Comparative Immunohistochemical Analysis of Ochratoxin A Tumourigenesis in Rats and Urinary Tract Carcinoma in Humans; Mechanistic Significance of p-S6 Ribosomal Protein Expression
Abstract
:1. Introduction
2. Results
2.1. Rat Controls
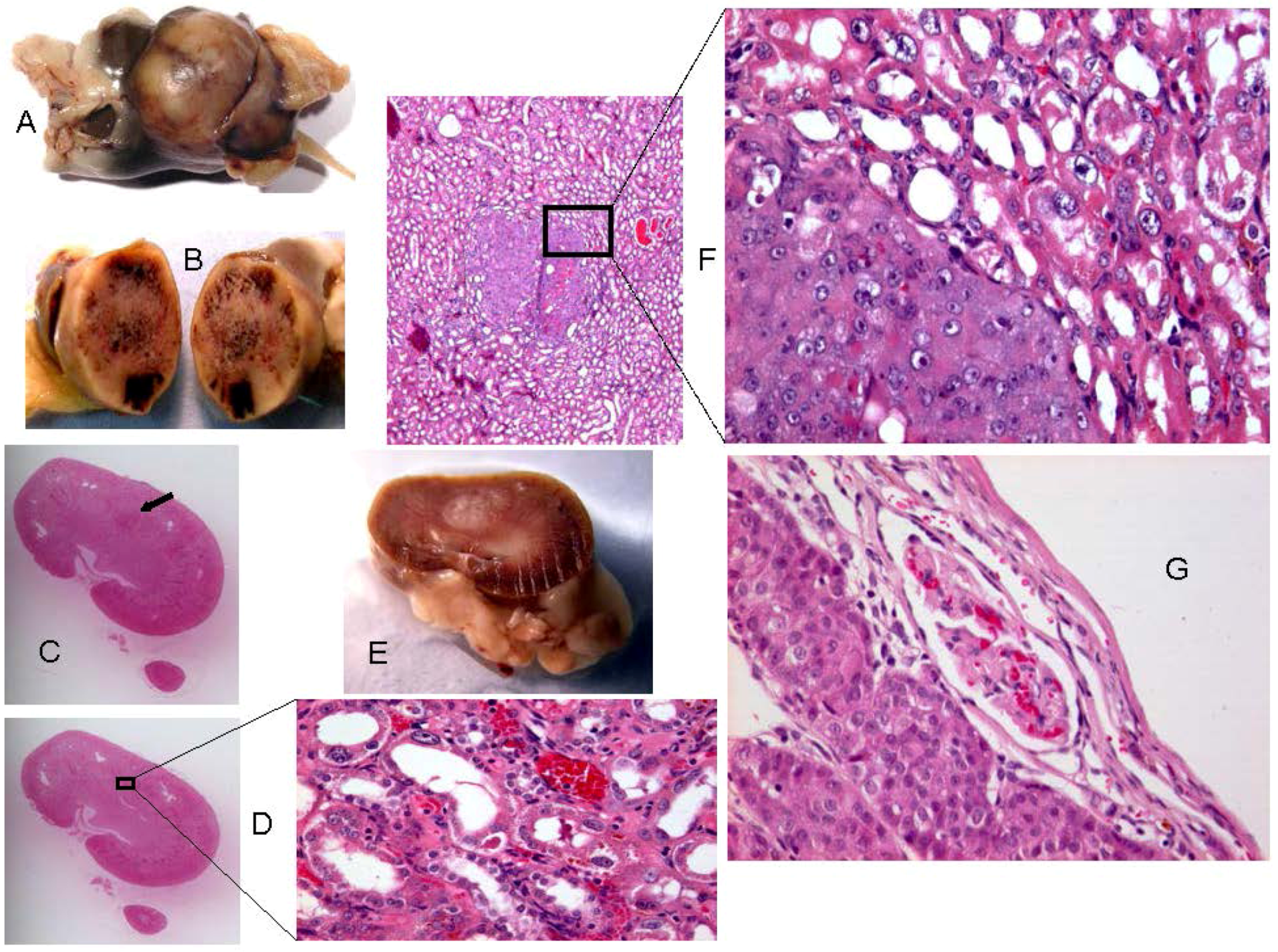
2.2. Rats Exposed Experimentally to OTA
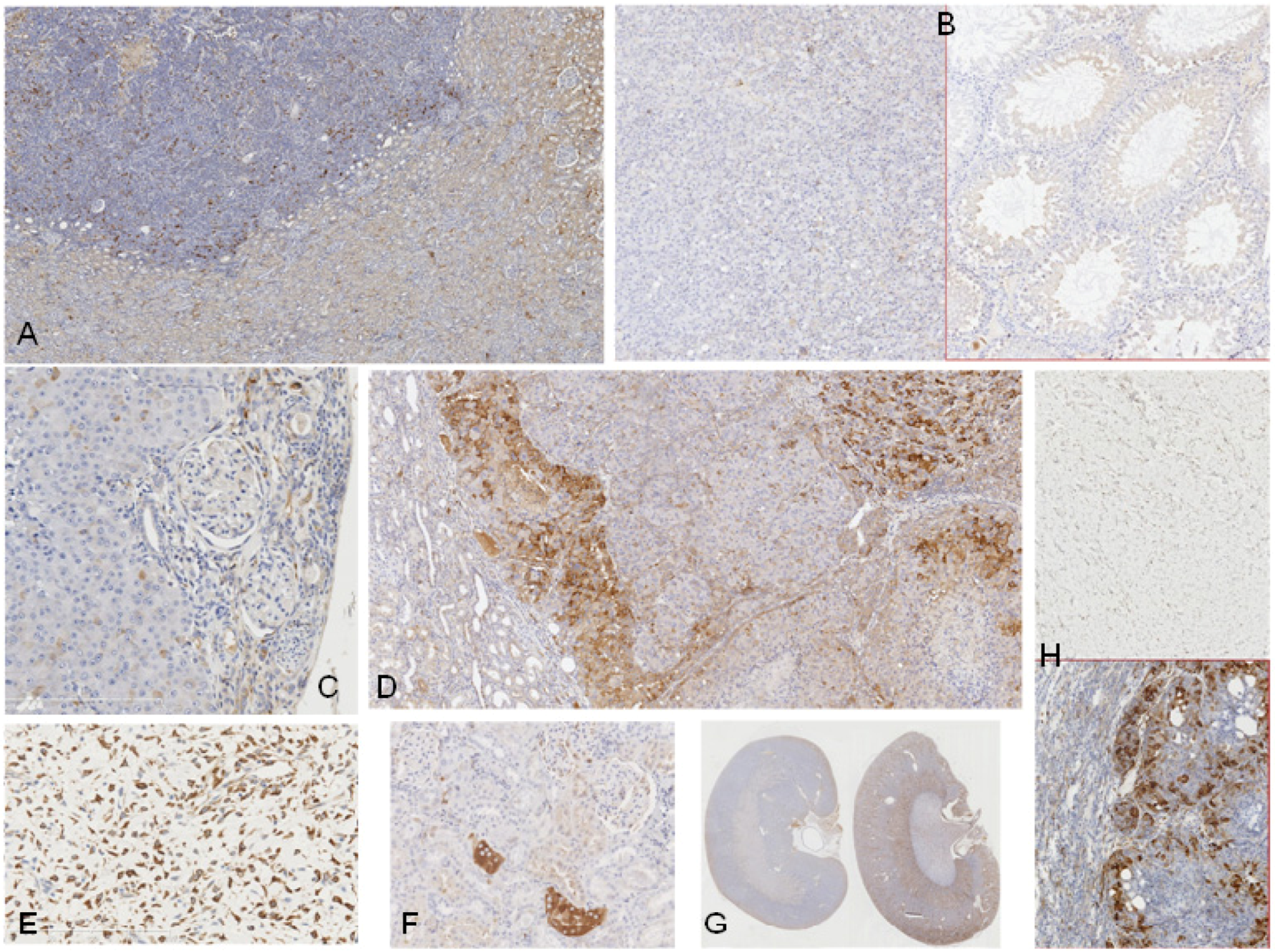
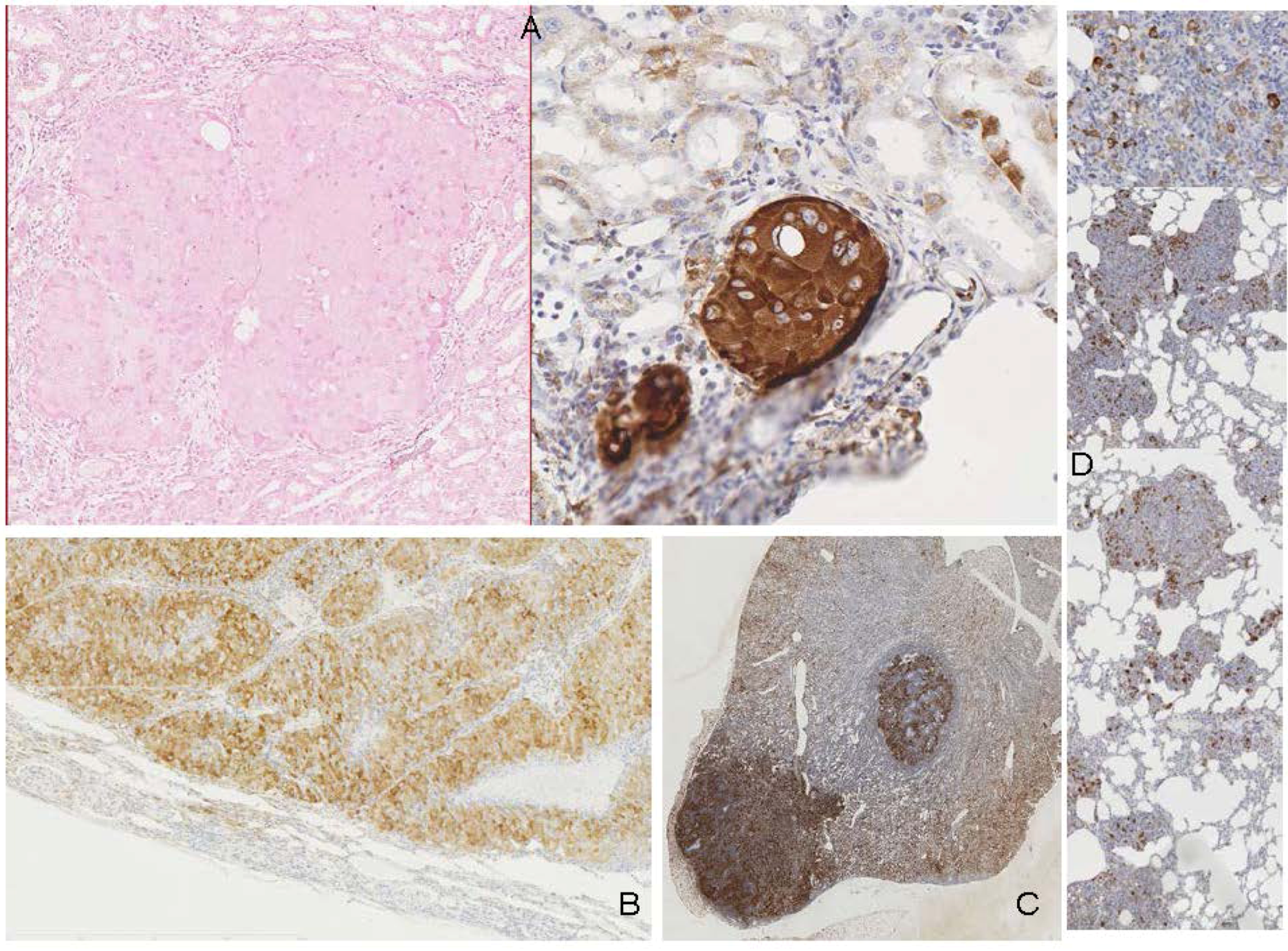

2.3. Human Urinary Tract Tumours
| Pathological finding | Total cases | Positive | Negative | |
|---|---|---|---|---|
| Rat | Kidney, carcinoma or adenocarcinoma | 13 | 13 | 0 |
| Rat | Lung, carcinomas (metastatic from kidney) | 1 | 1 | 0 |
| Rat | Kidney, small adenoma (in situ) | 3 | 0 | 3 |
| Rat | Mammary, angiosarcoma | 1 | 1 | 0 |
| Rat | Subcutaneous fibrosarcoma | 1 | 0 | 1 |
| Rat | Testis, seminomas | 2 | 0 | 2 |
| Human | Upper urinary tract, transitional cell carcinoma (Balkan endemic nephropathy) | 4 | 0 | 4 |
| Human | Kidney, renal cell carcinoma | 1 | 0 | 1 |
| Human | Angiosarcoma | 1 | 0 | 1 |
3. Discussion
4. Experimental Section
4.1. Rat and Human Tissues
4.2. Immunohistochemistry
5. Conclusions
Supplementary
| Rat ID | Strain/gender/age at OTA start | OTA dose µg/kg | Weeks On OTA | Latency from OTA to finding tumour | Age (wk) at death | Organ | Tumour type | Histopathology/DNA ploidy distribution | Cited * (Figure in this text) | Immunohistochemistry response |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DA | 250 | 26 | 93 w | 119 | kidney | carcinoma, possibly metastatic from other tumourous kidney | infiltrating (3.2 mm), enveloping glomeruli, not distorting the kidney. | A | Scattered intensely +ve elements |
