Emerging Opportunities for Serotypes of Botulinum Neurotoxins
Abstract
:1. Introduction
2. Clostridium botulinum and Botulism: Botulinum Neurotoxin as a Cause of Disease, and as a Medication
3. Botulinum Neurotoxin Structure Serotypes and Subtypes
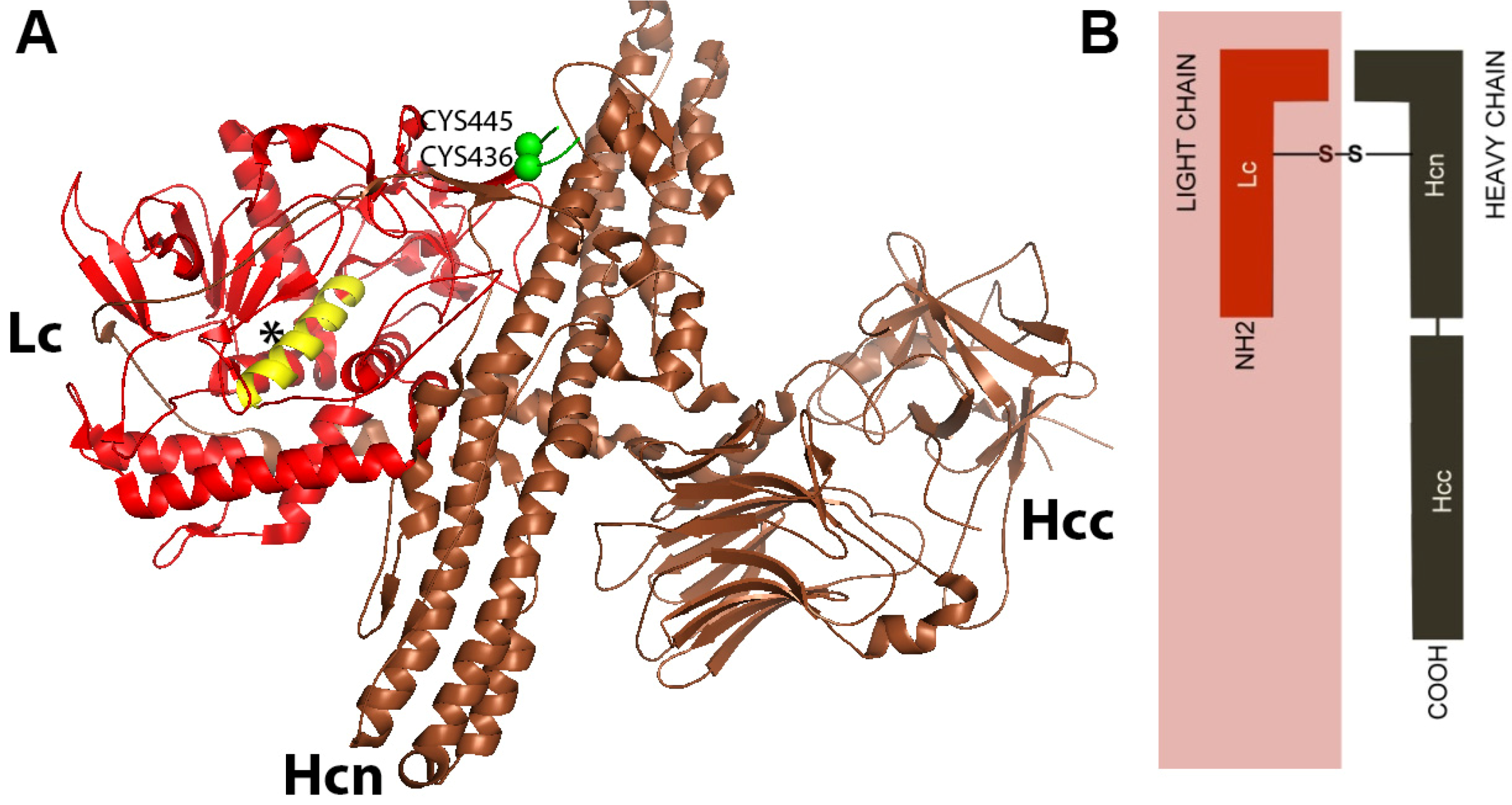
3.1. Structure

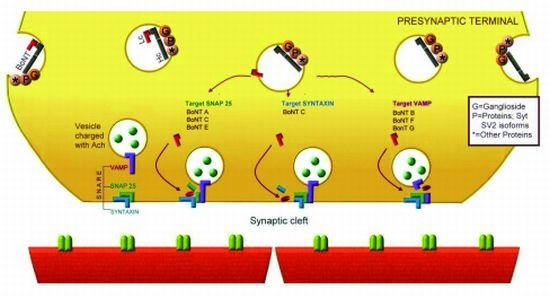
3.2. Serotypes
| Group | Toxin types produced | Additional strains closely related Clostridial species |
|---|---|---|
| I | A, Proteolytic B, F | C. Sporogenes |
| II | E, Non-proteolytic B, F | C. Novyi, C. haemolyticum |
| III | C, D | C. Subterminale, C. Proteolyticus |
| IV | G | C. Argentinense, C. Schirmacherense |
3.3. Subtypes of Neurotoxin Serotypes
4. Measuring the Potency of the Botulinum Neurotoxins
5. Mechanism of Action of Botulinum Neurotoxins
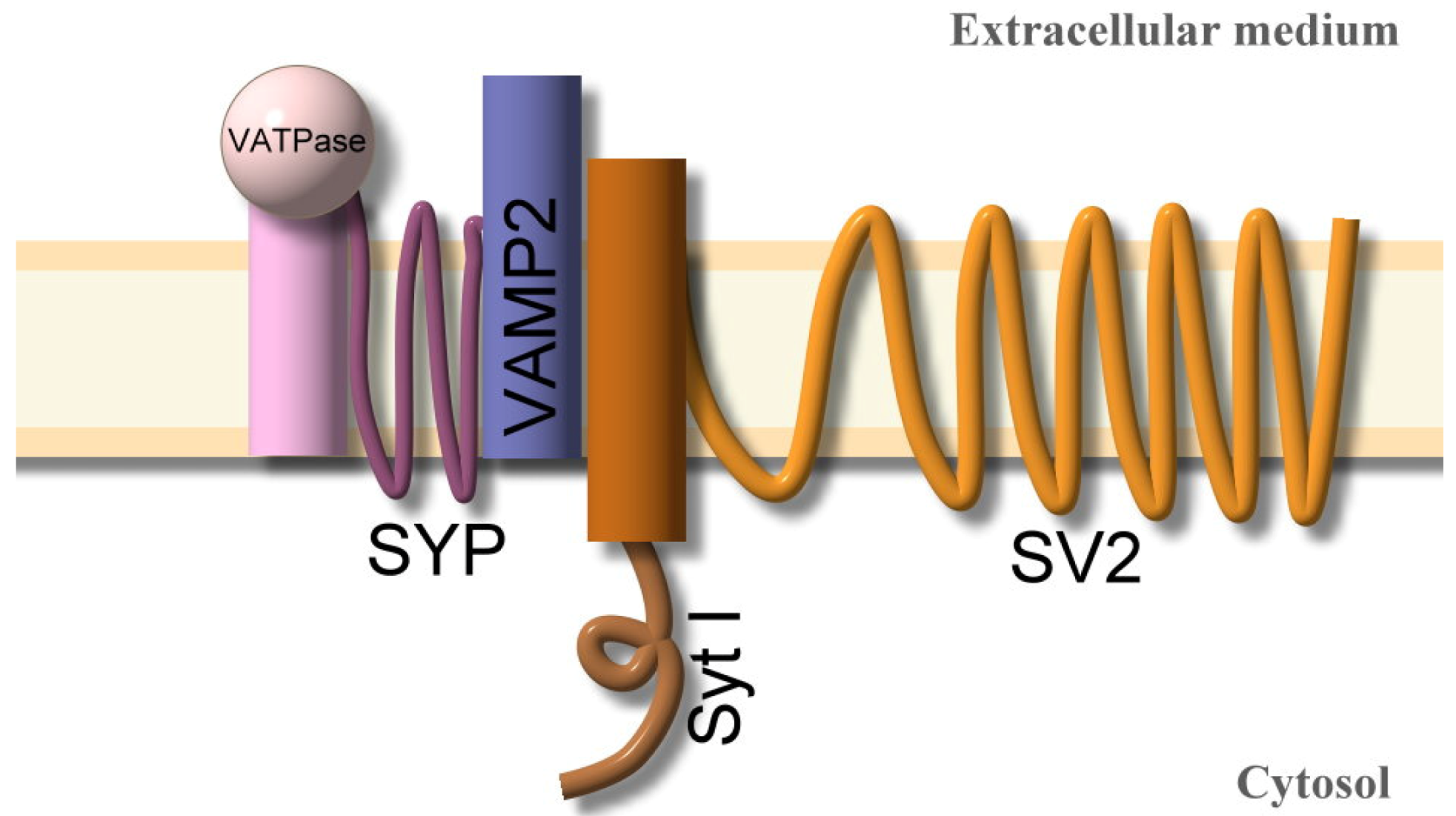
5.1. Binding
| BoNT (Receptors) | BoNT serotype Binding and Affinity to Presynaptic proteins | Neuromuscular junction [51,52] | Hippocampus [52,53] cortex excitatory neurons | Hippocampus [53] cortex inhibitory neurons | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A [54,55] | B [56,57] | C [58] | D [58,59] | E [49,60] | F [61] | G [62,63] | |||||
| Proteins | Syt-I | + | + | - | + | - | + | + | |||
| Syt-II | + | - | + | + | + | ||||||
| SV2A | + | - | + | + | + | + | + | + | |||
| SV2B | + | - | + | + | + | + | + | - | |||
| SV2C | + | - | + | - | + | + | - | + | |||
| Gangliosides | GD1a | + | + | + | |||||||
| GD1b | + | + | - | ||||||||
| GT1b | + | + | + | + | + | + | |||||
| GQ1a | |||||||||||
| GM1a | + | - | |||||||||
| GM3 | + | ||||||||||
5.2. Internalization
5.3. Membrane Translocation
5.4. Proteolysis of Specific SNARE Proteins
| BoNT Type | C. botulinum | Laboratory measurement | Affects | SNARE Cleavage target | Commercial | |||
|---|---|---|---|---|---|---|---|---|
| tSNARE | vSNARE | |||||||
| Potency LD50/mg | Duration * | SNAP 25 | Syntaxin | VAMP | ||||
| A | Group I | 3.7 × 107 | 31 days | Human | + | - | - | Yes |
| B | Group I-II | 1.2 × 107 | 10 days | Human | - | - | + | Yes |
| C | Group III | 5.8 × 106 | 25 days | Animal | + | + | - | No |
| D | Group III | 3.1 × 107 | Animal | - | - | + | No | |
| E | Group II | 3 × 107 | 0.8 days | Human, fish | + | - | - | No |
| F | Group I-II | 3.6 × 106 | 2 days | Human | - | - | + | No |
| G | Group IV | 4 × 106 | Human | - | - | + | No | |
6. Current Commercially Available Neurotoxins

| Physiochemical Characteristics of BoNT formulations | ||||
|---|---|---|---|---|
