A Novel Bradykinin-Related Dodecapeptide (RVALPPGFTPLR) from the Skin Secretion of the Fujian Large-Headed Frog (Limnonectes fujianensis) Exhibiting Unusual Structural and Functional Features
Abstract
:1. Introduction
2. Results
2.1. “Shotgun” Cloning of a cDNA Encoding the Biosynthetic Precursor of a Novel BRP
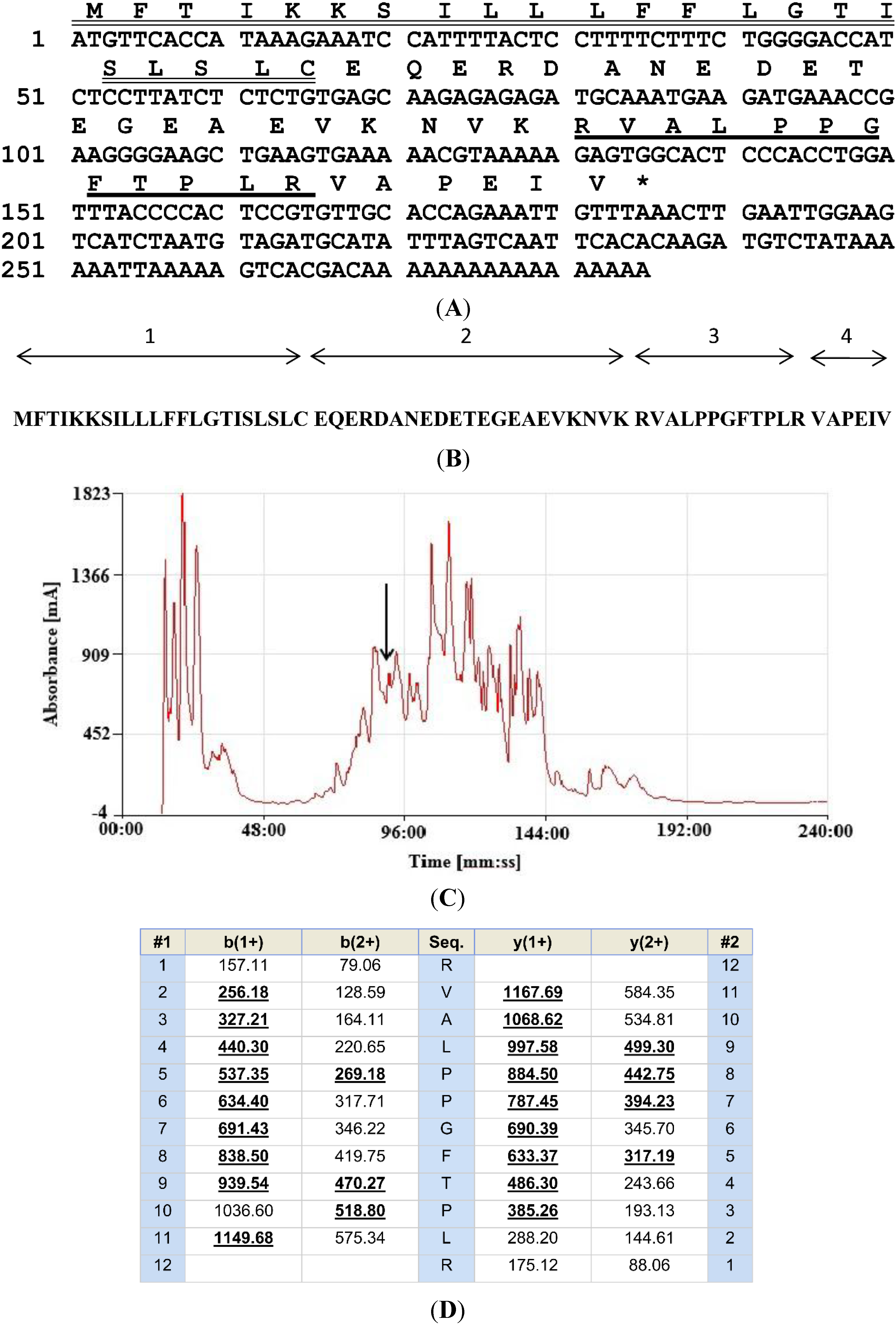
2.2. Isolation and Structural Characterisation of the Novel BRP
2.3. Bioinformatic Analyses of the Novel BRP, RVAL-(L1, T6, L8)-BK

2.4. Rat Arterial Smooth Muscle Pharmacology of Synthetic RVAL-(L1, T6, L8)-BK

3. Discussion
4. Experimental Section
4.1. Acquisition of Frogs and Harvesting of Skin Secretions
4.2. “Shotgun” Cloning of a L. Fujianensis Skin Secretion-Derived cDNA Library
4.3. Identification and Structural Analysis of the Novel BRP Deduced from Translation of the Open-Reading Frame of Cloned cDNA
4.4. Solid-Phase Peptide Synthesis of the Novel BRP
4.5. Pharmacological Evaluation of the Synthetic Novel BRP Using Rat Arterial Smooth Muscle
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Erspamer, V. Bioactive secretions of the amphibian integument. Amphib. Biol. 1994, 1, 179–350. [Google Scholar]
- Conlon, J.M. Bradykinin and its receptors in non-mammalian vertebrates. Regul. Pept. 1999, 79, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, A.; Erspamer, V.; Bertaccini, G. Occurence of bradykinin in the skin of Rana temporaria. Comp. Biochem. Physiol. 1965, 14, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Orr, D.F.; Bjourson, A.J.; McClean, S.; O’Rourke, M.; Hirst, D.G.; Rao, P.; Shaw, C. Novel bradykinins and their precursor cDNAs from European yellow-bellied toad (Bombina variegata) skin. Eur. J Biochem. 2002, 269, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; O’Rourke, M.; Orr, D.F.; Coulter, D.J.M.; Hirst, D.G.; Rao, P.; Shaw, C. Kinestatin: A novel bradykinin B2 receptor antagonist peptide from the skin secretion of the Chinese toad. Bombina maxima. Regul. Pept. 2003, 116, 139–146. [Google Scholar] [CrossRef]
- Conceição, K.; Konno, K.; de Melo, R.L.; Antoniazzi, M.M.; Jared, C.; Sciani, J.M.; Conceição, M.; Prezoto, B.C.; de Camargo, A.C.; Pimenta, D.C. Isolation and characterization of a novel bradykinin potentiating peptide (BPP) from the skin secretion of Phyllomedusa hypochondrialis. Peptides 2007, 28, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Yano, K. Kallikrein generates angiotensin II but not bradykinin in the plasma of the urodele Amphiuma tridactylum. Comp. Biochem. Physiol. C 1995, 110, 305–311. [Google Scholar] [PubMed]