| M | (1A) | |||||||||
| 8w | ||||||||||
| lung | scattered malignant foci | possibly metastatic from contralateral kidney’s carcinoma | A | Scattered intensely +ve elements | ||||||
| (2D) | ||||||||||
| 2 | DA | 250 | 39 | 83 w | 122 (losing weight) | kidney ‘a’ | none recognised initially | - | A | Intense staining of swollen nephron fragment at cortico-medullary junction. Implies early neoplasm. |
| M | (1F) | |||||||||
| 8w | ||||||||||
| kidney ‘b’: no tumour apparent | micro-adeno-carcinoma | (2.5 mm), already infiltrating | A | Clear differentiation of tumour from cortex, but not as striking a contrast in staining as in kidney ‘a’ | ||||||
| 3 | SD/F | 200 | 36 | 61 w | 114 | kidney, bilateral | adeno-carcinoma (two, small) | erupting tumour, seen in two parts in section. diploid | B, G | Contrasting intense +ve in tumour; some diffuse +ve elements in kidney periphery. |
| M | (2C) | |||||||||
| 8w | ||||||||||
| subcutaneous tumour | sarcoma (typical) | diploid | G | -ve, contrasting with kidney and renal tumour of same animal. | ||||||
| (1H) | ||||||||||
| 4 | SD/F | 150 | 36 | 61 w | 114 | mammary | angio-sarcoma in fibro-adenoma | typically sarcomatoid region of tumour. | B | Intense + ve staining in sarcomatoid elements only. |
