Phormidium autumnale Growth and Anatoxin-a Production under Iron and Copper Stress
Abstract
:1. Introduction
2. Results and Discussion
2.1. Media Concentrations
 control, ■ treatment (MLA2×Fe); (b) Copper
control, ■ treatment (MLA2×Fe); (b) Copper  control, ■ treatment (MLA2×Fe). (n = 3 for cultures, n = 2+ for controls).
control, ■ treatment (MLA2×Fe). (n = 3 for cultures, n = 2+ for controls).
 control, ■ treatment (MLA2×Fe); (b) Copper
control, ■ treatment (MLA2×Fe); (b) Copper  control, ■ treatment (MLA2×Fe). (n = 3 for cultures, n = 2+ for controls).
control, ■ treatment (MLA2×Fe). (n = 3 for cultures, n = 2+ for controls).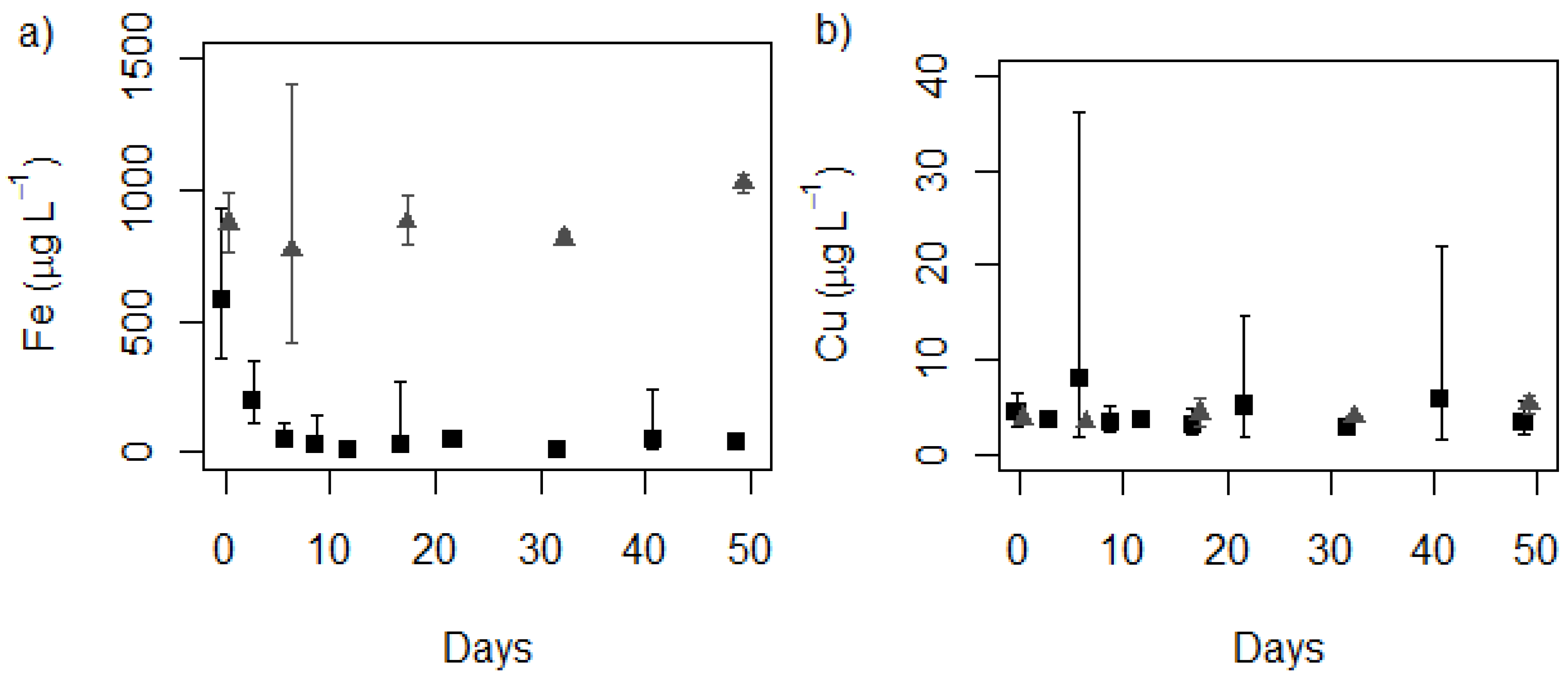
2.2. Growth Profile

2.3. Anatoxin-a Production


 statistically significant concentrations per day; nd, not detected.