| OnabotulinumtoxinA (BOTOX®) | AbobotulinumtoxinA (DYSPORT®) | RimabotulinumtoxinB (MYOBLOC®) | IncobotulinumtoxinA (XEOMIN®) | |
| Date introduced | 1989 | 1991 | 2000 | 2005 |
| Serotype | BoNT A | BoNT A | BoNT B | BoNT A |
| Total weight kDa | 900 | >500 | 700 | 150 |
| Excipients | Sodium chloride | Lactose | Sodium chloride | Sucrose |
| Albumin | Albumin | Sodium succinate | Albumin | |
| Albumin | ||||
| Final formulation | Vacuum dried | Freeze | Solution | Freeze dried |
| pH value | 7 | 7 | 5.6 | 7 |
| Trading name® and Non proprietary name | Year | FDA Approved | OFF Label Indications |
|---|---|---|---|
| BOTOX® OnabotulinumtoxinA Units/vial: 50, 100, 200 | 1989 | Strabismus Blepharospasm | |
| Disease related to facial nerve | Focal hand dystonia | ||
| 2000 | Cervical dystonia | ||
| 2002 | Glabellar lines | ||
| 2004 | Primary axillary hyperhidrosis | Lower Limb Spasticity | |
| 2010 | Chronic Migraine Upper limb spasticity | Myokymia | |
| 2011 | Hyperactive bladder | ||
| DYSPORT® AbobotulinumtoxinA Units/vial:300, 500 | 2009 | Cervical dystonia Glabellar lines | Tensional Headache |
| Head tremor | |||
| XEOMIN® IncobotulinumtoxinA Units/vial:50, 100 | 2010 | Cervical Dystonia | |
| Blepharospasm | |||
| 2011 | Glabellar lines | Intractable Hyperkinesias | |
| MYOBLOC®/NEUROBLOC® RimabotulinumtoxinB Units/vial:2500, 5000, 10000 | 2010 | Blepharospasm | |
| Cervical Dystonia | |||
| 2011 | Glabellar lines |
7. Immunogenicity

8. Future Challenges and Opportunities for Use of Botulinum Neurotoxins
9. Conclusions
Acknowledgments
Conflict of Interest
References
- Jankovic, J. Disease-oriented approach to botulinum toxin use. Toxicon 2009, 54, 614–623. [Google Scholar] [CrossRef]
- Olesen, J.; Tfelt-Hansen, P. Licence for Botox in so-called chronic migraine. Lancet 2010, 376, 1825–1826, discussion 1826. [Google Scholar]
- FDA approves Botox to treat specific form of urinary incontinence. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm269509.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=BOTOX&utm_content=10 (accessed on 24 October 2011).
- Hasler, W.L. The expanding spectrum of clinical uses for botulinum toxin: Healing of chronic anal fissures. Gastroenterology 1999, 116, 221–223. [Google Scholar] [CrossRef]
- Dykstra, D.D.; Sidi, A.A. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: A double-blind study. Arch. Phys. Med. Rehabil. 1990, 71, 24–26. [Google Scholar]