| F | (1E) | |||||||||
| 8w | ||||||||||
| kidneys | none | - | B | Scattered + ve elements in cortex. -ve in medulla and papilla. | ||||||
| 5 | F344 | 300 | 43 | 46 w | 97 | kidney | Carcinoma (large) | infiltrating into kidney. aneuploid | G | Contrasting +ve in non-necrotic regions of tumour, -ve in distorted kidney. |
| M | (3A) | |||||||||
| 8w | ||||||||||
| testis | typical rat age-related seminomas | diploid | H | -ve in both seminoma and seminiferous tubules | ||||||
| (1B) | ||||||||||
| 6 | F344 | 300 | 91 | 0 | 99 (pre-mature; s/c sarcoma) | kidney | carcinoma (large) | infiltrating into kidney | Contrasting +ve of tumour; -ve in all remaining kidney. | |
| M | ||||||||||
| 8w | ||||||||||
| 7 | F344 | 300 | 83 | 0 | 91 | kidney | adeno-carcinoma | compression tumour with some adenoma regions | (1D) | -ve in adenomatous regions of tumour as in renal cortex. Contrasting +ve in carcinoma regions of tumour. |
| M | ||||||||||
| 8w | ||||||||||
| testis | seminomas | typical of ageing rats | H | -ve | ||||||
| 8 | F344 | 300 | 43 | 49 w | 100 (weight loss, moribund) | kidney (other kidney with major metastasising carcinoma) | carcinoma (tumourous kidney 3g) | tumour infiltrating into and erupting from kidney. | Tumour clearly differentiated from kidney by +ve elements particularly around the tumour periphery, but also scattered throughout and more abundant than in the surrounding kidney. | |
| M | ||||||||||
