Clinical Uses of Botulinum Neurotoxins: Current Indications, Limitations and Future Developments
Abstract
:1. Introduction
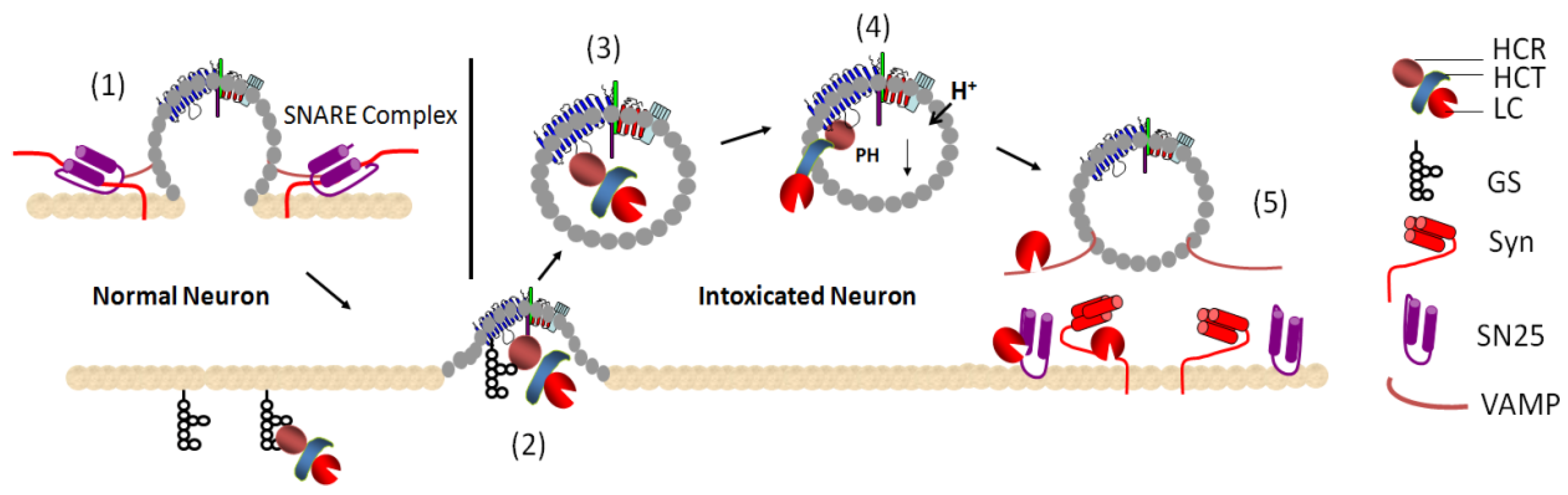
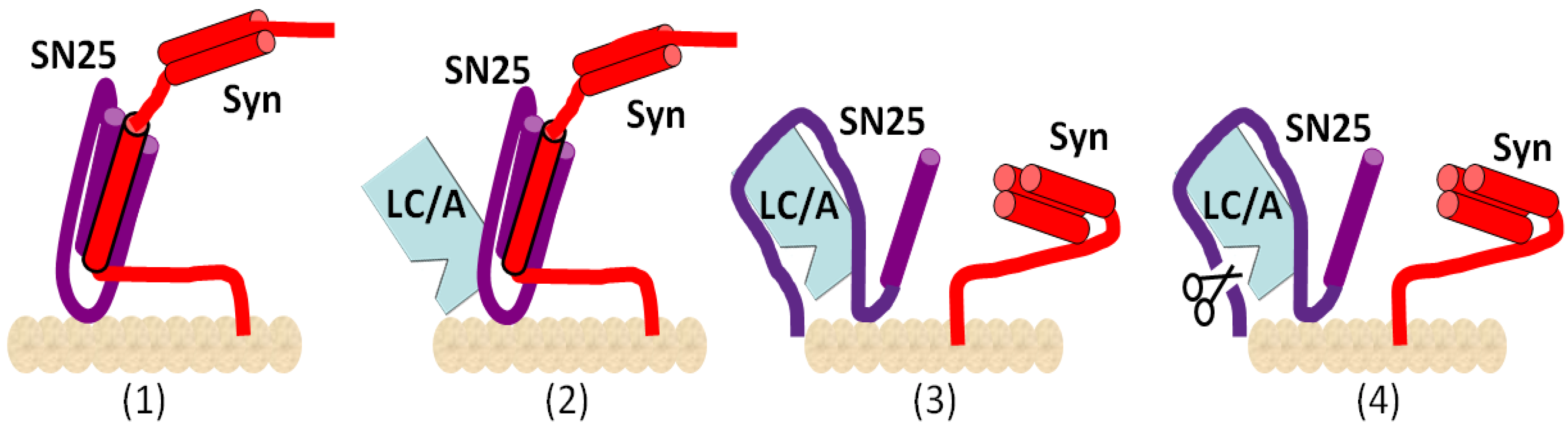
2. Mechanisms of Action of BoNTs

| Status | Indications | BoNT product (Year of approval) | Remarks |
|---|---|---|---|
| FDA approved indications | Strabismus | Oculinum/BOTOX (1989) | Very effective but repetitive injections are required, therefore more suitable for temporary uses |
| Blepharospasm | Oculinum/BOTOX (1989) | Very effective and no more trials are required | |
| Hemifacial spasm | Oculinum/BOTOX (1989) | Very effective and no more trials are required | |
| Cervical dystonia | BOTOX (2001), Dysport (2009), Xeomin (2010), NeuroBloc (2000) | Very effective but larger doses may be needed, therefore immune-resistance might sometimes develop in some patients | |
| Cosmetic use | BOTOX (2000, Canada)BOTOX (2012, US) | Very effective and safe for long-term use | |
| Axillary hyperhidrosis | BOTOX (2001, UK and Canada), BOTOX(2004, US) | Effective and safe, but painful at the injection sites | |
| Chronic migraine | BOTOX (2010) | Safe and effective for randomized studies but not placebo controlled trials | |
| Neurogenic detrusor overactivity | BOTOX (2012) | Remarkable efficacy and minimal side effects | |
| Off-labeled use indications | Lower urinary tract disorders | BOTOX | Remarkable efficacy and minimal side effects |
| Gastrointestinal tract disorders | BOTOX | Commonly used for some indications, but effects are relatively short-lived | |
| Spasticity | BOTOX | Can be considered as a first-line treatment, but should be used at the early stage | |
| Spasmodic dysphonia | BOTOX | Effective but more controlled studies are needed | |
| Sialorrhea | BOTOX | Effective on reducing excessive salivation but effective therapeutic dosages and the ideal form of application remain to be established | |
| Temporomandibular disorder | BOTOX | Correct injection technique and appropriate dosing guidelines are very important for successful results | |
| Chronic musculoskeletal pain | BOTOX | Effective for some patients who have not responded favorably to first-line treatments | |
| Vaginism | BOTOX | Effective but reports are limited | |
| Wound healing | BOTOX | Improvement of wound healing | |
| Diabetic neuropathy | BOTOX | Effective and safe treatment but reports are limited |
3. Clinical Applications of Botulinum Neurotoxins
3.1. FDA Approved Clinical Use of BoNTs
3.1.1. Strabismus
3.1.2. Blepharospasm
3.1.3. Hemifacial Spasm
3.1.4. Cervical Dystonia
3.1.5. Cosmetic Use
3.1.6. Axillary Hyperhidrosis
3.1.7. Chronic Migraine
3.1.8. Detrusor Overactivity
3.2. Off-Labeled Use of BoNTs
3.2.1. Lower Urinary Tract Disorders
3.2.2. Gastrointestinal Tract Disorders
3.2.3. Spasticity
3.2.4. Spasmodic Dysphonia
3.2.5. Sialorrhea
3.2.6. Temporomandibular Disorder
3.2.7. Chronic Musculoskeletal Pain
3.2.8. Other Indications
4. Limitations of Currently Available BoNT Therapies and Novel Product Development
4.1. Engineering Novel BoNT to Target non-Neuronal Substrate

4.2. Combating BoNT Immunoresistance Issues
5. Persistence of BoNT
6. Conclusion
Acknowledgments
Conflict of Interest
References
- Cherington, M. Clinical spectrum of botulism. Muscle Nerve 1998, 21, 701–710. [Google Scholar] [CrossRef]
- Dembek, Z.F.; Smith, L.A.; Rusnak, J.M. Botulism: Cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med. Public Health Prep. 2007, 1, 122–134. [Google Scholar] [CrossRef]
- Schiavo, G.; Rossetto, O.; Benfenati, F.; Poulain, B.; Montecucco, C. Tetanus and botulinum neurotoxins are zinc proteases specific for components of the neuroexocytosis apparatus. Ann. N. Y.Acad. Sci. 1994, 710, 65–75. [Google Scholar] [CrossRef]
- Montecucco, C.; Schiavo, G. Mechanism of action of tetanus and botulinum neurotoxins. Mol. Microbiol. 1994, 13, 1–8. [Google Scholar] [CrossRef]
- Poulain, B.; Humeau, Y. Mode of action of botulinum neurotoxin: Pathological, cellular and molecular aspect. Ann. Readapt Med. Phys. 2003, 46, 265–275. [Google Scholar] [CrossRef]
- Davletov, B.; Bajohrs, M.; Binz, T. Beyond BOTOX: Advantages and limitations of individual botulinum neurotoxins. Trends Neurosci 2005, 28, 446–452. [Google Scholar] [CrossRef]
- Brunger, A.T. Structure and function of SNARE and SNARE-interacting proteins. Q. Rev. Biophys. 2005, 38, 1–47. [Google Scholar] [CrossRef]
- Pickett, A.; Perrow, K. Towards new uses of botulinum toxin as a novel therapeutic tool. Toxins 2011, 3, 63–81. [Google Scholar]