- Snow, B.J.; Tsui, J.K.; Bhatt, M.H.; Varelas, M.; Hashimoto, S.A.; Calne, D.B. Treatment of spasticity with botulinum toxin: a double-blind study. Ann. Neurol. 1990, 28, 512–515. [Google Scholar]
- Das, T.K.; Park, D.M. Effect of treatment with botulinum toxin on spasticity. Postgrad. Med. J. 1989, 65, 208–210. [Google Scholar] [CrossRef]
- Rollnik, J.D.; Tanneberger, O.; Schubert, M.; Schneider, U.; Dengler, R. Treatment of tension-type headache with botulinum toxin type A: A double-blind, placebo-controlled study. Headache 2000, 40, 300–305. [Google Scholar] [CrossRef]
- Carruthers, A.; Carruthers, J. Botulinum toxin products overview. Skin Ther. Lett. 2008, 13, 1–4. [Google Scholar]
- Erbguth, F.J. Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov. Disord. 2004, 19, S2–S6. [Google Scholar] [CrossRef]
- Horowitz, B.Z. Type E botulism. Clin. Toxicol. (Phila) 2010, 48, 880–895. [Google Scholar] [CrossRef]
- Shapiro, R.L.; Hatheway, C.; Swerdlow, D.L. Botulism in the United States: A clinical and epidemiologic review. Ann. Intern. Med. 1998, 129, 221–228. [Google Scholar]
- Hambleton, P. Clostridium botulinum toxins: A general review of involvement in disease, structure, mode of action and preparation for clinical use. J. Neurol. 1992, 239, 16–20. [Google Scholar] [CrossRef]
- Morris, J.G., Jr.; Hatheway, C.L. Botulism. In Infectious Diseases, 3rd; Hoeprich, P.D., Ed.; Harper & Row: Philadelphia, PA, USA, 1983. [Google Scholar]
- Botulism—Information from the World Health Organization. J. Environ. Health. 2003, 65, 51–52.
- Dressler, D.; Adib Saberi, F. Botulinum toxin: Mechanisms of action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar]
- Minton, N.P. Molecular genetics of clostridial neurotoxins. Curr. Top Microbiol. Immunol. 1995, 195, 161–194. [Google Scholar]
- Swaminathan, S.; Eswaramoorthy, S. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat. Struct. Biol. 2000, 7, 693–699. [Google Scholar] [CrossRef]
- Eswaramoorthy, S.; Kumaran, D.; Keller, J.; Swaminathan, S. Role of metals in the biological activity of Clostridium botulinum neurotoxins. Biochemistry 2004, 43, 2209–2216. [Google Scholar] [CrossRef]
- Mushrush, D.J.; Koteiche, H.A.; Sammons, M.A.; Link, A.J.; McHaourab, H.S.; Lacy, D.B. Studies of the mechanistic details of the pH-dependent association of botulinum neurotoxin with membranes. J. Biol. Chem. 2011, 286, 27011–27018. [Google Scholar]
- Greensboro Xeomin®: Summary of product characteristics. Merz Pharmaceuticals 2010. Available online: http://www.medicines.org.uk/emc/document.aspx?documentId=20666 (accessed on 10 September 2011).
