Insights into Diphthamide, Key Diphtheria Toxin Effector
Abstract
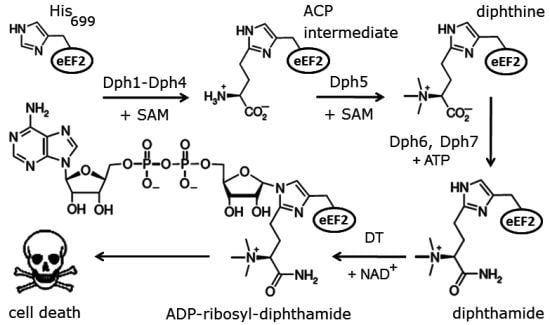
:1. Introduction
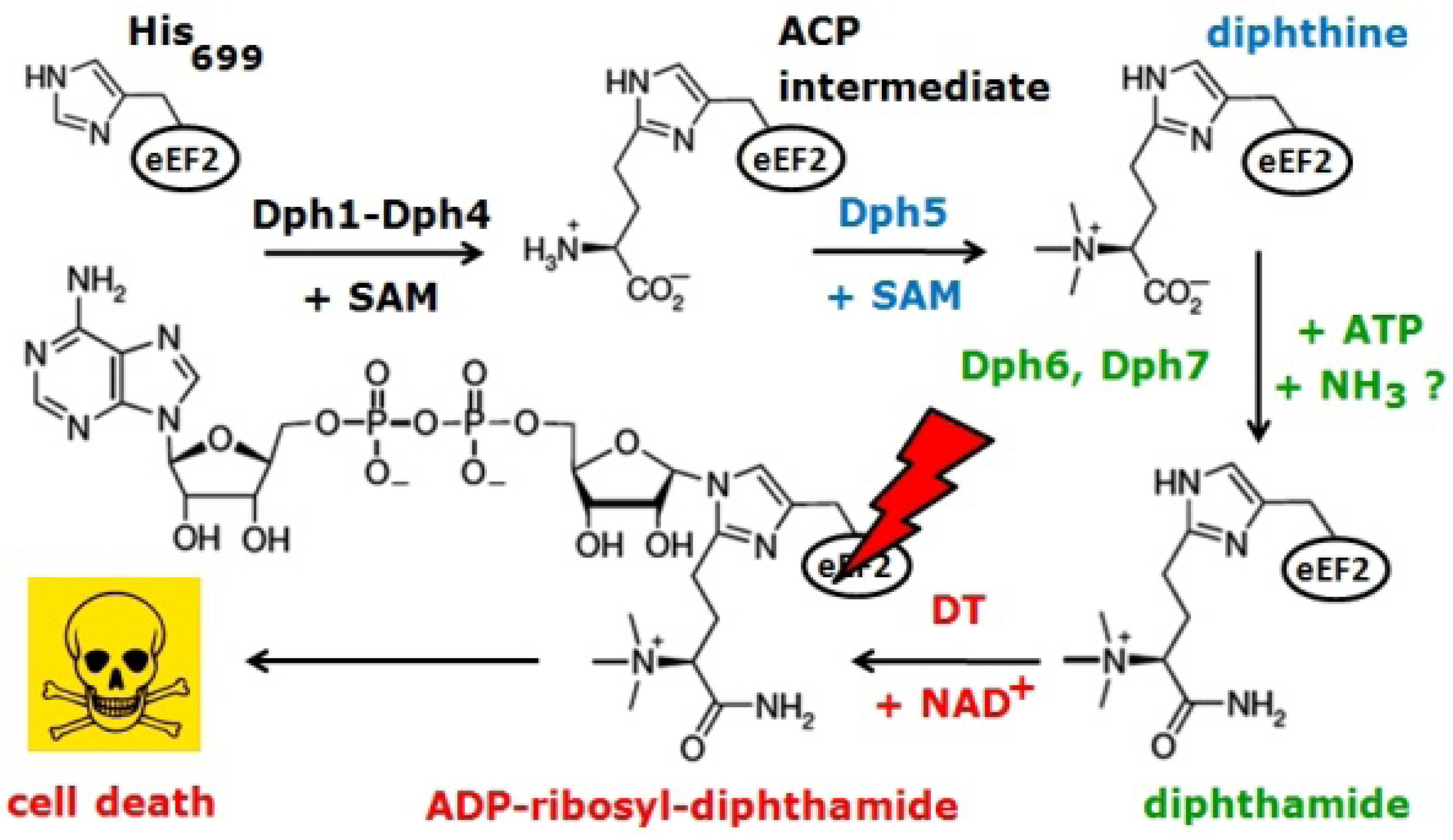
2. Results and Discussion
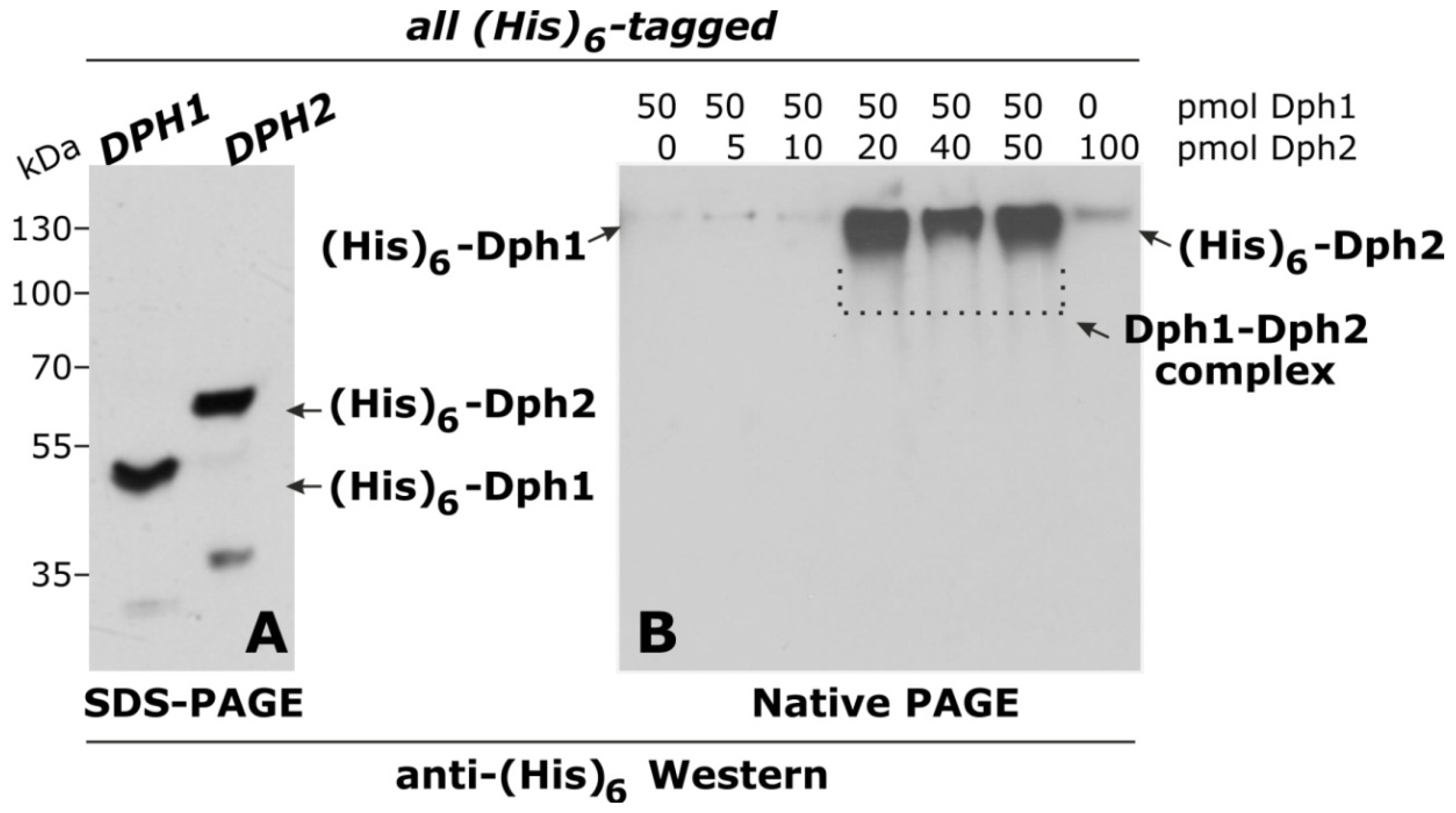
2.1. Dph1 Protein-Protein Interactions

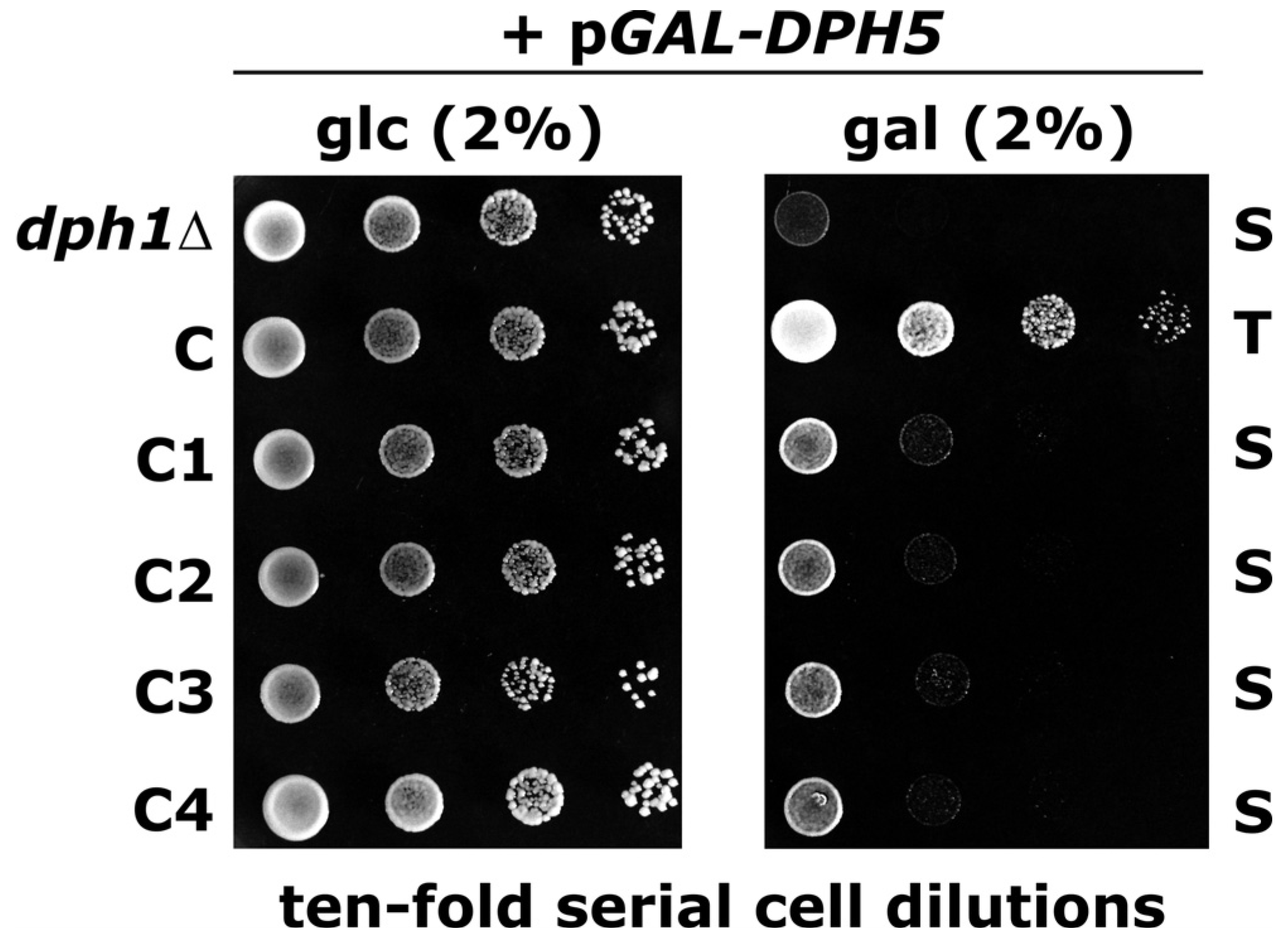
2.2. DPH5 Overexpression Toxicity Effects
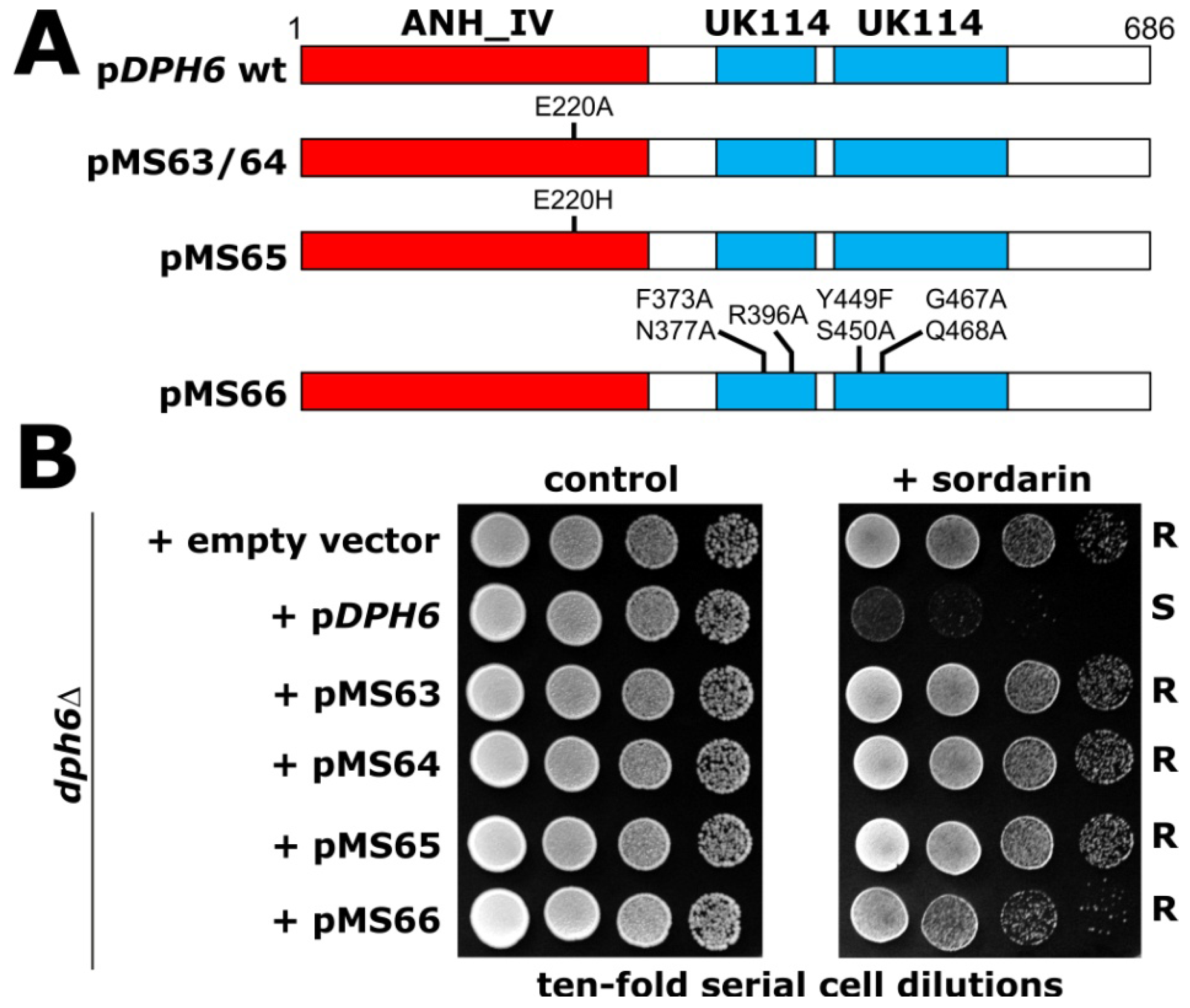
2.3. DPH6 Mutagenesis
3. Experimental Section
3.1. DPH1 and DPH2 Overexpression in E. coli
3.2. DPH1 Truncation Mutants
| Name | Sequence (5’➔3’) | Use |
|---|---|---|
| S2-DPH1 | GAATATGATACTAACTATTTATACATATGTAACAGGAAGACAAGTGACAACAAAAACTATTTAAAATCGATGAATTCGAGCTCG | DPH1 C-terminal HA tagging |
| S3-DPH1 | ATCCAATGGATTATTACGAAGCTAAAGGATACGGGCGTGGGGAAACTCCGAAACATGCGATTGAACGTACGCTGCAGGTCGAC | DPH1 C-terminal HA tagging |
| S3.1-DPH1 | TCAATAAACCACTATTAACACCATATGAGGCTAGTGTCTTACTAAAGAAACGTACGCTGCAGGTCGAC | DPH1 HA tagging & C1-truncation |
| S3.2-DPH1 | TTATTCTAAGTGAAGTTTTTCCCCAAAAGCTCGCAATGTTCGATCAAATTGATGTTTTTGTTCAGCGTACGCTGCAGGTCGAC | DPH1 HA tagging & C2-truncation |
| S3.3-DPH1 | GTAGACAAGGTAATTTAAACACTGTAAAAAACTTGGAAAAAAACCTGATCCGTACGCTGCAGGTCGAC | DPH1 HA tagging & C3-truncation |
| S3.4-DPH1 | TCACTAGAGAAGGATACGATCAAAAGCAACTCGTGGAAGTTAGAGCAGAGGCCATTGAAGTCGCTCGTACGCTGCAGGTCGAC | DPH1 HA tagging & C4-truncation |
