Occurrence of Ochratoxin A in the Wild Boar (Sus scrofa): Chemical and Histological Analysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples
2.2. Analytical Reagents and Calibration
2.3. Apparatus and Chromatographic Conditions
2.4. Sample Extraction and Clean-up of Tissues and Organs
2.5. Samples for Histological Testing
3. Results
| Samples | Kidney | Liver | Urinary bladder | Muscle |
|---|---|---|---|---|
| 1 | 1.9 | 0,9 | 0.3 | 0.5 |
| 2 | 1.4 | 1.0 | 1.0 | 0.3 |
| 3 | 0.3 | 0.2 | 0.9 | 0.3 |
| 4 | 1.1 | 0.3 | 0.2 | 0.2 |
| 5 | 0.3 | 0.2 | 0.2 | 0.1 |
| 6 | 3.2 | 1.8 | 2.6 | 1.3 |
| 7 | 0.4 | 0.2 | 0.4 | 0.3 |
| 8 | 2.2 | 0.2 | 0.2 | 0.2 |
| 9 | 0.3 | 0.2 | 0.3 | 0.1 |
| 10 | 0.7 | 0.1 | 0.6 | 0.4 |
| 11 | 0.4 | 0.1 | 0.8 | 0.3 |
| 12 | 0.2 | 0.9 | 0.2 | 0.1 |
| 13 | 0.9 | 0.2 | 0.3 | 0.2 |
| 14 | 0.1 | 0.9 | 0.2 | 0.3 |
| 15 | 1.0 | 0.3 | 0.8 | 0.2 |
| 16 | 3.9 | 0.7 | 0.3 | 0.2 |
| 17 | 0.3 | 0.1 | 0.3 | 0.1 |
| 18 | 0.3 | 0.1 | 0.2 | 0.1 |
| 19 | 0.8 | 0.2 | 0.3 | 0.1 |
| 20 | 3.8 | 2.0 | 1.7 | 0.5 |
| 21 | 0.4 | 0.2 | 0.3 | 0.1 |
| 22 | 0.8 | 0.2 | 0.6 | 0.4 |
| 23 | 0.3 | 0.1 | 0.1 | 0.1 |
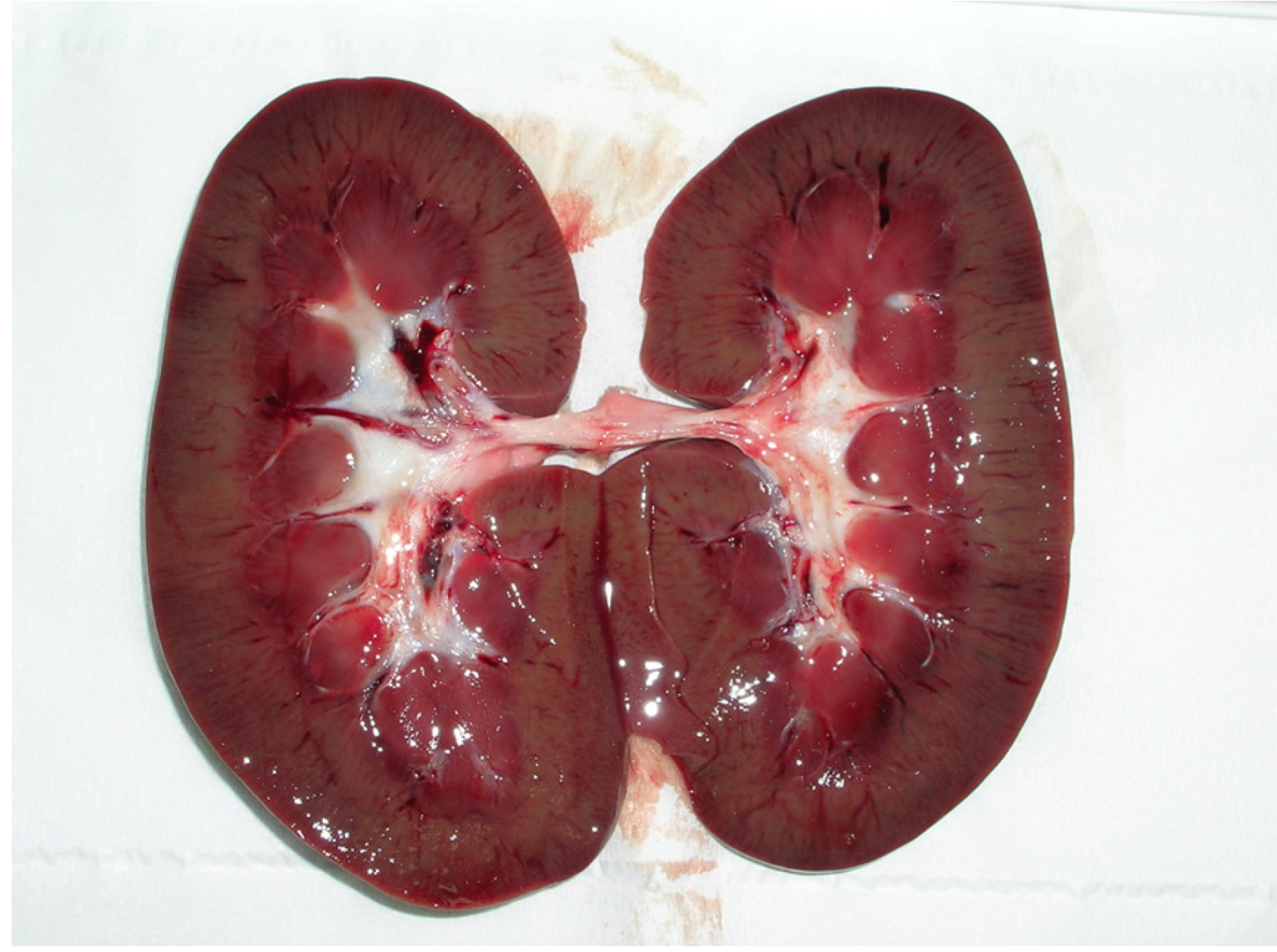
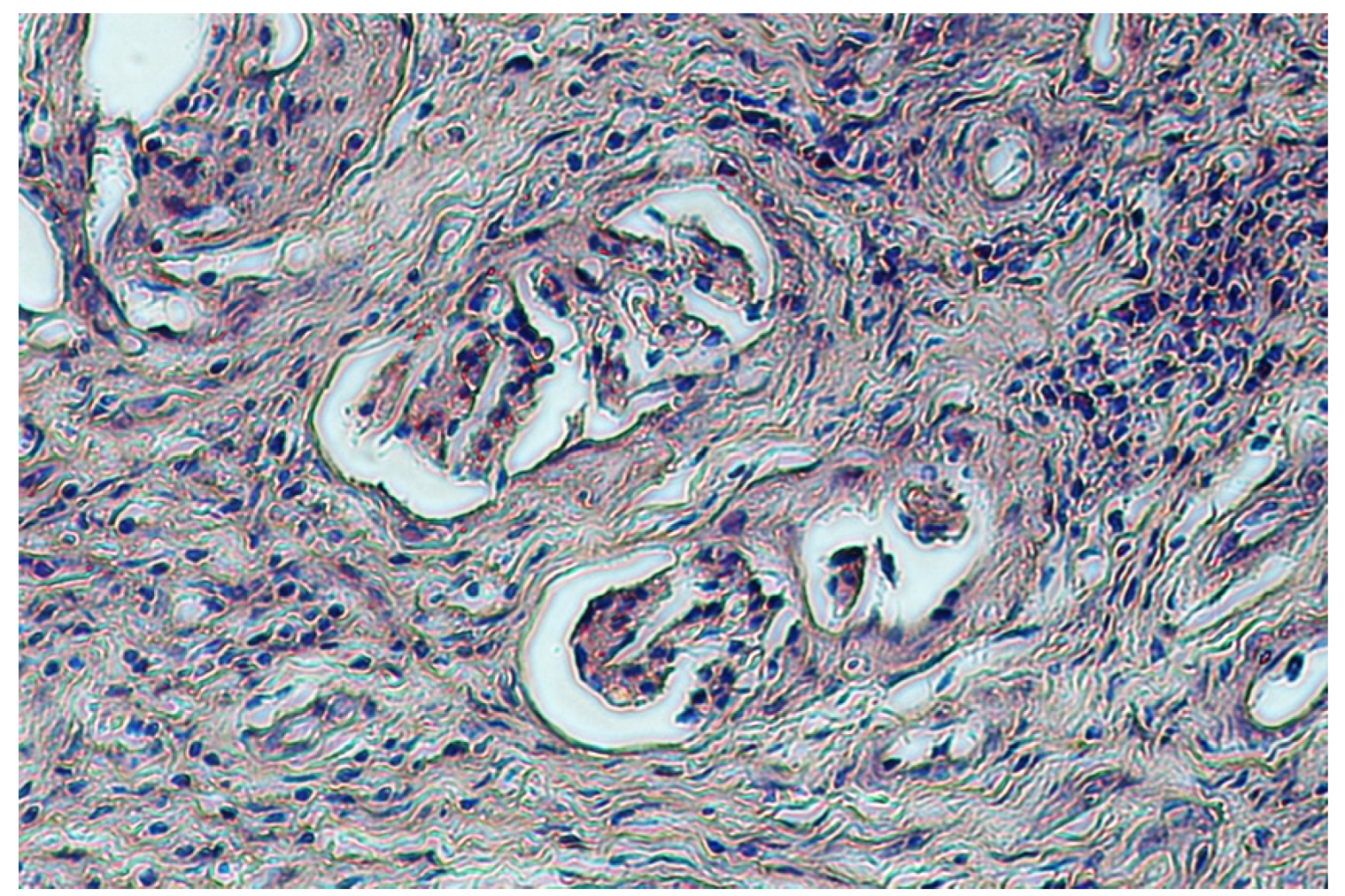


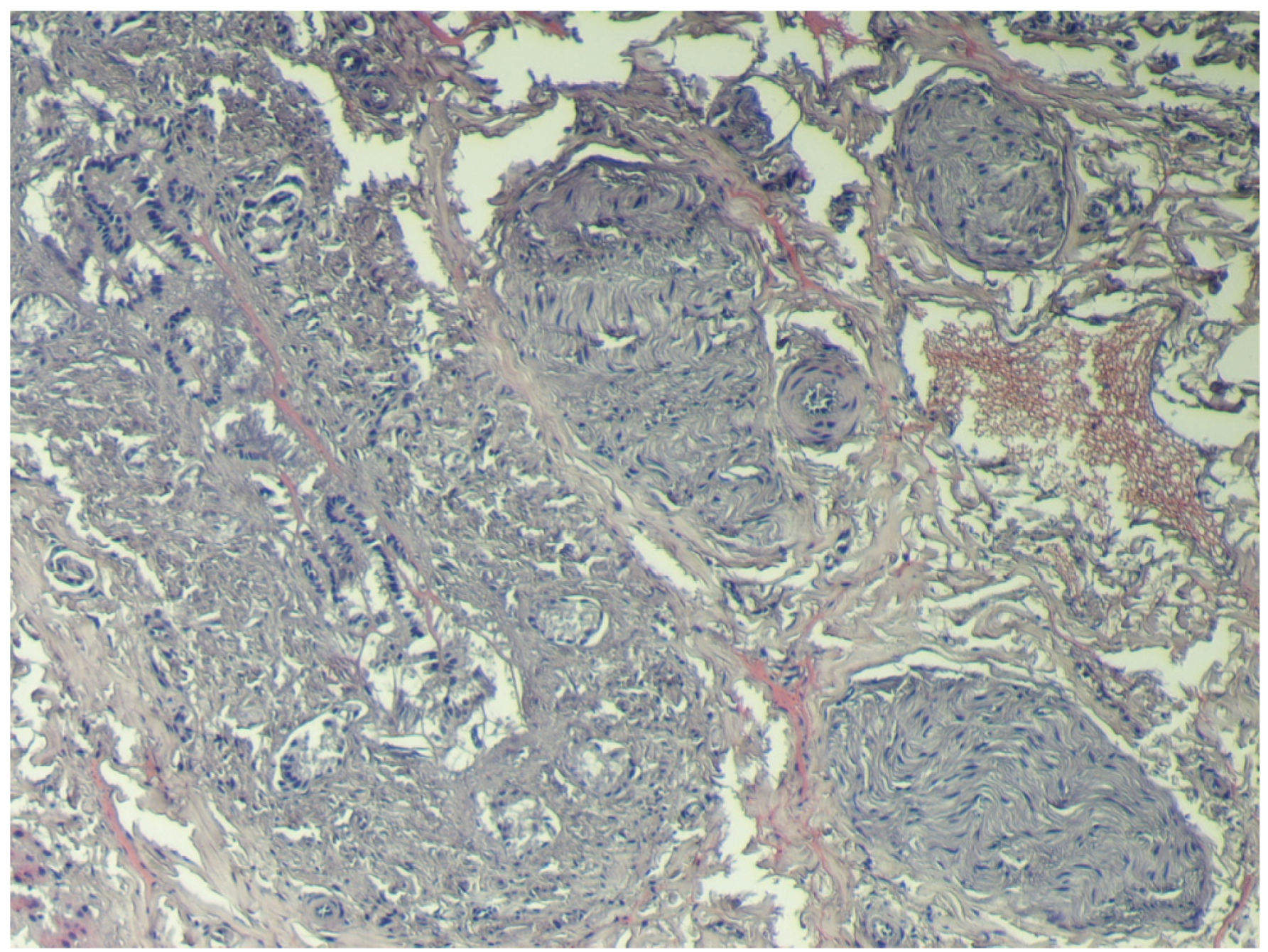

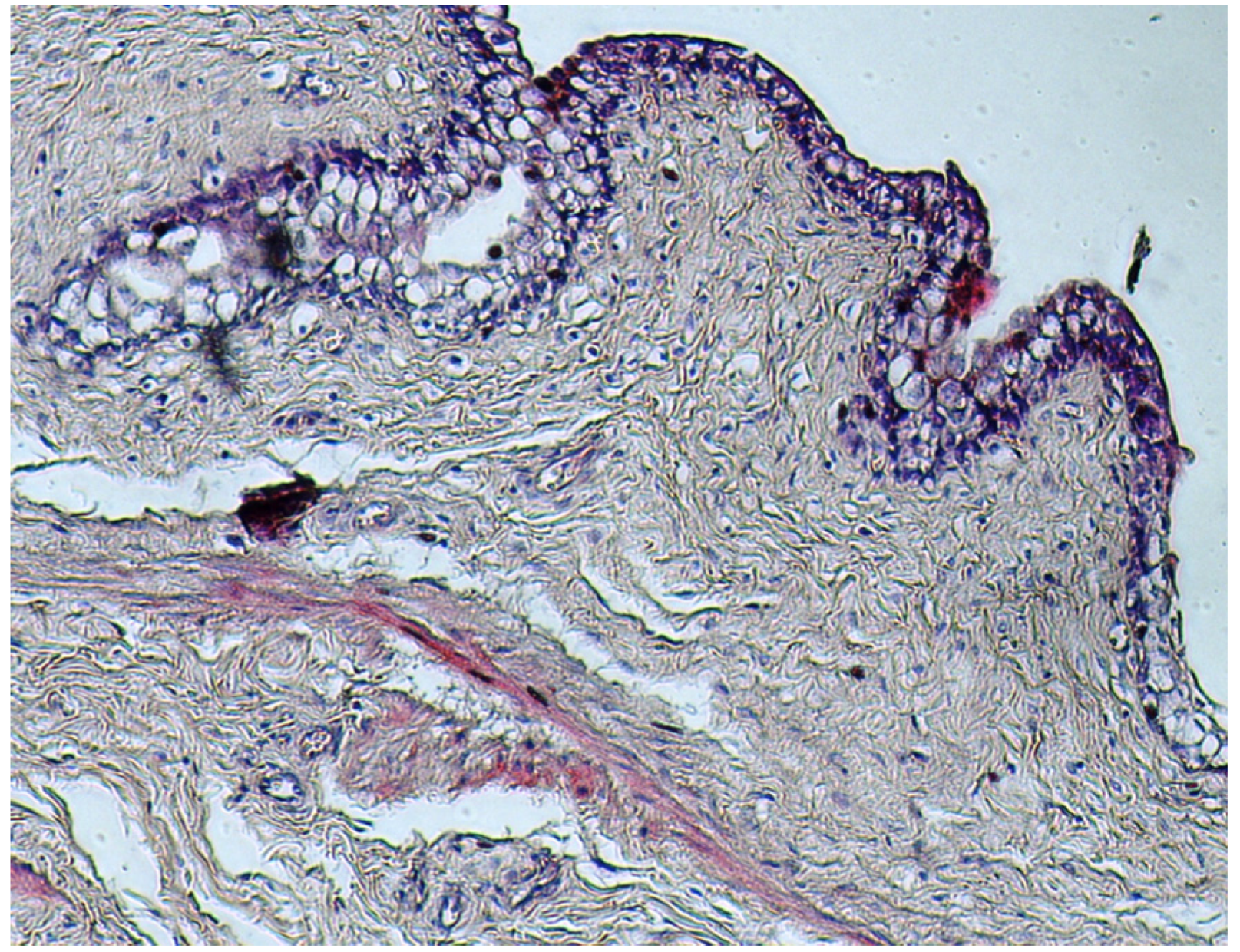
4. Discussion
5. Conclusions
References