statistically significant concentrations per day; nd, not detected.
 statistically significant concentrations per day; nd, not detected.
statistically significant concentrations per day; nd, not detected. 
2.4. Biological Role of Anatoxin-a
3. Methods
3.1. Experimental Design and Harvest
3.2. Growth Analysis
3.3. Culture Medium Analysis
3.4. Anatoxin-a Analysis
3.5. Statistical Analysis
4. Conclusions
Conflicts of Interest
Acknowledgements
References
- Bernard, C.; Froscio, S.; Campbell, R.; Monis, P.; Humpage, A.; Fabbro, L. Novel toxic effects associated with a tropical Limnothrix/Geitlerinema-like cyanobacterium. Environ. Toxicol. 2011, 26, 260–270. [Google Scholar] [CrossRef]
- Smith, F.M.J.; Wood, S.A.; Van Ginkel, R.; Broady, P.A.; Gaw, S. First report of saxitoxin production by a species of the freshwater benthic cyanobacterium, Scytonema Agardh. Toxicon 2011, 57, 566–573. [Google Scholar] [CrossRef]
- Quiblier, C.; Wood, S.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.-F. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Wood, S.A.; Heath, M.W.; Holland, P.T.; Munday, R.; McGregor, G.B.; Ryan, K.G. Identification of a benthic microcystin-producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef]
- Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health, Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; World Health Organization: London, UK, 1999; pp. 41–111. [Google Scholar]
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, P.T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First report of homoanatoxin-a and associated dog neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. [Google Scholar] [CrossRef]
- Heath, M.W.; Wood, S.A.; Ryan, K.G. Polyphasic assessment of fresh-water benthic mat-forming cyanobacteria isolated from New Zealand. FEMS Microbiol. Ecol. 2010, 73, 95–109. [Google Scholar]
- Wood, S.A.; Holland, P.T.; MacKenzie, L. Development of solid phase adsorption toxin tracking (SPATT) for monitoring anatoxin-a and homoanatoxin-a in river water. Chemosphere 2011, 82, 888–894. [Google Scholar] [CrossRef]
- Codd, G.A. Cyanobacterial toxins: Occurrence, properties and biological significance. Water Sci. Technol. 1995, 32, 149–156. [Google Scholar] [CrossRef]
- Sivonen, K.; Börner, T. Bioactive compounds produced by cyanobacteria. In The Cyanobacteria Molecular Biology, Genomic and Evolution; Herrero, A., Flores, E., Eds.; Caister Academic Press: Norfolk, UK, 2008; pp. 159–197. [Google Scholar]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms—Toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar]
- Codd, G.; Lindsay, J.; Young, F.; Morrison, L.; Metcalf, J. Harmful cyanobacteria—From mass mortalities to management measures. In Harmful Cyanobacteria; Huisman, J., Matthijs, H., Visser, P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2005; Volume 3, pp. 1–23. [Google Scholar]
- Kardinaal, W.E.A.; Visser, P.M. Dyanimics of cyanobacterial toxins—Sources of variability in microcystin concentrations. In Harmful Cyanobacteria; Huisman, J., Matthijs, H., Visser, P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2005; Volume 3, pp. 41–63. [Google Scholar]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef]
- Tyler, A.; Hunter, P.; Carvalho, L.; Codd, G.; Elliott, J.A.; Ferguson, C.; Hanley, N.; Hopkins, D.; Maberly, S.; Mearns, K.; et al. Strategies for monitoring and managing mass populations of toxic cyanobacteria in recreational waters: A multi-interdisciplinary approach. Environ. Health 2009, 8, S11. [Google Scholar] [CrossRef]