| F4- DPH1 | AGAAATATAAATTCCTCATCCTGTGTTATAGAGAATCTTGGTGTTATCATTATAGTTCAGAAGTGGAATTCGAGCTCGTTTAAAC | DPH1 N-terminal HA tagging |
| R3- DPH1 | CCAATAAATCTTCTTCTTGGTTGTTTTTTAGATTCTGTAGAGCCACTCATGCACTGAGCAGCGTAATCTG | DPH1 N-terminal HA tagging |
| R3.1- DPH1 | TTGTAGTTAGAGGGCAATAATTTGATGGCTTCATTCAACTCTTTGTCATTGCACTGAGCAGCGTAATCTG | DPH1 HA tagging & N1-truncation |
| R3.2- DPH1 | TCACTTATAATCAATGAGTAAATCAGCAAACCTTCAGGCATCTGTAGGGCTATTCTTTTAGCATTGCACTGAGCAGCGTAATCTG | DPH1 HA tagging & N2-truncation |
| R3.3- DPH1 | TCATCAATACAGCATGCACCATAAGACACATCCCCCATTACTAGAGTTTCGCACTGAGCAGCGTAATCTG | DPH1 HA tagging & N3-truncation |
| R3.4- DPH1 | AGTACTTTAATCTTTGTAACGTCAATAGGAACTAAACACGAATGAGCGTAGCACTGAGCAGCGTAATCTG | DPH1 HA tagging & N4-truncation |
3.3. DPH6 Mutagenesis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Collier, R.J. Understanding the mode of action of diphtheria toxin: A perspective on progress during the 20th century. Toxicon 2001, 39, 1793–1803. [Google Scholar]
- Liu, S.; Milne, G.T.; Kuremsky, J.G.; Fink, G.R.; Leppla, S.H. Identification of the proteins required for biosynthesis of diphthamide, the target of bacterial ADP-ribosylating toxins on translation elongation factor 2. Mol. Cell. Biol. 2004, 24, 9487–9497. [Google Scholar]