- Romani, S.; Sacchetti, G.; Chaves López, C.; Pinnavaia, G.; Dalla Rosa, M. Screening on the occurrence of ochratoxin A in green coffee beans of different origins and types. J. Agric. Food Chem. 2000, 48, 3616–3619. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Review on Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Kuiper-Godman, T.; Hilts, C.; Billiard, S.M.; Kiparissis, Y.; Richard, I.D.; Hayward, S. Health risk assessment of ochratoxin A for all age-sex strata in a market economy. Food Addit. Contam. A 2010, 27, 212–240. [Google Scholar]
- Peterson, S.W. Phylogenetic Relationships in Aspergillus Based on rDNA Sequence Analysis. In Classification of Penicillium and Aspergillus: Integration of Modern Taxonomic Methods; Samson, R.A., Pitt, J.R., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 323–356. [Google Scholar]
- Abarca, M.L.; Bragulat, M.R.; Castellà, G.; Cabañes, F.J. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 1994, 60, 2650–2652. [Google Scholar]
- Téren, J.; Varga, J.; Hamari, Z.; Rinyu, E.; Kevei, F. Immunochemical detection of ochratoxin A in black Aspergillus strains. Mycopathologia 1996, 134, 171–176. [Google Scholar] [CrossRef]
- Varga, J.; Kevei, E.; Rinyu, E.; Téren, J.; Kozakiewicz, Z. Ochratoxin production by Aspergillus species. Appl. Environ. Microbiol. 1996, 62, 4461–4464. [Google Scholar]