- Sin, Y.; Zhou, M.; Chen, W.; Wang, L.; Chen, T.B.; Walker, B.; Shaw, C. Skin bradykinin related peptides (BRPs) and their biosynthetic precursors (kininogens): Comparisons between various taxa of Chinese and North American ranid frogs. Peptides 2013, 29, 393–403. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Zhou, M.; Chen, T.; Ding, A.; Rao, P.; Walker, B.; Shaw, C. Amolopkinins W1 and W2—Novel bradykinin-related peptides (BRPs) from the skin of the Chinese torrent frog, Amolops wuyiensis: Antagonists of bradykinin-induced smooth muscle contraction of the rat ileum. Peptides 2009, 30, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, L.J.; Huang, H.; Ma, F.; Li, Q. Phylogenetic analysis of vertebrate kininogen genes. Genomics 2008, 91, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.; Camargo, A.C. The bradykinin-potentiating peptides from venom gland and brain of Bothrops jararaca contain highly site specific inhibitors of the somatic angiotensin-converting enzyme. Toxicon 2005, 45, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Regoli, D.; Barabe, J. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 1980, 32, 1–46. [Google Scholar] [PubMed]
- Zhang, R.; Zhou, M.; Wang, L.; McGrath, S.; Chen, T.; Chen, X.; Shaw, C. Phylloseptin-1 (PSN-1) from Phyllomedusa sauvagei skin secretion: A novel broad-spectrum antimicrobial peptide with antibiofilm activity. Mol. Immunol. 2010, 47, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Li, R.; Jiang, Y.; Lin, Y.; Wu, Y.; Zhou, M.; Xu, J.; Wang, L.; Chen, T.; Shaw, C. Medusins: A new class of antimicrobial peptides from the skin secretions of phyllomedusine frogs. Biochimie 2013, 95, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Ge, L.; Wang, L.; Guo, X.; Zhang, H.; Li, Y.; Zhou, Y.; Zhou, M.; Chen, T.; Shaw, C. Ornithokinin (avian bradykinin) from the skin of the Chinese bamboo odorous frog, Odorrana versabilis. J. Pept. Sci. 2014. [Google Scholar] [CrossRef]
- Boles, W.E. The world’s oldest songbird (Aves: Passeriformes). Nature 1995, 374, 21–22. [Google Scholar] [CrossRef]
- Barker, F.K.; Barrowclough, G.F.; Groth, J.G. A phylogenetic hypothesis for passerine birds: Taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Pro. Biol. Sci. 2002, 269, 295–308. [Google Scholar] [CrossRef]
- Eng, J.; Huang, C.G.; Pan, Y.C.E.; Hulmes, J.D.; Yalow, R.S. Guinea pig pancreatic polypeptide: Structure and pancreatic content. Peptides 1987, 8, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Sueyoshi, T.; Takada, K.; Tanaka, K.; Morita, T.; Iwanaga, S. Isolation and characterization of ornitho-kininogen. Eur. J. Biochem. 1987, 168, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Marks, N.J.; Shaw, C.; Halton, D.W.; Maule, A.G.; Curry, W.J.; Verhaert, P.; Thim, L. Isolation and primary structure of a novel avian pancreatic polypeptide from five species of Eurasian crow. Regul. Pept. 1993, 47, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Marks, N.J.; Shaw, C.; Halton, D.W.; Thim, L. The corrected primary structure of chicken (avian) pancreatic polypeptide. Regul. Pept. 1994, 52, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, N.; Zhang, Y.S. Isolation and sequence determination of goose pancreatic polypeptide. Sci. Sin., Ser. B Chem. Biol. Agric. Med. Earth Sci. 1984, 27, 590–592. [Google Scholar]