- Marvaud, J.C.; Eisel, U.; Binz, T.; Niemann, H.; Popoff, M.R. TetR is a positive regulator of the tetanus toxin gene in Clostridium tetani and is homologous to botR. Infect. Immun. 1998, 66, 5698–5702. [Google Scholar]
- Johnson, E.A.; Bradshaw, M. Clostridium botulinum and its neurotoxins: A metabolic and cellular perspective. Toxicon 2001, 39, 1703–1722. [Google Scholar] [CrossRef]
- Schantz, E.J.; Johnson, E.A. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol. Rev. 1992, 56, 80–99. [Google Scholar]
- Sakaguchi, G. Clostridium botulinum toxins. Pharmacol. Ther. 1982, 19, 165–194. [Google Scholar] [CrossRef]
- Chen, F.; Kuziemko, G.M.; Stevens, R.C. Biophysical characterization of the stability of the 150-kilodalton botulinum toxin, the nontoxic component, and the 900-kilodalton botulinum toxin complex species. Infect. Immun. 1998, 66, 2420–2425. [Google Scholar]
- Grein, S.; Mander, G.J.; Taylor, H.V. XEOMIN is stable without refrigeration: Complexing proteins are not required for stability of botulinum neurotoxin type A preparations. Toxicon 2008, 51, 13. [Google Scholar]
- Hill, K.K.; Smith, T.J.; Helma, C.H.; Ticknor, L.O.; Foley, B.T.; Svensson, R.T.; Brown, J.L.; Johnson, E.A.; Smith, L.A.; Okinaka, R.T.; et al. Genetic diversity among Botulinum Neurotoxin-producing clostridial strains. J. Bacteriol. 2007, 189, 818–832. [Google Scholar]
- Skarin, H.; Hafstrom, T.; Westerberg, J.; Segerman, B. Clostridium botulinum group III: A group with dual identity shaped by plasmids, phages and mobile elements. BMC Genomics 2011, 12, 185. [Google Scholar] [CrossRef]
- Collins, M.D.; East, A.K. Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. J. Appl. Microbiol. 1998, 84, 5–17. [Google Scholar] [CrossRef]
- Shukla, H.D.; Sharma, S.K. Clostridium botulinum: A bug with beauty and weapon. Crit. Rev. Microbiol. 2005, 31, 11–18. [Google Scholar] [CrossRef]
- Lindstrom, M.; Keto, R.; Markkula, A.; Nevas, M.; Hielm, S.; Korkeala, H. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 2001, 67, 5694–5699. [Google Scholar] [CrossRef]
- Montecucco, C.; Schiavo, G.; Dasgupta, B.R. Effect of pH on the interaction of botulinum neurotoxins A, B and E with liposomes. Biochem. J. 1989, 259, 47–53. [Google Scholar]
- Jacobson, M.J.; Lin, G.; Tepp, W.; Dupuy, J.; Stenmark, P.; Stevens, R.C.; Johnson, E.A. Purification, modeling, and analysis of botulinum neurotoxin subtype A5 (BoNT/A5) from Clostridium botulinum strain A661222. Appl. Environ. Microbiol. 2011, 77, 4217–4222. [Google Scholar] [CrossRef]
- Umeda, K.; Seto, Y.; Kohda, T.; Mukamoto, M.; Kozaki, S. Genetic characterization of Clostridium botulinum associated with type B infant botulism in Japan. J. Clin. Microbiol. 2009, 47, 2720–2728. [Google Scholar] [CrossRef]
- Henkel, J.S.; Jacobson, M.; Tepp, W.; Pier, C.; Johnson, E.A.; Barbieri, J.T. Catalytic properties of botulinum neurotoxin subtypes A3 and A4. Biochemistry 2009, 48, 2522–2528. [Google Scholar] [CrossRef]
- Pier, C.L.; Chen, C.; Tepp, W.H.; Lin, G.; Janda, K.D.; Barbieri, J.T.; Pellett, S.; Johnson, E.A. Botulinum neurotoxin subtype A2 enters neuronal cells faster than subtype A1. FEBS Lett. 2011, 585, 199–206. [Google Scholar] [CrossRef]
- Chen, Y.; Korkeala, H.; Aarnikunnas, J.; Lindstrom, M. Sequencing the botulinum neurotoxin gene and related genes in Clostridium botulinum type E strains reveals orfx3 and a novel type E neurotoxin subtype. J. Bacteriol. 2007, 189, 8643–8650. [Google Scholar] [CrossRef]
- Kubota, T.; Watanabe, T.; Yokosawa, N.; Tsuzuki, K.; Indoh, T.; Moriishi, K.; Sanda, K.; Maki, Y.; Inoue, K.; Fujii, N. Epitope regions in the heavy chain of Clostridium botulinum type E neurotoxin recognized by monoclonal antibodies. Appl. Environ. Microbiol. 1997, 63, 1214–1218. [Google Scholar]
- Lacy, D.B.; Stevens, R.C. Sequence homology and structural analysis of the clostridial neurotoxins. J. Mol. Biol. 1999, 291, 1091–1104. [Google Scholar] [CrossRef]
- Smith, T.J.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Tsai, R.; Laporte, S.L.; Tepp, W.H.; Bradshaw, M.; Johnson, E.A.; Smith, L.A.; et al. Sequence variation within botulinum neurotoxin serotypes impacts antibody binding and neutralization. Infect. Immun. 2005, 73, 5450–5457. [Google Scholar] [CrossRef]