- Krogh, P. Mycotoxic Nephropathy. In Advances in Veterinary Science and Comparative; Academic Press: New York, NY, USA, 1976; pp. 147–170. [Google Scholar]
- Szczech, G.M.; Carlton, W.W.; Tuite, J. Ochratoxin A toxicosis in swine. Vet. Pathol. 1973, 10, 347–364. [Google Scholar] [CrossRef]
- IARC, Some Naturally Occurring Substances; Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In IARC Monographs on the Evolution of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Geneva, Switzerland, 1993; Volume 57, pp. 489–521.
- Krogh, P. Ochratoxins: Occurrence, Biological Effects and Casual Role in Disease. In Natural Toxins; Eaker, D., Wadstrom, T., Eds.; Pergamon Press: Oxford, UK, 1980; pp. 673–680. [Google Scholar]
- Marquardt, R.R.; Frohlich, A.A. A review of recent advances in understanding ochratoxicosis. J. Animal Sci. 1992, 70, 3968–3988. [Google Scholar]
- Pfohl-Leszkowicz, A.; Petkova-Bocharova, T.; Chernozemsky, I.N.; Castegnaro, M. Balkan endemic nephropathy and associated urinary tract tumours: A review on aetiological causes and the potential role of mycotoxins. Food Addit. Contam. 2002, 19, 282–302. [Google Scholar]
- Radavanovic, S.; Jankovic, S.; Jeremovic, J. Incidence of tumours of urinary organs in focus of Balkan endemic nephropathy. Kidney Int. 1991, 40, 75–77. [Google Scholar]