- Bullens, R.W.; O’Hanlon, G.M.; Wagner, E.; Molenaar, P.C.; Furukawa, K.; Plomp, J.J.; Willison, H.J. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J. Neurosci. 2002, 22, 6876–6884. [Google Scholar]
- Kitamura, M.; Igimi, S.; Furukawa, K. Different response of the knockout mice lacking b-series gangliosides against botulinum and tetanus toxins. Biochim. Biophys. Acta 2005, 1741, 1–3. [Google Scholar] [CrossRef]
- Rummel, A.; Eichner, T.; Weil, T.; Karnath, T.; Gutcaits, A.; Mahrhold, S.; Sandhoff, K.; Proia, R.L.; Acharya, K.R.; Bigalke, H.; et al. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc. Natl. Acad. Sci. USA 2007, 104, 359–364. [Google Scholar]
- Tsukamoto, K.; Kohda, T.; Mukamoto, M.; Takeuchi, K.; Ihara, H.; Saito, M.; Kozaki, S. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 2005, 280, 35164–35171. [Google Scholar]
- Marxen, P.; Fuhrmann, U.; Bigalke, H. Gangliosides mediate inhibitory effects of tetanus and botulinum A neurotoxins on exocytosis in chromaffin cells. Toxicon 1989, 27, 849–859. [Google Scholar] [CrossRef]
- Marxen, P.; Erdmann, G.; Bigalke, H. The translocation of botulinum A neurotoxin by chromaffin cells is promoted in low ionic strength solution and is insensitive to trypsin. Toxicon 1991, 29, 181–189. [Google Scholar] [CrossRef]
- Kozaki, S.; Kamata, Y.; Watarai, S.; Nishiki, T.; Mochida, S. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb. Pathog. 1998, 25, 91–99. [Google Scholar] [CrossRef]
- Dong, M.; Richards, D.A.; Goodnough, M.C.; Tepp, W.H.; Johnson, E.A.; Chapman, E.R. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell. Biol. 2003, 162, 1293–1303. [Google Scholar]
- Nishiki, T.; Kamata, Y.; Nemoto, Y.; Omori, A.; Ito, T.; Takahashi, M.; Kozaki, S. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J. Biol. Chem. 1994, 269, 10498–10503. [Google Scholar]
- Dong, M.; Tepp, W.H.; Liu, H.; Johnson, E.A.; Chapman, E.R. Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J. Cell. Biol. 2007, 179, 1511–1522. [Google Scholar] [CrossRef]
- Peng, L.; Berntsson, R.P.; Tepp, W.H.; Pitkin, R.M.; Johnson, E.A.; Stenmark, P.; Dong, M. Botulinum neurotoxin D-C uses synaptotagmin I/II as receptors and human synaptotagmin II is not an effective receptor for type B, D-C, and G toxins. J. Cell. Sci. 2012, 125, 3233–3242. [Google Scholar] [CrossRef]
- Dong, M.; Yeh, F.; Tepp, W.H.; Dean, C.; Johnson, E.A.; Janz, R.; Chapman, E.R. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006, 312, 592–596. [Google Scholar]
- Dong, M.; Liu, H.; Tepp, W.H.; Johnson, E.A.; Janz, R.; Chapman, E.R. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol. Biol Cell 2008, 19, 5226–5237. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, C.; Barbieri, J.T.; Kim, J.J.; Baldwin, M.R. Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F. Biochemistry 2009, 48, 5631–5641. [Google Scholar] [CrossRef]
- Peng, L.; Tepp, W.H.; Johnson, E.A.; Dong, M. Botulinum neurotoxin D uses synaptic vesicle protein SV2 and gangliosides as receptors. PLoS Pathog. 2011, 7, e1002008. [Google Scholar] [CrossRef]
- Brunger, A.T.; Rummel, A. Receptor and substrate interactions of clostridial neurotoxins. Toxicon 2009, 54, 550–560. [Google Scholar] [CrossRef]
- Binz, T.; Rummel, A. Cell entry strategy of clostridial neurotoxins. J. Neurochem. 2009, 109, 1584–1595. [Google Scholar] [CrossRef]
- Montal, M. Translocation of botulinum neurotoxin light chain protease by the heavy chain protein-conducting channel. Toxicon 2009, 54, 565–569. [Google Scholar] [CrossRef]
- Brunger, A.T.; Breidenbach, M.A.; Jin, R.; Fischer, A.; Santos, J.S.; Montal, M. Botulinum neurotoxin heavy chain belt as an intramolecular chaperone for the light chain. PLoS Pathog. 2007, 3, 1191–1194. [Google Scholar]
- Fischer, A.; Montal, M. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J. Biol. Chem. 2007, 282, 29604–29611. [Google Scholar] [CrossRef]
- Fernandez-Salas, E.; Steward, L.E.; Ho, H.; Garay, P.E.; Sun, S.W.; Gilmore, M.A.; Ordas, J.V.; Wang, J.; Francis, J.; Aoki, K.R. Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc. Natl. Acad. Sci. USA 2004, 101, 3208–3213. [Google Scholar]
- Tsai, Y.C.; Maditz, R.; Kuo, C.L.; Fishman, P.S.; Shoemaker, C.B.; Oyler, G.A.; Weissman, A.M. Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc. Natl. Acad. Sci. USA 2010, 107, 16554–16559. [Google Scholar]
- Chen, S.; Barbieri, J.T. Association of botulinum neurotoxin serotype A light chain with plasma membrane-bound SNAP-25. J. Biol. Chem. 2011, 286, 15067–15072. [Google Scholar] [CrossRef]