- Lithauer, D.; Oelofsen, W. Purification and primary structure of ostrich pancreatic polypeptide. Int. J. Pept. Protein Res. 1987, 29, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.L.; Shaw, C.; Halton, D.W.; Thim, L. Isolation and structural characterisation of herring gull (Larus argentatus) pancreatic polypeptide. Gen. Comp. Endocrinol. 1994, 93, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.J.; Stone, D.J.M.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Meth. 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Li, L.; Bjourson, A.J.; He, J.; Cai, G.; Rao, P.; Shaw, C. Bradykinins and their cDNA from piebald odorous frog, Odorrana schmackeri, skin. Peptides 2003, 24, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Wang, H.; Chen, H.; Zhou, M.; Chen, T.; Shaw, C. A fish bradykinin (Arg0, Trp5, Leu8-bradykinin) from the defensive skin secretion of the European edible frog; Pelophylax kl. esculentus: Structural characterization; molecular cloning of skin kininogen cDNA and pharmacological effects on mammalian smooth muscle. Peptides 2011, 32, 26–30. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Luo, Y.; Du, Q.; Wang, L.; Zhou, M.; Ma, J.; Li, R.; Chen, T.; Shaw, C. A Novel Bradykinin-Related Dodecapeptide (RVALPPGFTPLR) from the Skin Secretion of the Fujian Large-Headed Frog (Limnonectes fujianensis) Exhibiting Unusual Structural and Functional Features. Toxins 2014, 6, 2886-2898. https://doi.org/10.3390/toxins6102886
Shi D, Luo Y, Du Q, Wang L, Zhou M, Ma J, Li R, Chen T, Shaw C. A Novel Bradykinin-Related Dodecapeptide (RVALPPGFTPLR) from the Skin Secretion of the Fujian Large-Headed Frog (Limnonectes fujianensis) Exhibiting Unusual Structural and Functional Features. Toxins. 2014; 6(10):2886-2898. https://doi.org/10.3390/toxins6102886
Chicago/Turabian StyleShi, Daning, Yu Luo, Qiang Du, Lei Wang, Mei Zhou, Jie Ma, Renjie Li, Tianbao Chen, and Chris Shaw. 2014. "A Novel Bradykinin-Related Dodecapeptide (RVALPPGFTPLR) from the Skin Secretion of the Fujian Large-Headed Frog (Limnonectes fujianensis) Exhibiting Unusual Structural and Functional Features" Toxins 6, no. 10: 2886-2898. https://doi.org/10.3390/toxins6102886
APA StyleShi, D., Luo, Y., Du, Q., Wang, L., Zhou, M., Ma, J., Li, R., Chen, T., & Shaw, C. (2014). A Novel Bradykinin-Related Dodecapeptide (RVALPPGFTPLR) from the Skin Secretion of the Fujian Large-Headed Frog (Limnonectes fujianensis) Exhibiting Unusual Structural and Functional Features. Toxins, 6(10), 2886-2898. https://doi.org/10.3390/toxins6102886