- Baptista, M.S.; Vasconcelos, M.T. Cyanobacteria metal interactions: Requirements, toxicity, and ecological implications. Crit. Rev. Microbiol. 2006, 32, 127–137. [Google Scholar] [CrossRef]
- Gadd, G.M. Accumulation of metals by microorganisms and algae. In Biotechnology; Rehm, H.-J., Ed.; Verlag-Chemie: Weinheim, Germany, 1988; Volume 6b, pp. 401–433. [Google Scholar]
- Kikuchi, T.; Tanaka, S. Biological removal and recovery of toxic heavy metals in water environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1007–1057. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Rueter, J.G.; Petersen, R.R. Micronutrient effects on cyanobacterial growth and physiology. N. Z. J. Mar. Freshwat. Res. 1987, 21, 435–445. [Google Scholar] [CrossRef]
- Jamers, A.; Blust, R.; De Coen, W. Omics in algae: Paving the way for a systems biological understanding of algal stress phenomena? Aquat. Toxicol. 2009, 92, 114–121. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Hughes, M.P.; Rue, E.L.; Wells, M.L. The effect of Fe and Cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol. Oceanogr. 2002, 47, 515–526. [Google Scholar] [CrossRef]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Amé, M.V.; Díaz, M.D.P.; Wunderlin, D.A. Occurrence of toxic cyanobacterial blooms in San Roque Reservoir (Córdoba, Argentina): A field and chemometric study. Environ. Toxicol. 2003, 18, 192–201. [Google Scholar] [CrossRef]
- Amé, M.V.; Wunderlin, D.A. Effects of iron, ammonium and temperature on microcystin content by a natural concentrated Microcystis aeruginosa population. Water Air Soil Pollut. 2005, 168, 235–248. [Google Scholar] [CrossRef]
- Kaebernick, M.; Neilan, B.A. Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol. Ecol. 2001, 35, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Murphy, T.; Guo, J.; Parr, T.; Nalewajko, C. Iron-stimulated growth and microcystin production of Microcystis novacekii UAM 250. Limnol. Ecol. Manag. Inland Waters 2009, 39, 255–259. [Google Scholar] [CrossRef]
- Chuang, A.; Garnett, C.; Hargreaves, M.; Shaw, G.; Senogles, P.-J.; Smith, M.; Eaglesham, G.; Müller, J.F. Effects of iron and manganese concentration and their ratio on cell growth and cylindrospermopsin production of the cyanobacterium Cylindrospermopsis raciborskii. In Harmful Algal Blooms, Proceedings of 9th International Conference on Harmful Algal Blooms, Hobart, Australia, 7–11 February 2000; Hallegraeff, G.M., Blackburn, S.I., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; pp. 344–347. [Google Scholar]
- Wightman, P.G.; Fein, J.B. Iron adsorption by Bacillus subtilis bacterial cell walls. Chem. Geol. 2005, 216, 177–189. [Google Scholar] [CrossRef]
- Fiore, M.F.; Trevors, J.T. Cell composition and metal tolerance in cyanobacteria. BioMetals 1994, 7, 83–103. [Google Scholar]
- Singh, S.; Pradhan, S.; Rai, L.C. Comparative assessment of Fe3+ and Cu2+ biosorption by field and laboratory-grown Microcystis. Process Biochem. 1998, 33, 495–504. [Google Scholar] [CrossRef]
- Harding, J.S.; Winterbourn, M.J. Effects of contrasting land use on physico-chemical conditions and benthic assemblages of streams in a Canterbury (South Island, New Zealand) river system. N. Z. J. Mar. Freshwat. Res. 1995, 29, 479–492. [Google Scholar] [CrossRef]
- Mighanetara, K.; Braungardt, C.B.; Rieuwerts, J.S.; Azizi, F. Contaminant fluxes from point and diffuse sources from abandoned mines in the River Tamar catchment, UK. J. Geochem. Explor. 2009, 100, 116–124. [Google Scholar] [CrossRef]
