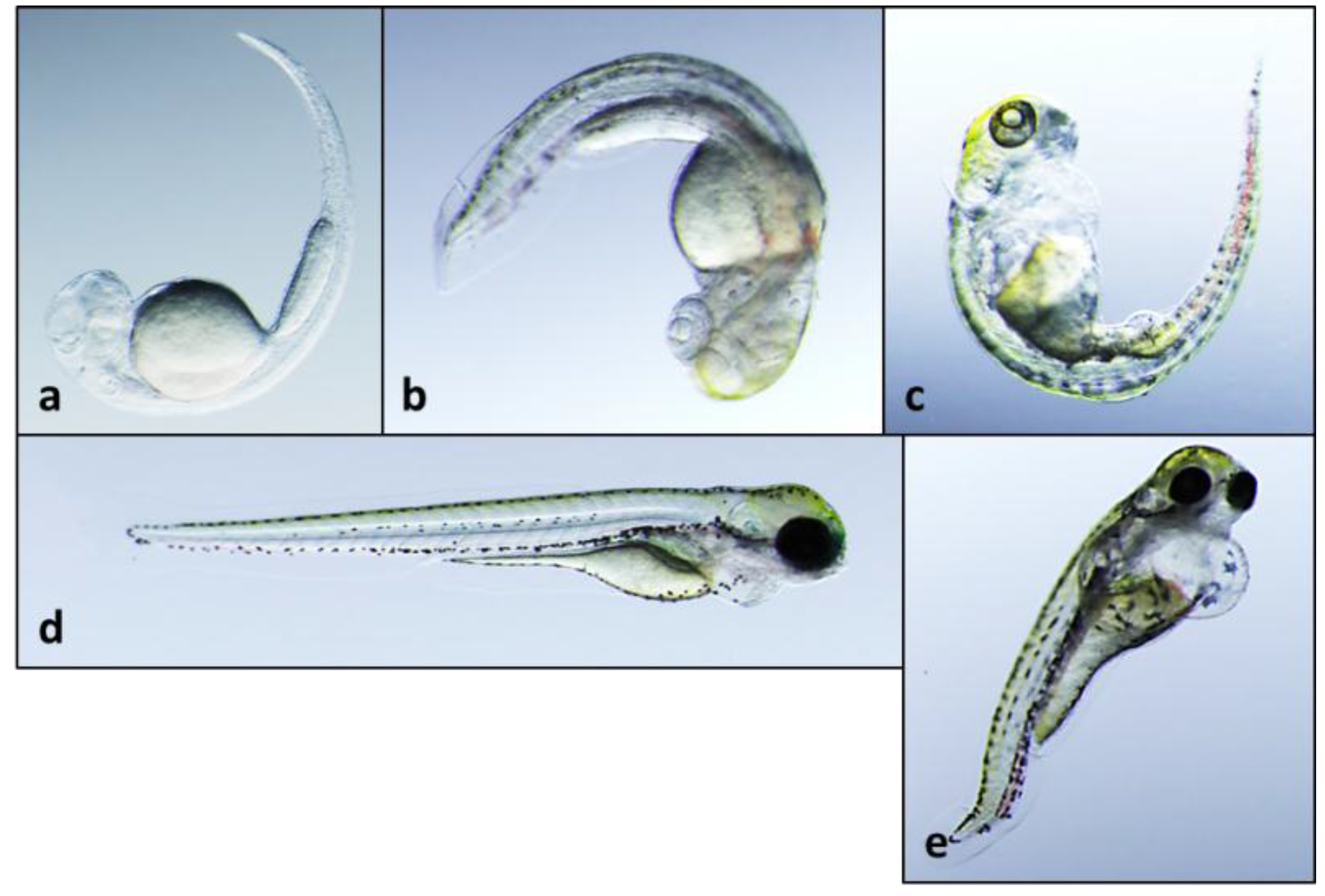
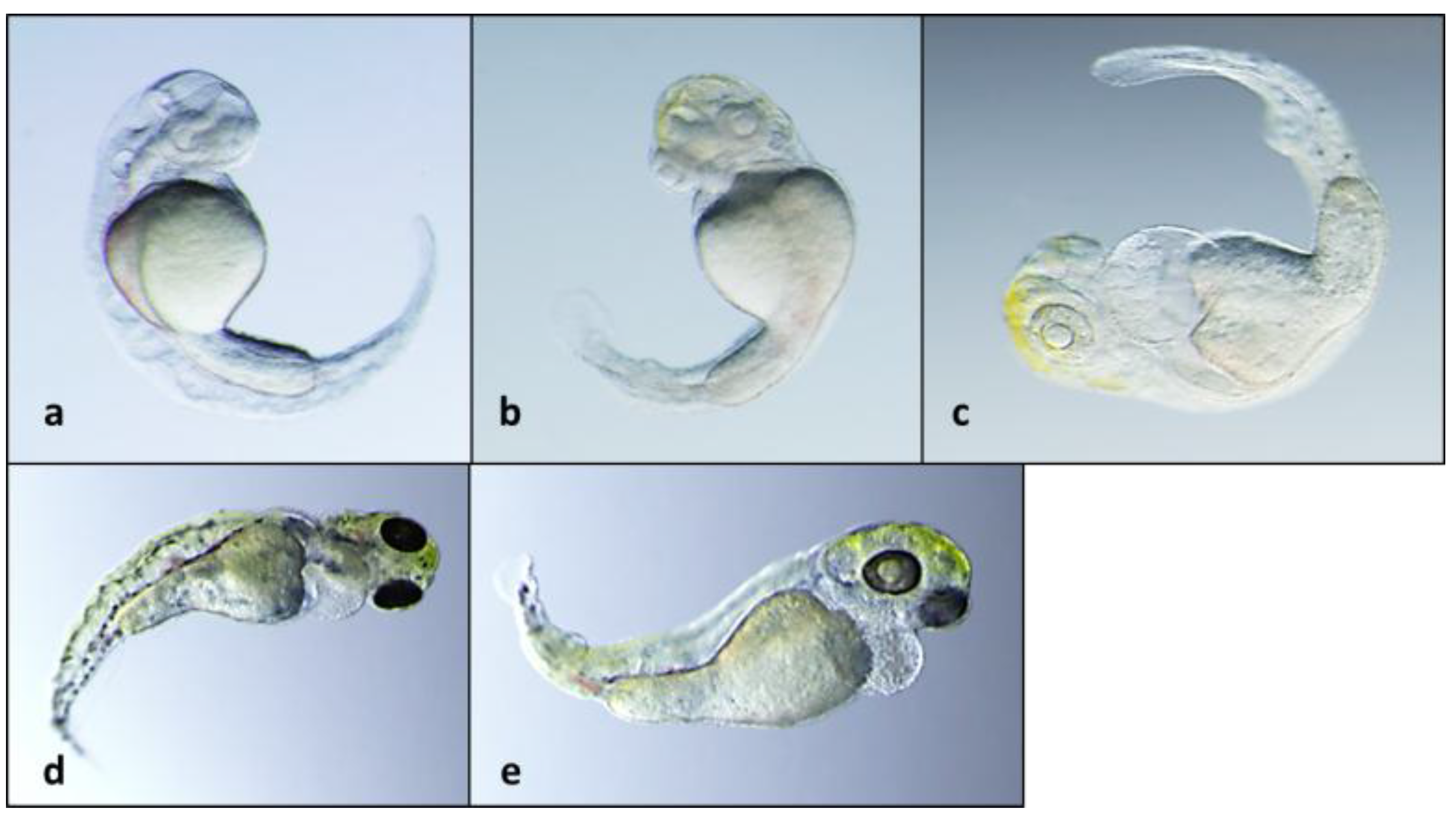
2.3. 12-Epi-Hapalindole H Isonitrile
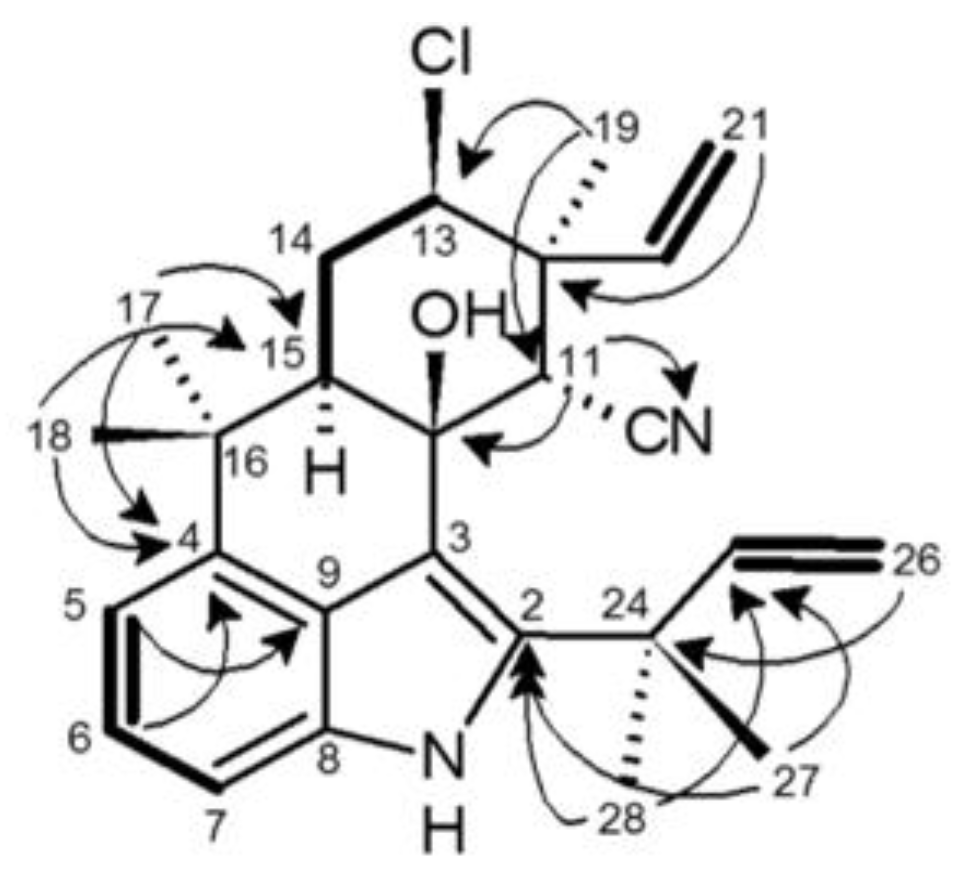
2.4. 12-Epi-Ambiguine B Nitrile
| Position | δC, type | δH (J in Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 7.32, s | 9 | |||
| 2 | 139.5 C | 27, 28 | |||
| 3 | 113.4 C | ||||
| 4 | 140.9 C | 6, 17, 18 | |||
| 5 | 115.6 CH | 7.10, dd (0.5, 7.3) | 6 | 6, 7 | 17 |
| 6 | 123.9 CH | 7.27, dd (7.3, 8) | 5, 7 | 4, 5 | 17 |
| 7 | 108.7 CH | 6.96, dd (0.5, 8) | 6 | 5, 9 | |
| 8 | 132.9 C | ||||
| 9 | 126.3 C | 7 | |||
| 10 | 73.9 C | 1.54, OH | 11 | 11,18, 27, 21E | |
| 11eq | 54.4 CH | 4.13, s | 10, 12, 13, 15, 19, 20, 23 | 27, 28 | |
| 12 | 44.9 C | 11, 19, 21 | |||
| 13ax | 67.4 CH | 4.16, dd (4.4, 12.6) | 14ax, 14eq | 11, 19 | 15 |
| 14ax | 30.5 CH2 | 2.65, q (12.5) | 13, 14eq, 15ax | 18, 20 | |
| 14eq | 30.5 CH2 | 2.25, ddd (2.2, 4.3) | 13, 14ax, 15ax | 15, 17 | |
| 15ax | 52 CH | 2.45, dd (2, 12.6) | 13, 14ax, 14eq | 11, 17, 18 | 13, 17 |
| 16 | 37.6 C | 17, 18 | |||
| 17 | 27.2 CH3 | 1.28, s | 4, 15, 16 | 5,6 | |
| 18 | 27.7 CH3 | 1.18, s | 4, 15, 16 | ||
| 19 | 27.6 CH3 | 1.58, s | 11, 12, 13, 20 | 11, 13, 21E | |
| 20 | 142.7 CH | 6.78, dd (11, 17.6) | 21E, 21Z | 11, 19 | 14ax |
| 21E | 114.5 CH2 | 5.05, dd (1, 17.6) | 20, 21Z | 12 | 19 |
| 21Z | 114.5 CH2 | 5.09, dd (1, 11) | 20, 21E | 12 | |
| 23 | 120.1 C | 11 | |||
| 24 | 39.5 C | 26, 27, 28 | |||
| 25 | 148 CH | 6.05, dd (10.5, 17.6) | 26E, 26Z | 27, 28 | |
| 26E | 113.3 CH2 | 5.00, dd (1, 17.6) | 25, 26Z | 24 | 27, 28 |
| 26Z | 113.3 CH2 | 4.95, dd (1, 10.5) | 25, 26E | 24 | |
| 27 | 29.4 CH3 | 1.05, s | 2, 24, 25 | 11 | |
| 28 | 28.1 CH3 | 1.26, s | 2, 24, 25 | 11 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Cyanobacterial Material