- Silvaggi, N.R.; Boldt, G.E.; Hixon, M.S.; Kennedy, J.P.; Tzipori, S.; Janda, K.D.; Allen, K.N. Structures of Clostridium botulinum Neurotoxin Serotype A Light Chain complexed with small-molecule inhibitors highlight active-site flexibility. Chem. Biol. 2007, 14, 533–542. [Google Scholar] [CrossRef]
- Arndt, J.W.; Chai, Q.; Christian, T.; Stevens, R.C. Structure of botulinum neurotoxin type D light chain at 1.65 A resolution: Repercussions for VAMP-2 substrate specificity. Biochemistry 2006, 45, 3255–3262. [Google Scholar] [CrossRef]
- Agarwal, R.; Binz, T.; Swaminathan, S. Structural analysis of botulinum neurotoxin serotype F light chain: Implications on substrate binding and inhibitor design. Biochemistry 2005, 44, 11758–11765. [Google Scholar] [CrossRef]
- Arndt, J.W.; Yu, W.; Bi, F.; Stevens, R.C. Crystal structure of botulinum neurotoxin type G light chain: Serotype divergence in substrate recognition. Biochemistry 2005, 44, 9574–9580. [Google Scholar] [CrossRef]
- Agarwal, R.; Binz, T.; Swaminathan, S. Analysis of active site residues of botulinum neurotoxin E by mutational, functional, and structural studies: Glu335Gln is an apoenzyme. Biochemistry 2005, 44, 8291–8302. [Google Scholar] [CrossRef]
- Jin, R.; Sikorra, S.; Stegmann, C.M.; Pich, A.; Binz, T.; Brunger, A.T. Structural and biochemical studies of botulinum neurotoxin serotype C1 light chain protease: Implications for dual substrate specificity. Biochemistry 2007, 46, 10685–10693. [Google Scholar] [CrossRef]
- Vaidyanathan, V.V.; Yoshino, K.; Jahnz, M.; Dorries, C.; Bade, S.; Nauenburg, S.; Niemann, H.; Binz, T. Proteolysis of SNAP-25 isoforms by botulinum neurotoxin types A, C, and E: domains and amino acid residues controlling the formation of enzyme-substrate complexes and cleavage. J. Neurochem. 1999, 72, 327–337. [Google Scholar]
- Binz, T.; Blasi, J.; Yamasaki, S.; Baumeister, A.; Link, E.; Sudhof, T.C.; Jahn, R.; Niemann, H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J. Biol. Chem. 1994, 269, 1617–1620. [Google Scholar]
- Foran, P.; Shone, C.C.; Dolly, J.O. Differences in the protease activities of tetanus and botulinum B toxins revealed by the cleavage of vesicle-associated membrane protein and various sized fragments. Biochemistry 1994, 33, 15365–15374. [Google Scholar]
- Breidenbach, M.A.; Brunger, A.T. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature 2004, 432, 925–929. [Google Scholar] [CrossRef]
- Agarwal, R.; Schmidt, J.J.; Stafford, R.G.; Swaminathan, S. Mode of VAMP substrate recognition and inhibition of Clostridium botulinum neurotoxin F. Nat. Struct. Mol. Biol. 2009, 16, 789–794. [Google Scholar] [CrossRef]
- Chen, S.; Kim, J.J.; Barbieri, J.T. Mechanism of substrate recognition by botulinum neurotoxin serotype A. J. Biol. Chem. 2007, 282, 9621–9627. [Google Scholar] [CrossRef]
- Atassi, M.Z.; Oshima, M. Structure, activity, and immune (T and B cell) recognition of botulinum neurotoxins. Crit. Rev. Immunol. 1999, 19, 219–260. [Google Scholar]
- Mahant, N.; Clouston, P.D.; Lorentz, I.T. The current use of botulinum toxin. J. Clin. Neurosci. 2000, 7, 389–394. [Google Scholar] [CrossRef]
- Glogau, R.G. Review of the use of botulinum toxin for hyperhidrosis and cosmetic purposes. Clin. J. Pain 2002, 18, S191–S197. [Google Scholar] [CrossRef]
- Thant, Z.S.; Tan, E.K. Emerging therapeutic applications of botulinum toxin. Med. Sci. Monit. 2003, 9, RA40–R48. [Google Scholar]
- Bell, K.R.; Williams, F. Use of botulinum toxin type A and type B for spasticity in upper and lower limbs. Phys. Med. Rehabil. Clin. N. Am. 2003, 14, 821–835. [Google Scholar] [CrossRef]
- Atassi, M.Z. Basic immunological aspects of botulinum toxin therapy. Mov. Disord. 2004, 19, S68–S84. [Google Scholar] [CrossRef]
- Ascher, B.; Rossi, B. Botulinum toxin and wrinkles: Few side effects and effective combining procedures with other treatments. Ann. Chir. Plast. Esthet. 2004, 49, 537–552. [Google Scholar] [CrossRef]
- Benedetto, A.V. Commentary: Botulinum toxin in clinical medicine: Part II. Clin. Dermatol. 2004, 22, 1–2. [Google Scholar] [CrossRef]
- Cheng, C.M.; Chen, J.S.; Patel, R.P. Unlabeled uses of botulinum toxins: A review, part 2. Am. J. Health Syst. Pharm. 2006, 63, 225–232. [Google Scholar] [CrossRef]
- Mahajan, S.T.; Brubaker, L. Botulinum toxin: From life-threatening disease to novel medical therapy. Am. J. Obstet. Gynecol. 2007, 196, 7–15. [Google Scholar] [CrossRef]
- Brashear, A. Botulinum toxin type B: A new injectable treatment for cervical dystonia. Expert Opin. Investig. Drugs 2001, 10, 2191–2199. [Google Scholar] [CrossRef]
- Neurotoxins Licensed as Treatment for Neck Neuromuscular Disorder. Am. J. Health Syst. Pharm. 2001. 58:200.