- Sekabira, K.; Origa, H.O.; Basamba, T.A.; Mutumba, G.; Kakudidi, E. Heavy metal assessment and water quality values in urban stream and rain water. Int. J. Environ. Sci. Technol. 2010, 7, 759–770. [Google Scholar] [CrossRef]
- Estevez, M.S.; Malanga, G.; Puntarulo, S. Iron-dependent oxidative stress in Chlorella vulgaris. Plant Sci. 2001, 161, 9–17. [Google Scholar] [CrossRef]
- Lukač, M.; Aegerter, R. Influence of trace metals on growth and toxin production of Microcystis aeruginosa. Toxicon 1993, 31, 293–305. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Xing, W.; Huang, W.-M.; Li, D.-H.; Liu, Y.-D. Effects of iron on growth, pigment content, photosystem II efficiency, and siderophores production of Microcystis aeruginosa and Microcystis wesenbergii. Curr. Microbiol. 2007, 55, 94–98. [Google Scholar] [CrossRef]
- Latifi, A.; Jeanjean, R.; Lemeille, S.; Havaux, M.; Zhang, C.-C. Iron starvation leads to oxidative stress in Anabaena sp. strain PCC 7120. J. Bacteriol. 2005, 187, 6596–6598. [Google Scholar] [CrossRef]
- McKnight, D.M.; Chisholm, S.W.; Harleman, D.R.F. CuSO4 treatment of nuisance algal blooms in drinking water reservoirs. Environ. Manage. 1983, 7, 311–320. [Google Scholar] [CrossRef]
- García-Villada, L.; Rico, M.; Altamirano, M.; Sánchez-Martín, L.; López-Rodas, V.; Costas, E. Occurrence of copper resistant mutants in the toxic cyanobacteria Microcystis aeruginosa: Characterisation and future implications in the use of copper sulphate as algaecide. Water Res. 2004, 38, 2207–2213. [Google Scholar] [CrossRef]
- Rapala, J.; Sivonen, K. Assessment of environmental conditions that favor hepatotoxic and neurotoxic Anabaena spp. strains cultured under light limitation at different temperatures. Microb. Ecol. 1998, 36, 181–192. [Google Scholar] [CrossRef]
- Rapala, J.; Sivonen, K.; Luukkainen, R.; Niemelä, S. Anatoxin-a concentration Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and non-toxic Anabaena-strains—A laboratory study. J. Appl. Phycol. 1993, 5, 581–591. [Google Scholar] [CrossRef]
- Gupta, N.; Bhaskar, A.S.B.; Rao, P.V.L. Growth characteristics and toxin production in batch cultures of Anabaena flos-aquae: Effects of culture media and duration. World J. Microbiol. Biotechnol. 2002, 18, 29–35. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Selwood, A.I.; Holland, P.T.; Wood, S.A.; Smith, K.F.; McNabb, P.S. Production of anatoxin-a and a novel biosynthetic precursor by the cyanobacterium Aphanizomenon issatschenkoi. Environ. Sci. Technol. 2007, 41, 506–510. [Google Scholar] [CrossRef]
- Briand, J.-F.; Jacquet, S.; Flinois, C.; Avois-Jacquet, C.; Maisonnette, C.; Leberre, B.; Humbert, J.-F. Variations in the microcystin production of Planktothrix rubescens (Cyanobacteria) assessed from a four-year survey of Lac du Bourget (France) and from laboratory experiments. Microb. Ecol. 2005, 50, 418–428. [Google Scholar] [CrossRef]
- Long, B.M.; Jones, G.J.; Orr, P.T. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 2001, 67, 278–283. [Google Scholar] [CrossRef]
- Vézie, C.; Rapala, J.; Vaitomaa, J.; Seitsonen, J.; Sivonen, K. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microb. Ecol. 2002, 43, 443–454. [Google Scholar] [CrossRef]
- Wiedner, C.; Visser, P.M.; Fastner, J.; Metcalf, J.S.; Codd, G.A.; Mur, L.R. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003, 69, 1475–1481. [Google Scholar] [CrossRef]