- Uthman, S.; Liu, S.; Giorgini, F.; Stark, M.J.R.; Costanzo, M.; Schaffrath, R. Diphtheria Disease and Genes Involved in Formation of Diphthamide, Key Effector of the Diphtheria Toxin. In Insight and Control of Infectious Disease in Global Scenario; Kumar, R., Ed.; INTECH Open Access Publisher: Rijeka, Croatia, 2012; pp. 333–356. [Google Scholar]
- Su, X.; Chen, W.; Lee, W.; Jiang, H.; Zhang, S.; Lin, H. YBR246W is required for the third step of diphthamide biosynthesis. J. Am. Chem. Soc. 2012, 134, 773–776. [Google Scholar]
- Su, X.; Lin, Z.; Chen, W.; Jiang, H.; Zhang, S.; Lin, H. Chemogenomic approach identified yeast YLR143W as diphthamide synthetase. Proc. Natl. Acad. Sci. USA 2012, 109, 19983–19987. [Google Scholar]
- Uthman, S.; Bär, C.; Scheidt, V.; Liu, S.; ten Have, S.; Giorgini, F.; Stark, M.J.R.; Schaffrath, R. The amidation step of diphthamide biosynthesis in yeast requires DPH6, a gene identified through mining the DPH1-DPH5 interaction network. PLoS Genet. 2013, 9, e1003334. [Google Scholar]
- Zhang, Y.; Zhu, X.; Torelli, A.T.; Lee, M.; Dzikovski, B.; Koralewski, R.M.; Wang, E.; Freed, J.; Krebs, C.; Ealick, S.E.; et al. Diphthamide biosynthesis requires an organic radical generated by an iron-sulphur enzyme. Nature 2010, 465, 891–896. [Google Scholar]