- Sesardic, D.; Das, R.E.; Corbel, M.J. Botulinum toxin. J. R. Soc. Med. 1994, 87, 307. [Google Scholar]
- McLellan, K.; Das, R.E.; Ekong, T.A.; Sesardic, D. Therapeutic botulinum type A toxin: Factors affecting potency. Toxicon 1996, 34, 975–985. [Google Scholar] [CrossRef]
- Sesardic, D.; Jones, R.G.; Leung, T.; Alsop, T.; Tierney, R. Detection of antibodies against botulinum toxins. Mov. Disord. 2004, 19, S85–S91. [Google Scholar] [CrossRef]
- Sesardic, D.; McLellan, K.; Ekong, T.A.; Das, R.G. Refinement and validation of an alternative bioassay for potency testing of therapeutic botulinum type A toxin. Pharmacol. Toxicol. 1996, 78, 283–288. [Google Scholar] [CrossRef]
- Golan, D.E. Principles of Autonomic and Pheripheral Nervous System Pharmacology. In Principles of Pharmacology; The Physiopathology of Drugs, 2nd; Wilkins, L.W., Ed.; Lippincott Williams: Philadelphia, PA, USA, 2008; pp. 107–125. [Google Scholar]
- Verderio, C.; Rossetto, O.; Grumelli, C.; Frassoni, C.; Montecucco, C.; Matteoli, M. Entering neurons: Botulinum toxins and synaptic vesicle recycling. EMBO Rep. 2006, 7, 995–999. [Google Scholar] [CrossRef]
- Dong, M.; Liu, H.; Tepp, W.H.; Johnson, E.A.; Janz, R.; Chapman, E.R. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol. Biol. Cell 2008, 19, 5226–5237. [Google Scholar] [CrossRef]
- Dolly, J.O.; Lawrence, G.W.; Meng, J.; Wang, J.; Ovsepian, S.V. Neuro-exocytosis: botulinum toxins as inhibitory probes and versatile therapeutics. Curr. Opin. Pharmacol. 2009, 9, 326–335. [Google Scholar] [CrossRef]
- Li, G.; Rungger-Brandle, E.; Just, I.; Jonas, J.C.; Aktories, K.; Wollheim, C.B. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol. Biol. Cell 1994, 5, 1199–1213. [Google Scholar]
- Marqueze, B.; Boudier, J.A.; Mizuta, M.; Inagaki, N.; Seino, S.; Seagar, M. Cellular localization of synaptotagmin I, II, and III mRNAs in the central nervous system and pituitary and adrenal glands of the rat. J. Neurosci. 1995, 15, 4906–4917. [Google Scholar]
- Geppert, M.; Archer, B.T., III; Sudhof, T. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J. Biol. Chem. 1991, 266, 13548–13552. [Google Scholar]
- Dong, M.; Yeh, F.; Tepp, W.H.; Dean, C.; Johnson, E.A.; Janz, R.; Chapman, E.R. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006, 312, 592–596. [Google Scholar] [CrossRef]
- Mahrhold, S.; Rummel, A.; Bigalke, H.; Davletov, B.; Binz, T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006, 580, 2011–2014. [Google Scholar] [CrossRef]
- Dong, M.; Richards, D.A.; Goodnough, M.C.; Tepp, W.H.; Johnson, E.A.; Chapman, E.R. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 2003, 162, 1293–1303. [Google Scholar] [CrossRef]
- Nishiki, T.; Tokuyama, Y.; Kamata, Y.; Nemoto, Y.; Yoshida, A.; Sato, K.; Sekiguchi, M.; Takahashi, M.; Kozaki, S. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 1996, 378, 253–257. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Kohda, T.; Mukamoto, M.; Takeuchi, K.; Ihara, H.; Saito, M.; Kozaki, S. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 2005, 280, 35164–35171. [Google Scholar]
- Peng, L.; Tepp, W.H.; Johnson, E.A.; Dong, M. Botulinum neurotoxin D uses synaptic vesicle protein SV2 and gangliosides as receptors. PLoS Pathog. 2011, 7, e1002008. [Google Scholar] [CrossRef]
- Rummel, A.; Hafner, K.; Mahrhold, S.; Darashchonak, N.; Holt, M.; Jahn, R.; Beermann, S.; Karnath, T.; Bigalke, H.; Binz, T. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J. Neurochem. 2009, 110, 1942–1954. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, C.; Barbieri, J.T.; Kim, J.J.; Baldwin, M.R. Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F. Biochemistry 2009, 48, 5631–5641. [Google Scholar]
- Rummel, A.; Karnath, T.; Henke, T.; Bigalke, H.; Binz, T. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J. Biol. Chem. 2004, 279, 30865–30870. [Google Scholar]
- Schmitt, J.; Karalewitz, A.; Benefield, D.A.; Mushrush, D.J.; Pruitt, R.N.; Spiller, B.W.; Barbieri, J.T.; Lacy, D.B. Structural analysis of botulinum neurotoxin type G receptor binding. Biochemistry 2010, 49, 5200–5205. [Google Scholar] [CrossRef]