- Figgitt, D.P.; Noble, S. Botulinum toxin B: A review of its therapeutic potential in the management of cervical dystonia. Drugs 2002, 62, 705–722. [Google Scholar] [CrossRef]
- Scott, A.B. Botulinum toxin injection of eye muscles to correct strabismus. Trans. Am. Ophthalmol. Soc. 1981, 79, 734–770. [Google Scholar]
- Scott, A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. J. Pediatric Ophthalmol. Strabismus 1980, 17, 21–25. [Google Scholar]
- Scott, A.B.; Rosenbaum, A.; Collins, C.C. Pharmacologic weakening of extraocular muscles. Investig. Ophthalmol. 1973, 12, 924–927. [Google Scholar]
- Kowal, L.; Wong, E.; Yahalom, C. Botulinum toxin in the treatment of strabismus. A review of its use and effects. Disabil. Rehabil. 2007, 29, 1823–1831. [Google Scholar] [CrossRef]
- Krzizok, T. Botulinum toxin injections for the treatment of strabismus. Which indications are still useful today? Ophthalmologe 2007, 104, 759–762. [Google Scholar] [CrossRef]
- Rowe, F.J.; Noonan, C.P. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Rowe, F.J.; Noonan, C.P. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- 64. Hancox, J.; Sharma, S.; Mackenzie, K.; Adams, G. The effect on quality of life of long-term botulinum toxin A injections to maintain ocular alignment in adult patients with strabismus. Br. J. Ophthalmol. 2012, doi: 10.1136/bjophthalmol-2011-301332.
- Chang, L.B.; Tsai, C.P.; Liao, K.K.; Kao, K.P.; Yuan, C.L.; Yen, D.J.; Lin, K.P. Use of botulinum toxin A in the treatment of hemifacial spasm and blepharospasm (In Chinese). Chin. Med. J. 1999, 62, 1–5. [Google Scholar]
- Drummond, G.T.; Hinz, B.J. Botulinum toxin for blepharospasm and hemifacial spasm: Stability of duration of effect and dosage over time. Can. J. Ophthalmol. 2001, 36, 398–403. [Google Scholar]
- Duzynski, W.; Slawek, J. Treatment of hemifacial spasm with botulinum A toxin. Neurol. Neurochir. Pol. 1998, 32, 61–69. [Google Scholar]
- Gouider-Khouja, N.; Turki, I.; Ben Hamida, M.; Hentati, F. Hemifacial spasm and its treatment with botulinum toxin. Tunis. Med. 1999, 77, 41–44. [Google Scholar]
- Costa, J.; Espirito-Santo, C.; Borges, A.; Ferreira, J.J.; Coelho, M.; Moore, P.; Sampaio, C. Botulinum toxin type A therapy for hemifacial spasm. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Jankovic, J.; Orman, J. Botulinum A toxin for cranial-cervical dystonia: A double-blind, placebo-controlled study. Neurology 1987, 37, 616–623. [Google Scholar] [CrossRef]
- Bhaumik, S.; Behari, M. Botulinum toxin A-Injection for cervical dystonia. J. Assoc. Physicians India 1999, 47, 267–270. [Google Scholar]
- Edwards, L.L.; Normand, M.M.; Wszolek, Z.K. Cervical dystonia: A review the role of botulinum toxin. Nebr. Med. J. 1995, 80, 109–115. [Google Scholar]
- Poewe, W.; Wissel, J. Use of botulinum toxin in the treatment of cervical dystonia. Baillieres Clin. Neurol. 1993, 2, 179–185. [Google Scholar]
- Jankovic, J.; Schwartz, K. Botulinum toxin injections for cervical dystonia. Neurology 1990, 40, 277–280. [Google Scholar] [CrossRef]
- Tsui, J.K.; Calne, D.B. Botulinum toxin in cervical dystonia. Adv. Neurol. 1988, 49, 473–478. [Google Scholar]
- Truong, D.; Duane, D.D.; Jankovic, J.; Singer, C.; Seeberger, L.C.; Comella, C.L.; Lew, M.F.; Rodnitzky, R.L.; Danisi, F.O.; Sutton, J.P.; et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: Results of the first US randomized, double-blind, placebo-controlled study. Mov. Disord. 2005, 20, 783–791. [Google Scholar] [CrossRef]
- Factor, S.A.; Molho, E.S.; Evans, S.; Feustel, P.J. Efficacy and safety of repeated doses of botulinum toxin type B in type A resistant and responsive cervical dystonia. Mov. Disord. 2005, 20, 1152–1160. [Google Scholar] [CrossRef]
- Costa, J.; Espirito-Santo, C.; Borges, A.; Ferreira, J.J.; Coelho, M.; Moore, P.; Sampaio, C. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Costa, J.; Borges, A.; Espirito-Santo, C.; Ferreira, J.; Coelho, M.; Moore, P.; Sampaio, C. Botulinum toxin type A versus botulinum toxin type B for cervical dystonia. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Comella, C.L.; Jankovic, J.; Shannon, K.M.; Tsui, J.; Swenson, M.; Leurgans, S.; Fan, W. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology 2005, 65, 1423–1429. [Google Scholar] [CrossRef]
- Benohanian, A. What stands in the way of treating palmar hyperhidrosis as effectively as axillary hyperhidrosis with botulinum toxin type A. Dermatol. Online J. 2009, 15, 12. [Google Scholar]
- Vadoud-Seyedi, J.; Simonart, T. Treatment of axillary hyperhidrosis with botulinum toxin type A reconstituted in lidocaine or in normal saline: A randomized, side-by-side, double-blind study. Br. J. Dermatol. 2007, 156, 986–989. [Google Scholar] [CrossRef]
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; DeGryse, R.E.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef]
- Aurora, S.K.; Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef]
- Aurora, S.K.; Winner, P.; Freeman, M.C.; Spierings, E.L.; Heiring, J.O.; DeGryse, R.E.; VanDenburgh, A.M.; Nolan, M.E.; Turkel, C.C. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-Week PREEMPT clinical program. Headache 2011, 51, 1358–1373. [Google Scholar] [CrossRef]