- Stoev, S.D.; Hald, B.; Mantle, P.G. Porcine nephropathy in Bulgaria: A progressive syndrome of complex of uncertain (mycotoxin) etiology. Vet. Rec. 1998, 142, 190–194. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food. Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef]
- Pardo, E.; Marin, S.; Sanchis, V.; Ramos, A.J. Prediction of fungal growth and ochratoxin A production by Aspergillus ochraceus on irradiated barley grain as influenced by temperature and water activity. Int. J. Food Microbiol. 2004, 95, 79–88. [Google Scholar] [CrossRef]
- Lindblad, M.; Johnsson, P.; Jonsson, N.; Lindqvist, R.; Olsen, M. Predicting noncompliant levels of ochratoxin A in cereal grain from Penicillium verrucosum counts. J. Appl. Microbiol. 2004, 97, 609–616. [Google Scholar] [CrossRef]
- Jorgensen, K. Survey of pork, poultry, coffee, beer and pulses for ochratoxin A. Food Addit. Contam. 1998, 15, 550–554. [Google Scholar] [CrossRef]
- Alexander, J.; Autrup, H.; Bard, D.; Benford, D.; Carere, A.; Costa, L.G.; Cravedi, J.; di Domenico, A.; Fanelli, R.; Fink-Gremmels, J.; et al. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ochratoxin A in food. EFSA J. 2006, 365, 1–56. [Google Scholar]
- Bozzo, G.; Ceci, E.; Pinto, P.; Bonerba, E.; Martella, V.; Terio, E.; Tantillo, G. Ochratoxin A in avicultural meat production: Chemical and histological effects. World Mycotoxin J. 2009, 2, 61–69. [Google Scholar] [CrossRef]