- Hansson, H.A.; Holmgren, J.; Svennerholm, L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc. Natl. Acad. Sci. USA 1977, 74, 3782–3786. [Google Scholar] [CrossRef]
- Bullens, R.W.; O’Hanlon, G.M.; Wagner, E.; Molenaar, P.C.; Furukawa, K.; Plomp, J.J.; Willison, H.J. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J. Neurosci. 2002, 22, 6876–6884. [Google Scholar]
- Kitamura, M. Identification of RNA species in the RNA-toxin complex and structure of the complex in Clostridium botulinum type E. Biochem. Biophys. Res. Commun. 2002, 291, 154–157. [Google Scholar] [CrossRef]
- Yowler, B.C.; Kensinger, R.D.; Schengrund, C.L. Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J. Biol. Chem. 2002, 277, 32815–32819. [Google Scholar]
- Keller, J.E.; Cai, F.; Neale, E.A. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry 2004, 43, 526–532. [Google Scholar] [CrossRef]
- Montecucco, C.; Rossetto, O.; Schiavo, G. Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol. 2004, 12, 442–446. [Google Scholar] [CrossRef]
- Nuemket, N.; Tanaka, Y.; Tsukamoto, K.; Tsuji, T.; Nakamura, K.; Kozaki, S.; Yao, M.; Tanaka, I. Structural and mutational analyses of the receptor binding domain of botulinum D/C mosaic neurotoxin: Insight into the ganglioside binding mechanism. Biochem. Biophys. Res. Commun. 2011, 411, 433–439. [Google Scholar] [CrossRef]
- Benson, M.A.; Fu, Z.; Kim, J.J.; Baldwin, M.R. Unique ganglioside recognition strategies for clostridial neurotoxins. J. Biol. Chem. 2011, 286, 34015–34022. [Google Scholar]
- Chai, Q.; Arndt, J.W.; Dong, M.; Tepp, W.H.; Johnson, E.A.; Chapman, E.R.; Stevens, R.C. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature 2006, 444, 1096–1100. [Google Scholar] [CrossRef]
- Poea-Guyon, S.; Amar, M.; Fossier, P.; Morel, N. Alternative splicing controls neuronal expression of v-ATPase subunit a1 and sorting to nerve terminals. J. Biol. Chem. 2006, 281, 17164–17172. [Google Scholar] [CrossRef]
- Sugita, S.; Janz, R.; Sudhof, T.C. Synaptogyrins regulate Ca2+-dependent exocytosis in PC12 cells. J. Biol. Chem. 1999, 274, 18893–18901. [Google Scholar]
- Lafourcade, C.; Sobo, K.; Kieffer-Jaquinod, S.; Garin, J.; van der Goot, F.G. Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization. PLoS One 2008, 3, e2758. [Google Scholar]
- Schiavo, G.; Boquet, P.; Dasgupta, B.R.; Montecucco, C. Membrane interactions of tetanus and botulinum neurotoxins: A photolabelling study with photoactivatable phospholipids. J. Physiol. (Paris) 1990, 84, 180–187. [Google Scholar]
- Sun, S.; Suresh, S.; Liu, H.; Tepp, W.H.; Johnson, E.A.; Edwardson, J.M.; Chapman, E.R. Receptor binding enables botulinum neurotoxin B to sense low pH for translocation channel assembly. Cell Host Microbe 2011, 10, 237–247. [Google Scholar] [CrossRef]
- Montal, M. Botulinum neurotoxin: A marvel of protein design. Annu. Rev. Biochem. 2010, 79, 591–617. [Google Scholar] [CrossRef]
- Swaminathan, S.; Eswaramoorthy, S. Crystallization and preliminary X-ray analysis of Clostridium botulinum neurotoxin type B. Acta Crystallogr. D Biol. Crystallogr. 2000, 56, 1024–1026. [Google Scholar] [CrossRef]
- Chen, S.; Barbieri, J.T. Multiple pocket recognition of SNAP25 by botulinum neurotoxin serotype E. J. Biol. Chem. 2007, 282, 25540–25547. [Google Scholar]
- Foran, P.G.; Mohammed, N.; Lisk, G.O.; Nagwaney, S.; Lawrence, G.W.; Johnson, E.; Smith, L.; Aoki, K.R.; Dolly, J.O. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem. 2003, 278, 1363–1371. [Google Scholar]
- Fernandez-Salas, E.; Ho, H.; Garay, P.; Steward, L.E.; Aoki, K.R. Is the light chain subcellular localization an important factor in botulinum toxin duration of action? Mov. Disord. 2004, 19, S23–S34. [Google Scholar] [CrossRef]
- Fernandez-Salas, E.; Steward, L.E.; Ho, H.; Garay, P.E.; Sun, S.W.; Gilmore, M.A.; Ordas, J.V.; Wang, J.; Francis, J.; Aoki, K.R. Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc. Natl. Acad. Sci. USA 2004, 101, 3208–3213. [Google Scholar]
- Sharma, S.K.; Whiting, R.C. Methods for detection of Clostridium botulinum toxin in foods. J. Food Protect. 2005, 68, 1256–1263. [Google Scholar]
- Metabiologics, I. Mouse Bioassays for Botulinum Toxins and Antibodies. Available online: http://www.metabiologics.com/ (accessed on 11 November 2011).