- Reitz, A.; Stohrer, M.; Kramer, G.; del Popolo, G.; Chartier-Kastler, E.; Pannek, J.; Burgdorfer, H.; Gocking, K.; Madersbacher, H.; Schumacher, S.; et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur. Urol. 2004, 45, 510–515. [Google Scholar] [CrossRef]
- Kuo, H.C. Therapeutic effects of suburothelial injection of botulinum a toxin for neurogenic detrusor overactivity due to chronic cerebrovascular accident and spinal cord lesions. Urology 2006, 67, 232–236. [Google Scholar] [CrossRef]
- Game, X.; Castel-Lacanal, E.; Bentaleb, Y.; Thiry-Escudie, I.; De Boissezon, X.; Malavaud, B.; Marque, P.; Rischmann, P. Botulinum toxin A detrusor injections in patients with neurogenic detrusor overactivity significantly decrease the incidence of symptomatic urinary tract infections. Eur. Urol. 2008, 53, 613–618. [Google Scholar] [CrossRef]
- Herschorn, S.; Gajewski, J.; Ethans, K.; Corcos, J.; Carlson, K.; Bailly, G.; Bard, R.; Valiquette, L.; Baverstock, R.; Carr, L.; et al. Efficacy of botulinum toxin A injection for neurogenic detrusor overactivity and urinary incontinence: A randomized, double-blind trial. J. Urol. 2011, 185, 2229–2235. [Google Scholar]
- Tincello, D.G.; Kenyon, S.; Abrams, K.R.; Mayne, C.; Toozs-Hobson, P.; Taylor, D.; Slack, M. Botulinum Toxin A Versus Placebo for Refractory Detrusor Overactivity in Women: A Randomised Blinded Placebo-Controlled Trial of 240 Women (the RELAX Study). Eur. Urol. 2012, 62, 507–514. [Google Scholar] [CrossRef]
- Giannantoni, A.; Mearini, E.; del Zingaro, M.; Porena, M. Six-year follow-up of botulinum toxin A intradetrusorial injections in patients with refractory neurogenic detrusor overactivity: Clinical and urodynamic results. Eur. Urol. 2009, 55, 705–711. [Google Scholar]
- Jabbari, B. Treatment of chronic low back pain with botulinum neurotoxins. Curr. Pain Headache Rep. 2007, 11, 352–358. [Google Scholar] [CrossRef]
- Ney, J.P.; Difazio, M.; Sichani, A.; Monacci, W.; Foster, L.; Jabbari, B. Treatment of chronic low back pain with successive injections of botulinum toxin a over 6 months: A prospective trial of 60 patients. Clin. J. Pain 2006, 22, 363–369. [Google Scholar] [CrossRef]
- De Andres, J.; Cerda-Olmedo, G.; Valia, J.C.; Monsalve, V.; Lopez, A.; Minguez, A. Use of botulinum toxin in the treatment of chronic myofascial pain. Clin. J. Pain 2003, 19, 269–275. [Google Scholar] [CrossRef]
- Difazio, M.; Jabbari, B. A focused review of the use of botulinum toxins for low back pain. Clin. J. Pain 2002, 18, S155–S162. [Google Scholar] [CrossRef]
- Foster, L.; Clapp, L.; Erickson, M.; Jabbari, B. Botulinum toxin A and chronic low back pain: A randomized, double-blind study. Neurology 2001, 56, 1290–1293. [Google Scholar] [CrossRef]
- Lacy, B.E.; Weiser, K.; Kennedy, A. Botulinum toxin and gastrointestinal tract disorders: Panacea, placebo, or pathway to the future? Gastroentero. Hepatol. 2008, 4, 283–295. [Google Scholar]
- Vittal, H.; Pasricha, P.F. Botulinum toxin for gastrointestinal disorders: Therapy and mechanisms. Neurotox Res. 2006, 9, 149–159. [Google Scholar] [CrossRef]
- Stankovic, N.; Mirkovic, D. Botulinum toxin A in the treatment of anal fissure. Vojnosanit Pregl. 2004, 61, 531–535. [Google Scholar] [CrossRef]
- Qureshi, W.A. Gastrointestinal uses of botulinum toxin. J. Clin. Gastroenterol. 2002, 34, 126–128. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A.; Pasricha, P.J. Botulinum toxin for spastic gastrointestinal disorders. Baillieres Best Pract Res. Clin. Gastroenterol. 1999, 13, 131–143. [Google Scholar] [CrossRef]
- Walton, J.M.; Tougas, G. Botulinum toxin use in pediatric esophageal achalasia: A case report. J. Pediatr. Surg. 1997, 32, 916–917. [Google Scholar] [CrossRef]
- Bhutani, M.S. Gastrointestinal uses of botulinum toxin. Am. J. Gastroenterol. 1997, 92, 929–933. [Google Scholar]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M. Botulinum toxin type A in post-stroke lower limb spasticity: A multicenter, double-blind, placebo-controlled trial. J. Neurol. 2010, 257, 1330–1337. [Google Scholar] [CrossRef]
- Shaw, L.; Rodgers, H. Botulinum toxin type A for upper limb spasticity after stroke. Expert Rev. Neurother. 2009, 9, 1713–1725. [Google Scholar] [CrossRef]
- Ward, A.B. Botulinum toxin in spasticity treatment in adults. Nervenarzt 2008, 79, 22–23. [Google Scholar]
- Marciniak, C.; Rader, L.; Gagnon, C. The use of botulinum toxin for spasticity after spinal cord injury. Am. J. Phys. Med. Rehabil. 2008, 87, 312–317. [Google Scholar] [CrossRef]
- Desloovere, K.; Molenaers, G. Treatment of juvenile spasticity with botulinum toxin type A. Nervenarzt 2008, 79, 19–21. [Google Scholar]
- Reichel, G. Botulinum toxin: A new dimension for spasticity. MMW Fortschr. Med. 2007, 149, 65–69. [Google Scholar]
- Sheean, G. Botulinum toxin treatment of adult spasticity : A benefit-risk assessment. Drug Saf. 2006, 29, 31–48. [Google Scholar]
- Watts, C.; Nye, C.; Whurr, R. Botulinum toxin for treating spasmodic dysphonia (laryngeal dystonia): A systematic Cochrane review. Clin. Rehabil. 2006, 20, 112–122. [Google Scholar] [CrossRef]