3.3. Extraction and Isolation
3.4. Zebrafish Embryo Toxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar]
- Holland, A.; Kinnear, S. Interpreting the possible ecological roles of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar]
- Berry, J.P.; Gantar, M.; Gawley, R.E.; Wang, M.; Rein, K.S. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from freshwater species of Lyngbya isolated from the Florida Everglades. Compt. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 231–238. [Google Scholar]
- Berry, J.P.; Gantar, M.; Gibbs, P.D.L.; Schmale, M.C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Compt. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 61–72. [Google Scholar]
- Berry, J.P.; Gibbs, P.D.L.; Schmale, M.C.; Saker, M.L. Toxicity of cylindrospermopsin, and other apparent metabolites from Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum, to the zebrafish (Danio rerio) embryo. Toxicon 2009, 53, 289–299. [Google Scholar]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar]
- Crawford, A.D.; Esguerra, C.V.; de Witte, P. Fishing for drugs from nature: zebrafish as a technology platform for natural product discovery. Planta Med. 2008, 74, 624–632. [Google Scholar]
- Oberemm, A.; Fastner, J.; Steinberg, C. Effects of MC-LR and cyanobacterial crude extracts on embryo-larval development of zebrafish. Water Res. 1997, 31, 2918–2921. [Google Scholar]
- Oberemm, A.; Becker, J.; Codd, G.A.; Steinberg, C. Effects of cyanobacterial toxins and aqueous crude extracts of cyanobacteria on the development of fish and amphibians. Environ. Toxicol. 1999, 14, 77–88. [Google Scholar]