- Zhu, X.; Dzikovski, B.; Su, X.; Torelli, A.T.; Zhang, Y.; Ealick, S.E.; Freed, J.H.; Lin, H. Mechanistic understanding of Pyrococcus. horikoshii Dph2, a [4Fe-4S] enzyme required for diphthamide biosynthesis. Mol. Biosyst. 2011, 7, 74–81. [Google Scholar]
- Liu, S.; Wiggins, J.F.; Sreenath, T.; Kulkarni, A.B.; Ward, J.M.; Leppla, S.H. Dph3, a small protein required for diphthamide biosynthesis, is essential in mouse development. Mol. Cell. Biol. 2006, 26, 3835–3841. [Google Scholar]
- Mattheakis, L.C.; Shen, W.H.; Collier, R.J. DPH5, a methyltransferase gene required for diphthamide biosynthesis in Saccharomyces. cerevisiae. Mol. Cell. Biol. 1992, 12, 4026–4037. [Google Scholar]
- Zhu, X.; Kim, J.; Su, X.; Lin, H. Reconstitution of diphthine synthase activity in vitro. Biochemistry 2010, 49, 9649–9657. [Google Scholar]
- Van Ness, B.G.; Howard, J.B.; Bodley, J.W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J. Biol. Chem. 1980, 255, 10710–10716. [Google Scholar]
- Sitikov, A.S.; Davydova, E.K.; Bezlepkina, T.A.; Ovchinnikov, L.P.; Spirin, A.S. Eukaryotic elongation factor 2 loses its non-specific affinity for RNA and leaves polyribosomes as a result of ADP-ribosylation. FEBS Lett. 1984, 176, 406–410. [Google Scholar]