- Watts, C.R.; Truong, D.D.; Nye, C. Evidence for the effectiveness of botulinum toxin for spasmodic dysphonia from high-quality research designs. J. Neural Transm. 2008, 115, 625–630. [Google Scholar] [CrossRef]
- Harris, S.R.; Purdy, A.H. Drooling and its management in cerebral palsy. Dev. Med. Child. Neurol. 1987, 29, 807–811. [Google Scholar] [CrossRef]
- Hyson, H.C.; Johnson, A.M.; Jog, M.S. Sublingual atropine for sialorrhea secondary to parkinsonism: A pilot study. Mov. Disord. 2002, 17, 1318–1320. [Google Scholar] [CrossRef]
- Garcia Ron, A.; Miranda, M.C.; Garriga Braun, C.; Jensen Veron, J.; Torrens Martinez, J.; Diez Hanbino, M.P. Efficacy and safety of botulinum toxin in the treatment of sialorrhea in children with neurological disorders. An. Pediatr. (Barc) 2012, 77, 289–291. [Google Scholar] [CrossRef]
- Schroeder, A.S.; Kling, T.; Huss, K.; Borggraefe, I.; Koerte, I.K.; Blaschek, A.; Jahn, K.; Heinen, F.; Berweck, S. Botulinum toxin type A and B for the reduction of hypersalivation in children with neurological disorders: A focus on effectiveness and therapy adherence. Neuropediatrics 2012, 43, 27–36. [Google Scholar]
- Chinnapongse, R.; Gullo, K.; Nemeth, P.; Zhang, Y.; Griggs, L. Safety and efficacy of botulinum toxin type B for treatment of sialorrhea in Parkinson’s disease: A prospective double-blind trial. Mov. Disord. 2012, 27, 219–226. [Google Scholar] [CrossRef]
- Guidubaldi, A.; Fasano, A.; Ialongo, T.; Piano, C.; Pompili, M.; Masciana, R.; Siciliani, L.; Sabatelli, M.; Bentivoglio, A.R. Botulinum toxin A versus B in sialorrhea: A prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or Parkinson’s disease. Mov. Disord. 2011, 26, 313–319. [Google Scholar] [CrossRef]
- Freund, B.; Schwartz, M.; Symington, J.M. The use of botulinum toxin for the treatment of temporomandibular disorders: Preliminary findings. J. Oral Maxillofac. Surg. 1999, 57, 916–920. [Google Scholar] [CrossRef]
- Jabbari, B. Evidence based medicine in the use of botulinum toxin for back pain. J. Neural. Transm. 2008, 115, 637–640. [Google Scholar] [CrossRef]
- Zhang, T.; Adatia, A.; Zarin, W.; Moitri, M.; Vijenthira, A.; Chu, R.; Thabane, L.; Kean, W. The efficacy of botulinum toxin type A in managing chronic musculoskeletal pain: A systematic review and meta analysis. Inflammopharmacology 2011, 19, 21–34. [Google Scholar] [CrossRef]
- Bertolasi, L.; Frasson, E.; Cappelletti, J.Y.; Vicentini, S.; Bordignon, M.; Graziottin, A. Botulinum neurotoxin type A injections for vaginismus secondary to vulvar vestibulitis syndrome. Obstet. Gynecol. 2009, 114, 1008–1016. [Google Scholar] [CrossRef]
- Fageeh, W.M. Different treatment modalities for refractory vaginismus in western Saudi Arabia. J. Sex. Med. 2011, 8, 1735–1739. [Google Scholar] [CrossRef]
- Patti, R.; Almasio, P.L.; Muggeo, V.M.; Buscemi, S.; Arcara, M.; Matranga, S.; Di Vita, G. Improvement of wound healing after hemorrhoidectomy: A double-blind, randomized study of botulinum toxin injection. Dis. Colon Rectum 2005, 48, 2173–2179. [Google Scholar]
- Gassner, H.G.; Brissett, A.E.; Otley, C.C.; Boahene, D.K.; Boggust, A.J.; Weaver, A.L.; Sherris, D.A. Botulinum toxin to improve facial wound healing: A prospective, blinded, placebo-controlled study. Mayo Clin. Proc. 2006, 81, 1023–1028. [Google Scholar] [CrossRef]
- Wilson, A.M. Use of botulinum toxin type A to prevent widening of facial scars. Plast. Reconstr. Surg. 2006, 117, 1758–1766. [Google Scholar] [CrossRef]
- Wang, L.; Tai, N.Z.; Fan, Z.H. Effect of botulinum toxin type A on the expression of substance P, calcitonin gene-related peptide, transforming growth factor beta-1 and alpha smooth muscle actin A in wound healing in rats. Chin. Plast. Surg. 2009, 25, 50–53. [Google Scholar]
- Sahinkanat, T.; Ozkan, K.U.; Ciralik, H.; Ozturk, S.; Resim, S. Botulinum toxin-A to improve urethral wound healing: An experimental study in a rat model. Urology 2009, 73, 405–409. [Google Scholar] [CrossRef]
- Xiao, Z.; Qu, G. Effects of botulinum toxin type a on collagen deposition in hypertrophic scars. Molecules 2012, 17, 2169–2177. [Google Scholar] [CrossRef]
- Argoff, C.E. A focused review on the use of botulinum toxins for neuropathic pain. Clin. J. Pain 2002, 18, S177–S181. [Google Scholar] [CrossRef]
- Restivo, D.A.; Marchese-Ragona, R.; Lauria, G.; Squatrito, S.; Gullo, D.; Vigneri, R. Botulinum toxin treatment for oropharyngeal dysphagia associated with diabetic neuropathy. Diabetes Care 2006, 29, 2650–2653. [Google Scholar] [CrossRef]
- Hummel, M.; Geigenberger, G.; Brand, J.; Ziegler, A.G.; Fuchtenbusch, M. New therapeutic approaches to diabetic gastroparesis. Med. Klin (Munich) 2008, 103, 514–518. [Google Scholar] [CrossRef]
- Yuan, R.Y.; Sheu, J.J.; Yu, J.M.; Chen, W.T.; Tseng, I.J.; Chang, H.H.; Hu, C.J. Botulinum toxin for diabetic neuropathic pain: A randomized double-blind crossover trial. Neurology 2009, 72, 1473–1478. [Google Scholar]
- Bach-Rojecky, L.; Salkovic-Petrisic, M.; Lackovic, Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: Bilateral effect after unilateral injection. Eur. J. Pharmacol. 2010, 633, 10–14. [Google Scholar] [Green Version]