- El Ghazali, I.; Saqrane, S.; Carvalho, A.P.; Ouahid, Y.; Oudra, B.; Del Campo, F.F.; Vasconcelos, V. Compensatory growth induced in zebrafish larvae after pre-exposure to a Microcystis aeruginosa natural bloom extract containing microcystins. Int. J. Mol. Sci. 2009, 10, 133–146. [Google Scholar]
- Jaja-Chimedza, A.; Gantar, M.; Mayer, G.; Gibbs, P.D.; Berry, J.P. Effects of cyanobacterial lipopolysaccharides from Microcystis on glutathione-based detoxification pathways in the zebrafish (Danio rerio) embryo. Toxins 2012, 4, 390–404. [Google Scholar]
- Rogers, E.D.; Henry, T.B.; Twiner, M.J.; Gouffon, J.S.; McPherson, J.T.; Boyer, G.L.; Sayler, G.S.; Wilhelm, S.W. Global gene expression profiling in larval zebrafish exposed to microcystin-LR and Microcystis reveals endocrine disrupting effects of cyanobacteria. Environ. Sci. Technol. 2011, 45, 1962–1969. [Google Scholar]
- Acs, A.; Kovacs, A.W.; Csepregi, J.Z.; Törö, N.; Kiss, G.; Gyori, J.; Vehoszky, A.; Kovats, N.; Farkas, A. The ecotoxicological evaluation of Cylindrospermopsis raciborskii from Lake Balaton employing a battery of bioassays and chemical screening. Toxicon 2013, 70, 98–106. [Google Scholar]
- Jonas, A.; Buranova, V.; Scholz, S.; Fetter, E.; Novakova, K.; Kohoutek, J.; Hilscherova, K. Retinoid-like activity and teratogenic effects of cyanobacterial exudates. Aquat. Toxicol. 2014, 155, 283–290. [Google Scholar]
- Papendorf, O.; König, G.M.; Wright, A.D.; Chorus, I.; Oberemm, A. Muegellone, a novel inhibitor of fish development from the fresh water cyanobacterium Aphizomenon flos-aquae. J. Nat. Prod. 1997, 60, 1298–1300. [Google Scholar]
- Wright, A.D.; Papendorf, O.; König, G.M.; Oberemm, A. Effects of cyanobacterium Fischerella ambigua isolates and cell free culture media on zebrafish (Danio rerio) embryo development. Chemosphere 2006, 65, 604–608. [Google Scholar]