- Moehring, T.J.; Danley, D.E.; Moehring, J.M. In vitro biosynthesis of diphthamide, studied with mutant Chinese hamster ovary cells resistant to diphtheria toxin. Mol. Cell. Biol. 1984, 4, 642–650. [Google Scholar]
- Bär, C.; Zabel, R.; Liu, S.; Stark, M.J.; Schaffrath, R. A versatile partner of eukaryotic protein complexes that is involved in multiple biological processes: Kti11/Dph3. Mol. Microbiol. 2008, 69, 1221–1233. [Google Scholar]
- Botet, J.; Rodríguez-Mateos, M.; Ballesta, J.P.; Revuelta, J.L.; Remacha, M. A chemical genomic screen in Saccharomyces. cerevisiae reveals a role for diphthamidation of translation elongation factor 2 in inhibition of protein synthesis by sordarin. Antimicrob. Agents Chemother. 2008, 52, 1623–1629. [Google Scholar]
- Fichtner, L.; Jablonowski, D.; Schierhorn, A.; Kitamoto, H.K.; Stark, M.J.R.; Schaffrath, R. Elongator’s toxin-target (TOT) function is nuclear localization sequence dependent and suppressed by post-translational modification. Mol. Microbiol. 2003, 49, 1297–1307. [Google Scholar]
- Janke, C.; Magiera, M.M.; Rathfelder, N.; Taxis, C.; Reber, S.; Maekawa, H.; Moreno-Borchart, A.; Doenges, G.; Schwob, E.; Schiebel, E.; et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 2004, 21, 947–962. [Google Scholar]