- Lund, N.S.; Maxel, T.; Rungby, J. Successful treatment of diabetic gustatory hyperhidrosis with topical glycopyrrolate. Ugeskr Laeger 2011, 173, 2200–2201. [Google Scholar]
- Jahn, R.; Scheller, R.H. SNAREs-Engines for membrane fusion. Nat. Rev. Mol. Cell Biol 2006, 7, 631–643. [Google Scholar] [CrossRef]
- Sadoul, K.; Berger, A.; Niemann, H.; Weller, U.; Roche, P.A.; Klip, A.; Trimble, W.S.; Regazzi, R.; Catsicas, S.; Halban, P.A. SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J. Biol. Chem. 1997, 272, 33023–33027. [Google Scholar]
- Paumet, F.; le Mao, J.; Martin, S.; Galli, T.; David, B.; Blank, U.; Roa, M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: Functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J. Immunol. 2000, 164, 5850–5857. [Google Scholar]
- Hickson, G.R.; Chamberlain, L.H.; Maier, V.H.; Gould, G.W. Quantification of SNARE protein levels in 3T3-L1 adipocytes: Implications for insulin-stimulated glucose transport. Biochem. Biophys. Res. Commun. 2000, 270, 841–845. [Google Scholar] [CrossRef]
- Chen, D.; Bernstein, A.M.; Lemons, P.P.; Whiteheart, S.W. Molecular mechanisms of platelet exocytosis: Role of SNAP-23 and syntaxin 2 in dense core granule release. Blood 2000, 95, 921–929. [Google Scholar]
- Martin-Martin, B.; Nabokina, S.M.; Blasi, J.; Lazo, P.A.; Mollinedo, F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood 2000, 96, 2574–2583. [Google Scholar]
- Castle, J.D.; Guo, Z.; Liu, L. Function of the t-SNARE SNAP-23 and secretory carrier membrane proteins (SCAMPs) in exocytosis in mast cells. Mol. Immunol. 2002, 38, 1337–1340. [Google Scholar] [CrossRef]
- Pagan, J.K.; Wylie, F.G.; Joseph, S.; Widberg, C.; Bryant, N.J.; James, D.E.; Stow, J.L. The t-SNARE syntaxin 4 is regulated during macrophage activation to function in membrane traffic and cytokine secretion. Curr. Biol. 2003, 13, 156–160. [Google Scholar] [CrossRef]
- Reales, E.; Mora-Lopez, F.; Rivas, V.; Garcia-Poley, A.; Brieva, J.A.; Campos-Caro, A. Identification of soluble N-ethylmaleimide-sensitive factor attachment protein receptor exocytotic machinery in human plasma cells: SNAP-23 is essential for antibody secretion. J. Immunol. 2005, 175, 6686–6693. [Google Scholar]
- Saxena, S.K.; George, C.M.; Pinskiy, V.; McConnell, B. Epithelial sodium channel is regulated by SNAP-23/syntaxin 1A interplay. Biochem. Biophys. Res. Commun. 2006, 343, 1279–1285. [Google Scholar] [CrossRef]
- Foster, K.A.; Adams, E.J.; Durose, L.; Cruttwell, C.J.; Marks, E.; Shone, C.C.; Chaddock, J.A.; Cox, C.L.; Heaton, C.; Sutton, J.M.; et al. Re-engineering the target specificity of Clostridial neurotoxins-A route to novel therapeutics. Neurotox Res. 2006, 9, 101–107. [Google Scholar]
- Rogers, D.F. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir. Care 2007, 52, 1134–1146. [Google Scholar]
- Davis, C.W.; Dickey, B.F. Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol 2008, 70, 487–512. [Google Scholar] [CrossRef]
- Goschel, H.; Wohlfarth, K.; Frevert, J.; Dengler, R.; Bigalke, H. Botulinum A toxin therapy: Neutralizing and nonneutralizing antibodies-Therapeutic consequences. Exp. Neurol. 1997, 147, 96–102. [Google Scholar] [CrossRef]
- Dolimbek, B.Z.; Jankovic, J.; Atassi, M.Z. Cross reaction of tetanus and botulinum neurotoxins A and B and the boosting effect of botulinum neurotoxins A and B on a primary anti-tetanus antibody response. Immunol. Invest. 2002, 31, 247–262. [Google Scholar] [CrossRef]
- Jankovic, J.; Vuong, K.D.; Ahsan, J. Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology 2003, 60, 1186–1188. [Google Scholar] [CrossRef]
- Atassi, M.Z.; Dolimbek, B.Z. Mapping of the antibody-binding regions on the HN-domain (residues 449-859) of botulinum neurotoxin A with antitoxin antibodies from four host species. Full profile of the continuous antigenic regions of the H-chain of botulinum neurotoxin A. Protein J. 2004, 23, 39–52. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin in clinical practice. J. Neurol. Neurosurg. Psychiatry 2004, 75, 951–957. [Google Scholar] [CrossRef]
- Jankovic, J. Treatment of cervical dystonia with botulinum toxin. Mov. Disord. 2004, 19, S109–S115. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin therapy for cervical dystonia. Neurotox. Res. 2006, 9, 145–148. [Google Scholar] [CrossRef]
- Brashear, A.; Lew, M.F.; Dykstra, D.D.; Comella, C.L.; Factor, S.A.; Rodnitzky, R.L.; Trosch, R.; Singer, C.; Brin, M.F.; Murray, J.J.; et al. Safety and efficacy of NeuroBloc (botulinum toxin type B) in type A-responsive cervical dystonia. Neurology 1999, 53, 1439–1446. [Google Scholar] [CrossRef]
- Aoki, K.R. Immunologic and Other Properties of Therapeutic Botulinum Toxin Serotypes. In Scientific and Therapeutic Aspects of Botulinum Toxin; Brin, M.F., Hallett, M., Jankovic, J., Eds.; Lippincott Willams & Wilkins: Philadelphia, PA, USA, 2002; pp. 103–113. [Google Scholar]
- Swope, D.; Barbano, R. Treatment recommendations and practical applications of botulinum toxin treatment of cervical dystonia. Neurol. Clin. 2008, 26, 54–65. [Google Scholar] [CrossRef]
- Comella, C.L. The treatment of cervical dystonia with botulinum toxins. J. Neural. Transm. 2008, 115, 579–583. [Google Scholar] [CrossRef]