| 8w | ||||||||||
| 9 | F344 | 300 | 93 | 0 | 101 | kidney | carcinoma | tumourous kidney 8g | Contrasting +ve in tumour. | |
| M | ||||||||||
| 8w | ||||||||||
| 10 | F344 | 200 | 102 | 0 | 110 | kidney | Carcinoma surrounding a cyst | compression tumour distorting kidney near pelvis. | C | Contrasting +ve in tumour; -ve in kidney. Isolated neoplastic focus revealed by intense staining. |
| M | (3B) | |||||||||
| 8w | ||||||||||
| 11 | F344 | 200 | 105 | 0 | 113 | kidney | carcinoma (large) | Metastasising. Aneuploid. Infiltrating under capsule. | C,G | Contrasting +ve in non-necrotic regions of tumour; -ve in necrotic tumour and in distorted kidney. |
| M | (3D) | |||||||||
| 8w | ||||||||||
| 12 | F344 | 200 | 84 | 0 | 92 | kidney | Adenoma, according to H & E stained histology. | spherical compression tumour (17 mm diameter). Diploid. | C,G | Contrasting +ve in tumour periphery; -ve in stretched kidney cortex. Tumour centre -ve, correlated with necrosis and haematoma. |
| M | (4,2B) | |||||||||
| 8w | ||||||||||
| 13 | F344 | 200 | 60 | 0 | 110 | kidney | adenoma | small (6 mm) compression tumour | D | -ve in tumour |
| M | (1C) | |||||||||
| 50w | ||||||||||
| 14 | F344 | 200 | 35 | 35 w | 120 | kidney | micro-adenoma | compression tumour (3 mm) | D | -ve in tumour |
| M | (3C) | |||||||||
| 50w | ||||||||||
| 15 | F344 | 200 | 35 | 29 w | 114 | kidney | micro-adenoma | compression tumour (0.1 mm) | D | -ve in tumour |
| M | ||||||||||