- Jaja-Chimedza, A.; Gantar, M.; Gibbs, P.D.; Schmale, M.C.; Berry, J.P. Polymethoxy-1-alkenes form Aphanizomenon ovalisporum inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Mar. Drugs 2012, 10, 2322–2336. [Google Scholar]
- Kastovsky, J.; Johansen, J.R. Mastigocladus laminosus (Stigonematales, Cyanobacteria): Phylogenetic relationship of strains from thermal springs to soil-inhabiting genera of the order and taxonomic implications for the genus. Phycologia 2008, 47, 307–320. [Google Scholar]
- Moore, R.E.; Cheuk, C.; Yang, X.Q.G.; Patterson, G.M.L. Hapalindoles, antibacterial and antimycotic alkaloids form the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987, 52, 1036–1043. [Google Scholar]
- Moore, R.E.; Yang, X.Q.G.; Patterson, G.M.L. Fontonamide and anhydrohapaloxidinole A, two new alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Org. Chem. 1987, 52, 3773–3777. [Google Scholar]
- Falch, B.S.; König, G.M.; Wright, A.D.; Rüegger, H.; Sticher, O.; Bernardinelli, G. Ambigol A and B: New biologically active polychlorinated aromatic compounds from the terrestrial blue green alga Fischerella ambigua. J. Org. Chem. 1993, 58, 6570–6575. [Google Scholar]
- Stratmann, K.; Burgoyne, D.L.; Moore, R.E.; Patterson, G.M.L.; Smith, C.D. Hapalosin, a cyanobacterial cyclic depsipeptide with multidrug-resistance reversing activity. J. Org. Chem. 1994, 59, 7219–7226. [Google Scholar]
- Stratmann, K.; Moore, R.; Bonjouklian, R.; Deeter, J.; Patterson, G.; Shaffer, S.; Smith, C.; Smitka, T. Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricate. Relationship to fischerindoles and hapalindoles. J. Am. Chem. Soc. 1994, 116, 9935–9942. [Google Scholar]