- Chaddock, J.A.; Purkiss, J.R.; Duggan, M.J.; Quinn, C.P.; Shone, C.C.; Foster, K.A. A conjugate composed of nerve growth factor coupled to a non-toxic derivative of Clostridium botulinum neurotoxin type A can inhibit neurotransmitter release in vitro. Growth Factors 2000, 18, 147–155. [Google Scholar] [CrossRef]
- Chaddock, J.A.; Purkiss, J.R.; Friis, L.M.; Broadbridge, J.D.; Duggan, M.J.; Fooks, S.J.; Shone, C.C.; Quinn, C.P.; Foster, K.A. Inhibition of vesicular secretion in both neuronal and nonneuronal cells by a retargeted endopeptidase derivative of Clostridium botulinum neurotoxin type A. Infect. Immun. 2000, 68, 2587–2593. [Google Scholar]
- Duggan, M.J.; Quinn, C.P.; Chaddock, J.A.; Purkiss, J.R.; Alexander, F.C.; Doward, S.; Fooks, S.J.; Friis, L.M.; Hall, Y.H.; Kirby, E.R.; et al. Inhibition of release of neurotransmitters from rat dorsal root ganglia by a novel conjugate of a Clostridium botulinum toxin A endopeptidase fragment and Erythrina cristagalli lectin. J. Biol. Chem. 2002, 277, 34846–34852. [Google Scholar]
- Chaddock, J.A.; Purkiss, J.R.; Alexander, F.C.; Doward, S.; Fooks, S.J.; Friis, L.M.; Hall, Y.H.; Kirby, E.R.; Leeds, N.; Moulsdale, H.J.; et al. Retargeted clostridial endopeptidases: Inhibition of nociceptive neurotransmitter release in vitro, and antinociceptive activity in in vivo models of pain. Mov. Disord. 2004, 19, S42–S47. [Google Scholar]
- Foster, K.A. A new wrinkle on pain relief: Re-engineering clostridial neurotoxins for analgesics. Drug Discov. Today 2005, 10, 563–569. [Google Scholar] [CrossRef]
- Chen, S.; Barbieri, J.T. Engineering botulinum neurotoxin to extend therapeutic intervention. Proc. Natl. Acad. Sci. USA 2009, 106, 9180–9184. [Google Scholar]
- Foster, K.A. Engineered toxins: New therapeutics. Toxicon 2009, 54, 587–592. [Google Scholar] [CrossRef]
- Rummel, A. Transport Protein which is used to Introduce Chemical Compounds into Nerve Cells. International patent application WO 2006/027207, 6 September 2004. [Google Scholar]
- Chen, S.; Karalewitz, A.P.; Barbieri, J.T. Insights into the Different Catalytic Activities of Clostridium Neurotoxins. Biochemistry 2012, 51, 3941–3947. [Google Scholar]
- Ahmed, S.A.; Olson, M.A.; Ludivico, M.L.; Gilsdorf, J.; Smith, L.A. Identification of residues surrounding the active site of type A botulinum neurotoxin important for substrate recognition and catalytic activity. Protein J. 2008, 27, 151–162. [Google Scholar] [CrossRef]
- Chen, S. Unpublished work, The Hong Kong Polytechnic University: Hong Kong, 2012.
- Dolimbek, B.Z.; Aoki, K.R.; Steward, L.E.; Jankovic, J.; Atassi, M.Z. Mapping of the regions on the heavy chain of botulinum neurotoxin A (BoNT/A) recognized by antibodies of cervical dystonia patients with immunoresistance to BoNT/A. Mol. Immunol. 2007, 44, 1029–1041. [Google Scholar] [CrossRef]
- Atassi, M.Z.; Dolimbek, B.Z.; Jankovic, J.; Steward, L.E.; Aoki, K.R. Molecular recognition of botulinum neurotoxin B heavy chain by human antibodies from cervical dystonia patients that develop immunoresistance to toxin treatment. Mol. Immunol. 2008, 45, 3878–3888. [Google Scholar] [CrossRef]
- Atassi, M.Z.; Jankovic, J.; Steward, L.E.; Aoki, K.R.; Dolimbek, B.Z. Molecular immune recognition of botulinum neurotoxin B. The light chain regions that bind human blocking antibodies from toxin-treated cervical dystonia patients. Antigenic structure of the entire BoNT/B molecule. Immunobiology 2012, 217, 17–27. [Google Scholar] [CrossRef]
- Atassi, M.Z.; Ruan, K.H.; Jinnai, K.; Oshima, M.; Ashizawa, T. Epitope-specific suppression of antibody response in experimental autoimmune myasthenia gravis by a monomethoxypolyethylene glycol conjugate of a myasthenogenic synthetic peptide. Proc. Natl. Acad. Sci. USA 1992, 89, 5852–5856. [Google Scholar] [CrossRef]
- Dolimbek, B.Z.; Aoki, K.R.; Atassi, M.Z. Reduction of antibody response against botulinum neurotoxin A by synthetic monomethoxypolyethylene glycol-peptide conjugates. Immunol. Lett. 2011, 137, 46–52. [Google Scholar] [CrossRef]
- Fernandez-Salas, E.; Ho, H.; Garay, P.; Steward, L.E.; Aoki, K.R. Is the light chain subcellular localization an important factor in botulinum toxin duration of action? Mov. Disord. 2004, 19, S23–S34. [Google Scholar] [CrossRef]
- Wang, J.; Zurawski, T.H.; Meng, J.; Lawrence, G.; Olango, W.M.; Finn, D.P.; Wheeler, L.; Dolly, J.O. A dileucine in the protease of botulinum toxin A underlies its long-lived neuroparalysis: Transfer of longevity to a novel potential therapeutic. J. Biol. Chem. 2011, 286, 6375–6385. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, S. Clinical Uses of Botulinum Neurotoxins: Current Indications, Limitations and Future Developments. Toxins 2012, 4, 913-939. https://doi.org/10.3390/toxins4100913
Chen S. Clinical Uses of Botulinum Neurotoxins: Current Indications, Limitations and Future Developments. Toxins. 2012; 4(10):913-939. https://doi.org/10.3390/toxins4100913
Chicago/Turabian StyleChen, Sheng. 2012. "Clinical Uses of Botulinum Neurotoxins: Current Indications, Limitations and Future Developments" Toxins 4, no. 10: 913-939. https://doi.org/10.3390/toxins4100913