| 50w | ||||||||||
| 16 | F 344 | 200 | 35 | 38 w | 113 | kidney | adeno-carcinoma | compression tumour (16 x 12 mm) | D | Diffuse +ve in tumour contrasting with surrounding kidney |
| M | ||||||||||
| 50w | ||||||||||
| 17 | F344 | 50 | 93 | 0 | 101 | kidney | micro-adeno-carcinoma | (0.5 mm) at cortico-medullary junction; evolving to carcinoma | E | Intense +ve staining of remaining tumour fragment in a nearby section. |
| M | (2A) | |||||||||
| 8w | ||||||||||
| 18 | DA | ~ 30 thresh-old | 30 | 0 | pneu-monia | kidney | - | - | A | Faint diffuse differentiation of 3 medullary regions and the cortex |
| M | (1G) | |||||||||
| 8w | ||||||||||
| 19 | DA | ~ 30 thresh-old | 75 | 0 | (weight loss) | kidney | - | - | A | Clearer diffuse differentiation of the 4 regions |
| M | (1G) | |||||||||
| 8w | ||||||||||
| 20 | SD | - | - | - | 10 | kidney | none | - | - | -ve |
| M | ||||||||||
| - | ||||||||||
| 21 | SD | - | - | - | 0 neonatal | kidney (3 animals) | none | - | F | -ve |
| - | ||||||||||
| - |
Acknowledgments
Conflict of Interest
References
- Krogh, P. Porcine Nephropathy Associated with Ochratoxin A. In Mycotoxinsand Animal Foods; Smith, J.E., Henderson, R.S., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 627–645. [Google Scholar]
- The European Food Safety Authority. Opinion of the scientific panel on contaminants in the food chain on a request from the Commission related to ochratoxin A in Food. EFSA J. 2006, 365, 1–56.