- Roy, V.; Ghani, K.; Caruso, M. A dominant-negative approach that prevents diphthamide formation confers resistance to Pseudomonas exotoxin A and diphtheria toxin. PLoS One 2010, 5, e15753. [Google Scholar]
- Gelperin, D.M.; White, M.A.; Wilkinson, M.L.; Kon, Y.; Kung, L.A.; Wise, K.J.; Lopez-Hoyo, N.; Jiang, L.; Piccirillo, S.; Yu, H.; et al. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 2005, 19, 2816–2826. [Google Scholar]
- Longtine, M.S.; McKenzie, A., 3rd; Demarini, D.J.; Shah, N.G.; Wach, A.; Brachat, A.; Philippsen, P.; Pringle, J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 1998, 14, 953–961. [Google Scholar]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdel-Fattah, W.; Scheidt, V.; Uthman, S.; Stark, M.J.R.; Schaffrath, R. Insights into Diphthamide, Key Diphtheria Toxin Effector. Toxins 2013, 5, 958-968. https://doi.org/10.3390/toxins5050958
Abdel-Fattah W, Scheidt V, Uthman S, Stark MJR, Schaffrath R. Insights into Diphthamide, Key Diphtheria Toxin Effector. Toxins. 2013; 5(5):958-968. https://doi.org/10.3390/toxins5050958
Chicago/Turabian StyleAbdel-Fattah, Wael, Viktor Scheidt, Shanow Uthman, Michael J. R. Stark, and Raffael Schaffrath. 2013. "Insights into Diphthamide, Key Diphtheria Toxin Effector" Toxins 5, no. 5: 958-968. https://doi.org/10.3390/toxins5050958