- Stefanaki, I.; Foufa, E.; Tsatsou-Dritsa, A.; Dais, P. Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Addit. Contam. 2003, 20, 74–83. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Official Journal of the European Union: Brussels, Belgum, 2006.
- Italian Ministry of Health Circular No 10; Italian Ministry of Health: Rome, Italy, 1999.
- Ceci, E.; Bozzo, G.; Bonerba, E.; di Pinto, A.; Tantillo, G. Ochratoxin A detection by HPLC in target tissues of swine and cytological and histological analysis. Food Chem. 2007, 105, 364–368. [Google Scholar] [CrossRef]
- Monaci, L.; Tantillo, G.; Palmisano, F. Determination of ochratoxin A in pig tissue by liquid-liquid extraction/partition and high performance liquid chromatography. Anal. Bioanal. Chem. 2004, 378, 1777–1782. [Google Scholar] [CrossRef]
- Matrella, R.; Monaci, L.; Milillo, M.A.; Palmisano, F.; Tantillo, M.G. Ochratoxin A determination in paired kidneys and muscle samples from swine slaughtered in southern Italy. Food Control 2006, 17, 114–117. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 669/2009 implementing Regulation (EC) No. 882/2004 of the European Parliament and of the Council as Regards the Increased Level of Official Controls on Imports of Certain Feed and Food of Non-Animal Origin and Amending Decision 2006/504/EC; Official Journal of the European Union: Brussels, Belgum, 2004.