- Chen, S.; Karalewitz, A.P.; Barbieri, J.T. Insights into the different catalytic activities of Clostridium neurotoxins. Biochemistry 2012, 51, 3941–3947. [Google Scholar] [CrossRef]
- Kumaran, D.; Eswaramoorthy, S.; Furey, W.; Navaza, J.; Sax, M.; Swaminathan, S. Domain organization in Clostridium botulinum neurotoxin type E is unique: Its implication in faster translocation. J. Mol. Biol. 2009, 386, 233–245. [Google Scholar] [CrossRef]
- Janz, R.; Südhof, T.C. SV2C is a synaptic vesicle protein with an unusually restricted localization: Anatomy of a synaptic vesicle protein family. Neuroscience 1999, 94, 1279–1290. [Google Scholar] [CrossRef]
- Dressler, D.; Saberi, F.A.; Barbosa, E.R. Botulinum toxin: Mechanisms of action. Arq. Neuropsiquiatr. 2005, 63, 180–185. [Google Scholar] [CrossRef]
- Kuehn, B.M. FDA requires black box warnings on labeling for botulinum toxin products. J. Am. Med. Assoc. 2009, 301, 2316. [Google Scholar] [CrossRef]
- Brashear, A. Clinical comparisons of botulinum neurotoxin formulations. Neurologist 2008, 14, 289–298. [Google Scholar] [CrossRef]
- Freitag, F.G. Botulinum toxin type A in chronic migraine. Expert Rev. Neurother. 2007, 7, 463–470. [Google Scholar] [CrossRef]
- Lew, M.F. Botulinum toxin type B (Myobloc, NeuroBloc): A new choice in cervical dystonia. Expert Rev. Neurother. 2001, 1, 143–152. [Google Scholar] [CrossRef]
- Holzer, S.E.; Ludlow, C.L. The swallowing side effects of botulinum toxin type A injection in spasmodic dysphonia. Laryngoscope 1996, 106, 86–92. [Google Scholar] [CrossRef]
- Kalra, H.K.; Magoon, E.H. Side effects of the use of botulinum toxin for treatment of benign essential blepharospasm and hemifacial spasm. Ophthalmic. Surg. 1990, 21, 335–338. [Google Scholar]
- Price, J.; O’Day, J. Efficacy and side effects of botulinum toxin treatment for blepharospasm and hemifacial spasm. Aust. New Zeal. J. Ophthalmol. 1994, 22, 255–260. [Google Scholar] [CrossRef]
- Greene, P.; Fahn, S.; Diamond, B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov. Disord. 1994, 9, 213–217. [Google Scholar]
- Dressler, D.; Benecke, R. Autonomic side effects of botulinum toxin type B treatment of cervical dystonia and hyperhidrosis. Eur. Neurol. 2003, 49, 34–38. [Google Scholar] [CrossRef]
- Likhachev, S.A.; Rushkevich Iu, N.; Chernukha, T.N.; Veevnik, E.V. Efficacy and side-effects of the botulinum toxin A treatment in patients with focal dystonia (in Russian). Zh Nevrol Psikhiatr Im S S Korsakova 2009, 109, 27–31. [Google Scholar]
- Jankovic, J.; Schwartz, K.S. Clinical correlates of response to botulinum toxin injections. Arch. Neurol. 1991, 48, 1253–1256. [Google Scholar] [CrossRef]
- Dolimbek, B.Z.; Jankovic, J.; Atassi, M.Z. Cross reaction of tetanus and botulinum neurotoxins A and B and the boosting effect of botulinum neurotoxins A and B on a primary anti-tetanus antibody response. Immunol. Invest. 2002, 31, 247–262. [Google Scholar] [CrossRef]
- Teixeira-Clerc, F.; Menez, A.; Kessler, P. How do short neurotoxins bind to a muscular-type nicotinic acetylcholine receptor? J. Biol. Chem. 2002, 277, 25741–25747. [Google Scholar] [CrossRef]
- Truong, D.; Duane, D.D.; Jankovic, J.; Singer, C.; Seeberger, L.C.; Comella, C.L.; Lew, M.F.; Rodnitzky, R.L.; Danisi, F.O.; Sutton, J.P.; et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: Results of the first US randomized, double-blind, placebo-controlled study. Mov. Disord. 2005, 20, 783–791. [Google Scholar] [CrossRef]
- Frevert, J.; Dressler, D. Complexing proteins in botulinum toxin type A drugs: A help or a hindrance? Biologics 2010, 4, 325–332. [Google Scholar]
- Atassi, M.Z.; Jankovic, J.; Steward, L.E.; Aoki, K.R.; Dolimbek, B.Z. Molecular immune recognition of botulinum neurotoxin B. The light chain regions that bind human blocking antibodies from toxin-treated cervical dystonia patients. Antigenic structure of the entire BoNT/B molecule. Immunobiology 2012, 217, 17–27. [Google Scholar] [CrossRef]
- Critchfield, J. Considering the immune response to botulinum toxin. Clin. J. Pain. 2002, 18, S133–S141. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, C.; Geren, I.N.; Lou, J.; Conrad, F.; Forsyth, C.; Wen, W.; Chakraborti, S.; Zao, H.; Manzanarez, G.; Smith, T.J.; et al. Neutralizing human monoclonal antibodies binding multiple serotypes of botulinum neurotoxin. Protein Eng. Des. Sel. 2011, 24, 321–331. [Google Scholar] [CrossRef]
- Sankhla, C.; Jankovic, J.; Duane, D. Variability of the immunologic and clinical response in dystonic patients immunoresistant to botulinum toxin injections. Mov. Disord. 1998, 13, 150–154. [Google Scholar] [CrossRef]
- Jankovic, J.; Vuong, K.D.; Ahsan, J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology 2003, 60, 1186–1188. [Google Scholar] [CrossRef]
- Dressler, D.; Hallett, M. Immunological aspects of Botox, Dysport and Myobloc/NeuroBloc. Eur. J. Neurol. 2006, 13, 11–15. [Google Scholar] [CrossRef]
- Blumel, J.; Frever, J.; Schwaier, A. Comparative antigenicity of three preparations of botulinum neurotoxin type A in the rabbit. Neurotox. Res. 2006, 9, 238. [Google Scholar]