- Negri, A.P.; Jones, G.J.; Blackburn, S.I.; Oshima, Y.; Hideyuki, O. Effect of culture and bloom development and of sample storage on paralytic shellfish poisons in the cyanobacterium Anabaena circinalis. J. Phycol. 1997, 33, 26–35. [Google Scholar]
- Baker, P.; Humpage, A. Toxicity associated with commonly occurring cyanobacteria in surface waters of the Murray-Darling Basin, Australia. Aust. J. Mar. Freshw. Res. 1994, 45, 773–786. [Google Scholar] [CrossRef]
- Utkilen, H.; Gjølme, N. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 1995, 61, 797–800. [Google Scholar]
- Alexova, R.; Fujii, M.; Birch, D.; Cheng, J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation. Environ. Microbiol. 2011, 13, 1064–1077. [Google Scholar] [CrossRef]
- Stevens, D.K.; Krieger, R.I. Stability studies on the cyanobacterial nicotinic alkaloid anatoxin-a. Toxicon 1991, 29, 167–179. [Google Scholar] [CrossRef]
- Surosz, W.; Palinska, K.A. Effects of heavy-metal stress on cyanobacterium Anabaena flos-aquae. Arch. Environ. Contam. Toxicol. 2004, 48, 40–48. [Google Scholar] [CrossRef]
- Byth, S. Palm Island mystery disease. Med. J. Aust. 1980, 2, 40–42. [Google Scholar]
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 2003, 18, 78–93. [Google Scholar] [CrossRef]
- Pouria, S.; De Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.S.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Esson, D.; Wood, S.; Packer, M. Harnessing the self-harvesting capability of benthic cyanobacteria for use in benthic photobioreactors. AMB Express 2011, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, A.; Harel, M.; Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A.; Dittmann, E. The languages spoken in the water body (or the biological role of cyanobacterial toxins). Front. Microbiol. 2012, 3, 138. [Google Scholar]
- Wood, S.A.; Rueckert, A.; Hamilton, D.P.; Cary, S.C.; Dietrich, D.R. Switching toxin production on and off: Intermittent microcystin synthesis in a Microcystis bloom. Environ. Microbiol. Reports 2011, 3, 118–124. [Google Scholar] [CrossRef]
- Kearns, K.D.; Hunter, M.D. Toxin-producing Anabaena flos-aquae induces settling of Chlamydomonas reinhardtii, a competing motile alga. Microb. Ecol. 2001, 42, 80–86. [Google Scholar]
- Bolch, C.J.S.; Blackburn, S.I. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kütz. J. Appl. Phycol. 1996, 8, 5–13. [Google Scholar] [CrossRef]
- Random Sequence Generator. Available online: http://www.random.org/sequences (accessed on 11 March 2010).
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-methodik (Towards a perfection of quantitative phytoplankton methodology). Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: http://www.r-project.org (accessed on 11 August 2011).
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harland, F.M.J.; Wood, S.A.; Moltchanova, E.; Williamson, W.M.; Gaw, S. Phormidium autumnale Growth and Anatoxin-a Production under Iron and Copper Stress. Toxins 2013, 5, 2504-2521. https://doi.org/10.3390/toxins5122504
Harland FMJ, Wood SA, Moltchanova E, Williamson WM, Gaw S. Phormidium autumnale Growth and Anatoxin-a Production under Iron and Copper Stress. Toxins. 2013; 5(12):2504-2521. https://doi.org/10.3390/toxins5122504
Chicago/Turabian StyleHarland, Francine M. J., Susanna A. Wood, Elena Moltchanova, Wendy M. Williamson, and Sally Gaw. 2013. "Phormidium autumnale Growth and Anatoxin-a Production under Iron and Copper Stress" Toxins 5, no. 12: 2504-2521. https://doi.org/10.3390/toxins5122504