- Richter, J.M.; Ishihara, Y.; Masuda, T.; Whitefield, B.W.; Llamas, T.; Pohjakallio, A.; Baran, P.S. Enantiospecific total synthesis of the hapalindoles, fischerindoles, and welwitindolinones via a redox economic approach. J. Am. Chem. Soc. 2008, 130, 17938–17954. [Google Scholar]
- Gantar, M.; Berry, J.; Thomas, S.; Wang, M.; Perez, R.; Rein, K. Allelopathic activity among cyanobacteria and microalgae isolated from Florida freshwater habitats. FEMS Microbiol. Ecol. 2008, 64, 55–64. [Google Scholar]
- Raveh, A.; Carmeli, S. Antimicrobial ambiguines from the cyanobacterium Fischerella sp. collected in Israel. J. Nat. Prod. 2007, 70, 196–201. [Google Scholar]
- Klein, D.; Daloze, D.; Braekman, J.C.; Hoffmann, L.; Demoulin, V. New hapalindoles from the cyanophyte Hapalosiphon laingii. J. Nat. Prod. 1995, 58, 1781–1785. [Google Scholar]
- Bonjouklian, R.; Spangle, L.A.; Moore, R.E. Facile intramolecular photoaddition and oxidation dimerization of hapalindole E, a naturally occurring isonitrile-containing indole. J. Org. Chem. 1989, 54, 719–721. [Google Scholar]
- Smitka, T.; Bonjouklian, R.; Doolin, L.; Jones, N.; Deeter, J.; Yoshida, W.; Prinsep, M.; Moore, R.; Patterson, G. Ambiguine isonitriles, fungicidal hapalindole-type alkaloids form the three genera of blue-green algae belonging to the Stigonemataceae. J. Org. Chem. 1992, 57, 857–861. [Google Scholar]
- Park, A.; Moore, R.E.; Patterson, G.M.L. Fischerindole L, a new isonitrile from the terrestrial blue-green alga Fischerella muscicola. Tetrahedron Lett. 1992, 33, 3257–3260. [Google Scholar]
- Huber, U.; Moore, R.E.; Patterson, G.M.L. Isolation of a nitrile-containing indole alkaloid from the terrestrial blue-green alga Hapalosiphon delicatulus. J. Nat. Prod. 1998, 61, 1304–1306. [Google Scholar]