- Boorman, G.A. Toxicology and Carcinogenesis Studies of Ochratoxin A (CAS No. 303-47-9) in F344/N Rats (Gavage Studies); Technical Report 358; Research Triangle Park: Research Triangle region, NC, USA, 1989. [Google Scholar]
- Castegnaro, M.; Mohr, U.; Pfohl-Leszkowicz, A.; Esteve, J.; Steinmann, J.; Tillmann, T.; Michelon, J.; Bartsch, H. Sex- and strain-specific induction of renal tumors by ochratoxin A in rats correlates with DNA adduction. Int. J. Cancer 1998, 77, 70–75. [Google Scholar] [CrossRef]
- Bendele, A.M.; Carlton, W.W.; Krogh, P.; Lillehoi, E.B. Ochratoxin A carcinogenesis in the (C57BL/6J x C3H) F1 mouse. J. Natl. Cancer Inst. 1985, 75, 733–744. [Google Scholar]
- Mantle, P.; Kulinskaya, E.; Nestler, S. Renal tumourigenesis in male rats in response to chronic dietary ochratoxin A. Food Addit. Contam. 2005, 22, 58–64. [Google Scholar] [CrossRef]
- Maher, E.R. Identification of the Individual at Risk: The Molecular and Clinical Genetics of Renal Cell Carcinoma. In The Effective Management of Renal Cell Carcinoma; Aitchison, M., Oliver, R.T.D., Miles, A., Eds.; Aesculapius Medical Press: London, UK, 2004; pp. 19–28. [Google Scholar]
- Mantle, P.G. Minimum tolerable exposure period and maximum threshold dietary intake of ochratoxin A for causing renal cancer in male Dark Agouti rats. Food Chem. Toxicol. 2009, 47, 2419–2424. [Google Scholar] [CrossRef]
- Mantle, P.G.; Amerasinghe, C.; Brown, A.L.; Herman, D.; Horn, T.; Krogh, T.; Odell, E.W.; Rosenbaum, T.; Tatu, C.A. A pilot study of nuclear instability in archived renal and upper urinary tract tumours with putative ochratoxin aetiology. Toxins 2010, 2, 326–340. [Google Scholar] [CrossRef]
- Mantle, P.G.; Dobrota, M.; Gillett, C.E.; Odell, E.W.; Pinder, S.E. Oncological outcomes in rats given nephrocarcinogenic exposure to dietary ochratoxin A, followed by the tumour promoter sodium barbital for life: a pilot study. Toxins 2010, 2, 552–571. [Google Scholar] [CrossRef]
- Mantle, P.G.; Nolan, C.C. Pathological outcomes in kidney and brain in male Fischer rats given dietary ochratoxin A, commencing at one year of age. Toxins 2010, 2, 1100–1110. [Google Scholar] [CrossRef]
- Mantle, P.; Kulinskaya, E. Lifetime, low-dose ochratoxin A, dietary study on renal carcinogenesis in male Fischer rats. Food Add. Contam. 2010, 27, 1566–1573. [Google Scholar] [CrossRef]
- Maher, E.R.; Ricketts, C.J. personal communication. University of Birmingham: UK, 2012. [Google Scholar]
- Hasumi, Y.; Baba, M.; Ajima, R.; Hasumi, H.; Valera, V.A.; Klein, M.E.; Haines, D.C.; Merino, M.J.; Hong, S.-B.; Yamaguchi, T.P.; et al. Homozygous loss of BDH causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc. Natl. Acad. Sci. USA 2009, 106, 18722–18727. [Google Scholar]
- McDorman, K.S.; Wolf, D.C. Use of the spontaneous Tsc2 knockout (Eker) rat model of hereditary renal cell carcinoma for the study of renal carcinogens. Toxicol. Pathol. 2002, 30, 675–680. [Google Scholar] [CrossRef]
- Ruggero, D.; Pandolfi, P.P. Does the ribosome translate cancer? Nat. Rev. Cancer 2003, 3, 179–192. [Google Scholar] [CrossRef]
- Kasprzak, K.S.; Diwan, B.A.; Knonishi, N.; Misra, M.; Rice, J.M. Initiation by nickel(11) acetate and promotion by sodium barbital of renal cortical epithelial tumours in male F344 rats. Carcinogenesis 1990, 11, 647–652. [Google Scholar] [CrossRef]
- Brown, A.L.; Odell, E.W.; Mantle, P.G. DNA ploidy distribution in renal tumours induced in male rats by dietary ochratoxin A. Exp. Toxicol. Pathol. 2007, 59, 85–95. [Google Scholar] [CrossRef]
- Wilson, C.; Bonnet, C.; Guy, C.; Idziaszczyk, S.; Colley, J.; Humphreys, V.; Maynard, J.; Sampson, J.R.; Cheadle, J.P. Tsc1 haploinsufficiency without mammalian target of rapamycin activation is sufficient for renal cyst formation in Tsc1+/− mice. Cancer Res. 2006, 66, 7934–7938. [Google Scholar]