- Kanovsky, P.; Bares, M.; Severa, S.; Richardson, A. Long-term efficacy and tolerability of 4-monthly versus yearly botulinum toxin type A treatment for lower-limb spasticity in children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 436–445. [Google Scholar] [CrossRef]
- Flynn, D.D.; Ferrari-DiLeo, G.; Mash, D.C.; Levey, A.I. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer’s disease. J. Neurochem. 1995, 64, 1888–1891. [Google Scholar]
- Mrzljak, L.; Levey, A.I.; Goldman-Rakic, P.S. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: Morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 1993, 90, 5194–5198. [Google Scholar] [CrossRef]
- Weiner, D.M.; Levey, A.I.; Brann, M.R. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. USA 1990, 87, 7050–7054. [Google Scholar] [CrossRef]
- Vilaro, M.T.; Palacios, J.M.; Mengod, G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci. Lett. 1990, 114, 154–159. [Google Scholar] [CrossRef]
- Scarr, E. Muscarinic Receptors: Their Roles in Disorders of the Central Nervous System and Potential as Therapeutic Targets. CNS Neurosci. Ther. 2011, 18, 369–379. [Google Scholar] [CrossRef]
- Nakamura, K.; Kohda, T.; Umeda, K.; Yamamoto, H.; Mukamoto, M.; Kozaki, S. Characterization of the D/C mosaic neurotoxin produced by Clostridium botulinum associated with bovine botulism in Japan. Vet. Microbiol. 2010, 140, 147–154. [Google Scholar] [CrossRef]
- Antonucci, F.; Bozzi, Y.; Caleo, M. Intrahippocampal infusion of botulinum neurotoxin E (BoNT/E) reduces spontaneous recurrent seizures in a mouse model of mesial temporal lobe epilepsy. Epilepsia 2009, 50, 963–966. [Google Scholar] [CrossRef]
- Costantin, L.; Bozzi, Y.; Richichi, C.; Viegi, A.; Antonucci, F.; Funicello, M.; Gobbi, M.; Mennini, T.; Rossetto, O.; Montecucco, C.; et al. Antiepileptic effects of botulinum neurotoxin E. J. Neurosci. 2005, 25, 1943–1951. [Google Scholar] [CrossRef]
- Manno, I.; Antonucci, F.; Caleo, M.; Bozzi, Y. BoNT/E prevents seizure-induced activation of caspase 3 in the rat hippocampus. Neuroreport 2007, 18, 373–376. [Google Scholar]
- Antonucci, F.; di Garbo, A.; Novelli, E.; Manno, I.; Sartucci, F.; Bozzi, Y.; Caleo, M. Botulinum neurotoxin E (BoNT/E) reduces CA1 neuron loss and granule cell dispersion, with no effects on chronic seizures, in a mouse model of temporal lobe epilepsy. Exp. Neurol. 2008, 210, 388–401. [Google Scholar] [CrossRef]
- Hou, Y.P.; Zhang, Y.P.; Song, Y.F.; Zhu, C.M.; Wang, Y.C.; Xie, G.L. Botulinum toxin type A inhibits rat pyloric myoelectrical activity and substance P release in vivo. Can. J. Physiol. Pharmacol. 2007, 85, 209–214. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Lawrence, G.; Dolly, J.O. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J. Cell. Sci. 2007, 120, 2864–2874. [Google Scholar] [CrossRef]
- Holtje, M.; Hofmann, F.; Lux, R.; Veh, R.W.; Just, I.; Ahnert-Hilger, G. Glutamate uptake and release by astrocytes are enhanced by Clostridium botulinum C3 protein. J. Biol. Chem. 2008, 283, 9289–9299. [Google Scholar] [CrossRef]
- O’Sullivan, G.A.; Mohammed, N.; Foran, P.G.; Lawrence, G.W.; Oliver Dolly, J. Rescue of exocytosis in botulinum toxin A-poisoned chromaffin cells by expression of cleavage-resistant SNAP-25. Identification of the minimal essential C-terminal residues. J. Biol. Chem. 1999, 274, 36897–36904. [Google Scholar]
- Gazerani, P.; Pedersen, N.S.; Staahl, C.; Drewes, A.M.; Arendt-Nielsen, L. Subcutaneous Botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. Pain 2009, 141, 60–69. [Google Scholar] [CrossRef]
- Zuniga, C.; Diaz, S.; Piedimonte, F.; Micheli, F. Beneficial effects of botulinum toxin type A in trigeminal neuralgia. Arq. Neuropsiquiatr. 2008, 66, 500–503. [Google Scholar] [CrossRef]
- Piovesan, E.J.; Leite Lda, S.; Teive, H.G.; Kowacs, P.A.; Mulinari, R.A.; Radunz, V.; Utiumi, M.; Campos, H.G.; Werneck, L.C. Botulinum toxin type-A effect as a preemptive treatment in a model of acute trigeminal pain: A pre-clinical double-blind and placebo-controlled study. Arq. Neuropsiquiatr. 2011, 69, 56–63. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Dong, M.; Sun, S.; Chapman, E.R.; Jackson, M.B. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry 2011, 50, 2711–2713. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng Chen, Z.; Morris, J.G., Jr.; Rodriguez, R.L.; Shukla, A.W.; Tapia-Núñez, J.; Okun, M.S. Emerging Opportunities for Serotypes of Botulinum Neurotoxins. Toxins 2012, 4, 1196-1222. https://doi.org/10.3390/toxins4111196
Peng Chen Z, Morris JG Jr., Rodriguez RL, Shukla AW, Tapia-Núñez J, Okun MS. Emerging Opportunities for Serotypes of Botulinum Neurotoxins. Toxins. 2012; 4(11):1196-1222. https://doi.org/10.3390/toxins4111196
Chicago/Turabian StylePeng Chen, Zhongxing, J. Glenn Morris, Jr., Ramon L. Rodriguez, Aparna Wagle Shukla, John Tapia-Núñez, and Michael S. Okun. 2012. "Emerging Opportunities for Serotypes of Botulinum Neurotoxins" Toxins 4, no. 11: 1196-1222. https://doi.org/10.3390/toxins4111196