- Mo, S.; Krunic, A.; Santarsiero, B.D.; Franzblau, S.G.; Orjala, J. Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116–2123. [Google Scholar]
- Kim, H.; Krunic, A.; Lantvit, D.; Shen, Q.; Kroll, D.J.; Swanson, S.M.; Orjala, J. Nitrile-containing fischerindoles from the cultured cyanobacterium Fischerella sp. Tetrahedron 2012, 68, 3205–3209. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Modern approach to the classification system of cyanophytes, 5-Stigonematales. Arch. Hydrobiol. Algol. Studies 1986, 43, 157–226. [Google Scholar]
- Brand, M.; Granato, M.; Nüsslein-Volhard, C. Keeping and Raising Zebrafish. In Zebrafish; Nüsslein-Volhard, C., Dahm, R., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 7–37. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walton, K.; Gantar, M.; Gibbs, P.D.L.; Schmale, M.C.; Berry, J.P. Indole Alkaloids from Fischerella Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model. Toxins 2014, 6, 3568-3581. https://doi.org/10.3390/toxins6123568
Walton K, Gantar M, Gibbs PDL, Schmale MC, Berry JP. Indole Alkaloids from Fischerella Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model. Toxins. 2014; 6(12):3568-3581. https://doi.org/10.3390/toxins6123568
Chicago/Turabian StyleWalton, Katherine, Miroslav Gantar, Patrick D. L. Gibbs, Michael C. Schmale, and John P. Berry. 2014. "Indole Alkaloids from Fischerella Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model" Toxins 6, no. 12: 3568-3581. https://doi.org/10.3390/toxins6123568
APA StyleWalton, K., Gantar, M., Gibbs, P. D. L., Schmale, M. C., & Berry, J. P. (2014). Indole Alkaloids from Fischerella Inhibit Vertebrate Development in the Zebrafish (Danio rerio) Embryo Model. Toxins, 6(12), 3568-3581. https://doi.org/10.3390/toxins6123568