- Kenerson, H.L.; Aicher, L.D.; True, L.D.; Yeung, R.S. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumours. Cancer Res. 2002, 62, 5645–5650. [Google Scholar]
- Iwenofu, O.H.; Lackman, R.D.; Staddon, A.P.; Goodwin, D.G.; Haupt, H.M.; Brooks, J.S. Phospho-S6 ribosomal protein: A potential new predictive sarcoma marker for targeted mTOR therapy. Modern Pathol. 2008, 21, 231–237. [Google Scholar] [CrossRef]
- Stemmer, K.; Ellinger-Ziegelbauer, H.; Ahr, H.J.; Dietrich, D.R. Carcinogen-specific gene expression profiles in short-term treated Eker and wild-type rats indicative of pathways involved in renal tumourigenesis. Cancer Res. 2007, 67, 4052–4068. [Google Scholar] [CrossRef]
- Mally, A.; Dekant, W. Mycotoxins and the kidney: modes of action for renal tumor formation by ochratoxin A in rodents. Mol. Nutr. Food Res. 2009, 53, 467–478. [Google Scholar] [CrossRef]
- Czakai, K.; Muller, K.; Mosesso, P.; Pepe, G.; Schulz, M.; Gohla, A.; Patnaik, D.; Dekant, W.; Higgins, J.M.G.; Mally, A. Perturbation of mitosis through inhibition of histone acetyltransferases: The key to ochratoxin toxicity and carcinogenicity? Toxicol. Sci. 2011, 122, 317–329. [Google Scholar] [CrossRef]
- Mantle, P.G.; Faucet-Marquis, V.; Manderville, R.A.; Squillaci, B.; Pfohl-Leszkowicz, A. Structures of covalent adducts between DNA and ochratoxin A: A new factor in debate about genotoxicity and human risk assessment. Chem. Res. Toxicol. 2010, 23, 89–98. [Google Scholar] [CrossRef]
- Pfohl-Leszkiwicz, A.; Faucet-Marquis, V.; Tozlovanu, M.; Peraica, M.; Stefanovic, V.; Manderville, R. C8-2′-Deoxyguanosine ochratoxin A-adducts and OTA metabolites in biologic fluids as biomarkers of OTA exposure. In Proceedings of MycoRed International Conference, Mendoza, Argentina, 15–17 November 2011; p. 90.
- Jennings-Gee, J.E.; Tozlovanu, M.; Manderville, R.; Miller, M.S.; Pfohl-Leszkowicz, A.; Schwartz, G.G. Ochratoxin A: In utero exposure in mice induces adducts in testicular DNA. Toxins 2010, 2, 1428–1444. [Google Scholar] [CrossRef] [Green Version]
- Mantle, P.G. Comments on “Ochratoxin A: In utero exposure in mice induces adducts in testicular DNA. Toxins 2010, 2, 1428–1424”—Mis-citation of rat literature to justify a hypothetical role for ochratoxin A in testicular cancer. Toxins 2010, 2, 2333–2336. [Google Scholar]
- Arai, M.; Hibino, T. Tumorgenicity of citrinin in male F344 rats. Cancer Lett. 1983, 17, 281–287. [Google Scholar] [CrossRef]
- The European Food Safety Authority. Scientific opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 2605.
- Mantle, P.G.; McHugh, K.M.; Fincham, J.E. Contrasting nephropathic responses to oral administration of extract of cultured Penicillium polonicum in rat and primate. Toxins 2010, 2, 2082–2097. [Google Scholar]
- Mantle, P.G. Renal histopathological responses to nephrotoxic Penicillium aurantiogriseum in the rat during pregnancy, lactation and after weaning. Nephron 1994, 66, 93–98. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gazinska, P.; Herman, D.; Gillett, C.; Pinder, S.; Mantle, P. Comparative Immunohistochemical Analysis of Ochratoxin A Tumourigenesis in Rats and Urinary Tract Carcinoma in Humans; Mechanistic Significance of p-S6 Ribosomal Protein Expression. Toxins 2012, 4, 643-662. https://doi.org/10.3390/toxins4090643
Gazinska P, Herman D, Gillett C, Pinder S, Mantle P. Comparative Immunohistochemical Analysis of Ochratoxin A Tumourigenesis in Rats and Urinary Tract Carcinoma in Humans; Mechanistic Significance of p-S6 Ribosomal Protein Expression. Toxins. 2012; 4(9):643-662. https://doi.org/10.3390/toxins4090643
Chicago/Turabian StyleGazinska, Patrycja, Diana Herman, Cheryl Gillett, Sarah Pinder, and Peter Mantle. 2012. "Comparative Immunohistochemical Analysis of Ochratoxin A Tumourigenesis in Rats and Urinary Tract Carcinoma in Humans; Mechanistic Significance of p-S6 Ribosomal Protein Expression" Toxins 4, no. 9: 643-662. https://doi.org/10.3390/toxins4090643