- Stoev, S.D.; Paskalev, M.; Mac Donald, S.; Mantle, P.G. Experimental one year ochratoxin A toxicosis in pigs. Exp. Toxicol. Pathol. 2002, 53, 481–487. [Google Scholar] [CrossRef]
- Milicevic, D.; Juric, V.; Mandic, M.; Dordevic, M. The presence of ochratoxin A residue in blood plasma of slaughtered swine. Zbornik Matice Srpske Za Prirodne Nauke 2007, 113, 55–62. [Google Scholar]
- Regulation (EC) No. 178/2002 of the European Parliament and of the Council Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety; Official Journal of the European Union: Brussels, Belgum, 2002.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bozzo, G.; Ceci, E.; Bonerba, E.; Di Pinto, A.; Tantillo, G.; De Giglio, E. Occurrence of Ochratoxin A in the Wild Boar (Sus scrofa): Chemical and Histological Analysis. Toxins 2012, 4, 1440-1450. https://doi.org/10.3390/toxins4121440
Bozzo G, Ceci E, Bonerba E, Di Pinto A, Tantillo G, De Giglio E. Occurrence of Ochratoxin A in the Wild Boar (Sus scrofa): Chemical and Histological Analysis. Toxins. 2012; 4(12):1440-1450. https://doi.org/10.3390/toxins4121440
Chicago/Turabian StyleBozzo, Giancarlo, Edmondo Ceci, Elisabetta Bonerba, Angela Di Pinto, Giuseppina Tantillo, and Elvira De Giglio. 2012. "Occurrence of Ochratoxin A in the Wild Boar (Sus scrofa): Chemical and Histological Analysis" Toxins 4, no. 12: 1440-1450. https://doi.org/10.3390/toxins4121440







