Gi/o Protein-Dependent and -Independent Actions of Pertussis Toxin (PTX)
Abstract
:Abbreviations
| AC | adenylyl cyclase |
| CHO | Chinese hamster ovary |
| CaMKII | calmodulin kinase II |
| CREB | cAMP response element-binding |
| 8-CPT | 8-(4-chlorophenylthio)-2'-O-methyladenosine-3',5'-cyclic monophosphate |
| eEF2 | eukaryotic elongation factor |
| Epac | exchange protein directly activated by cAMP |
| ERK | extracellular signal-regulated kinase |
| GPCRs | G protein-coupled receptors |
| Gi/oPCRs | Gi/o protein-coupled receptors |
| GPIb | glycoprotein Ib |
| MAPK | mitogen-activated protein kinase |
| NAD+ | nicotinamide adenine dinucleotide |
| PKA | cAMP-dependent protein kinase |
| PTX | Pertussis toxin |
| TLR4 | Toll-like receptor 4 |
| HUVECs | human umbilical vein endothelial cells |
| SAA | serum amyloid A |
| SPC | sphingosylphosphorylcholine |
| TCR | T-cell receptor |
1. Introduction
| ADP-Ribosylating Toxin | Bacterium | Target | Pathological Effect |
|---|---|---|---|
| Pertussis toxin | Bordetella pertussis | Cysteine residue of Gαi subfamily (Gαi, Gαo, and Gαt) except Gαz | Gαi protein-receptor coupling is inhibited, and its signal transduction is blocked. |
| Cholera toxin | Vibrio cholerae | Arginine residue of Gαs subfamily (Gαs and Gαolf) | As GTPase activity of the stimulatory Gαs is inhibited, Gαs protein is permanently activated. |
| Heat-labile enterotoxin | Escherichia coli | Arginine residue of Gαs subfamily (Gαs and Gαolf) | As GTPase activity of stimulatory Gαs is inhibited, the Gαs protein is permanently activated. |
| Diphtheria toxin | Corynebacterium diphtheirae | Diphthamide of eEF2 | Protein synthesis is blocked. |
| Exotoxin A | Pseudomonas aeruginosa | Diphthamide of eEF2 | Protein synthesis is blocked. |
2. Structure of Pertussis Toxin
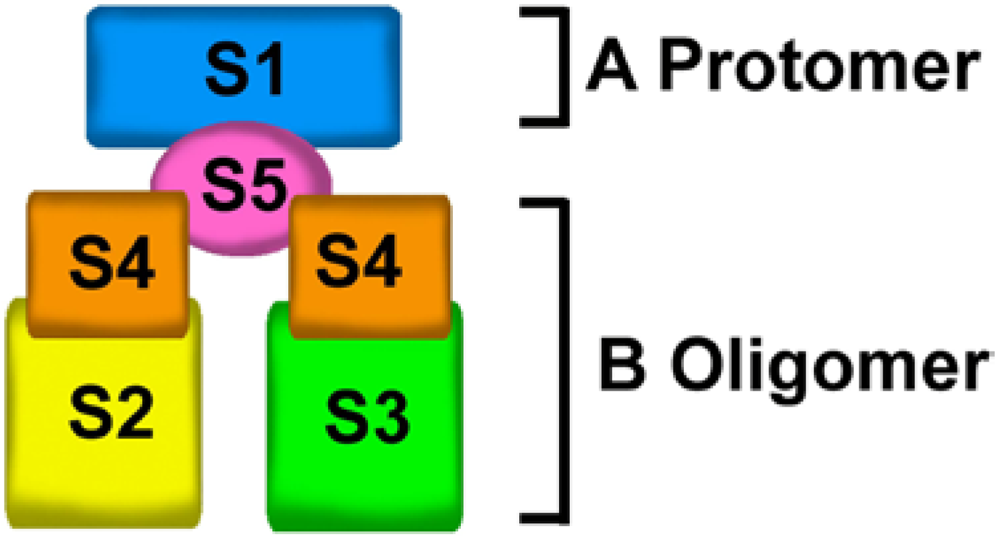
3. ADP-Ribosylation Mechanism of PTX
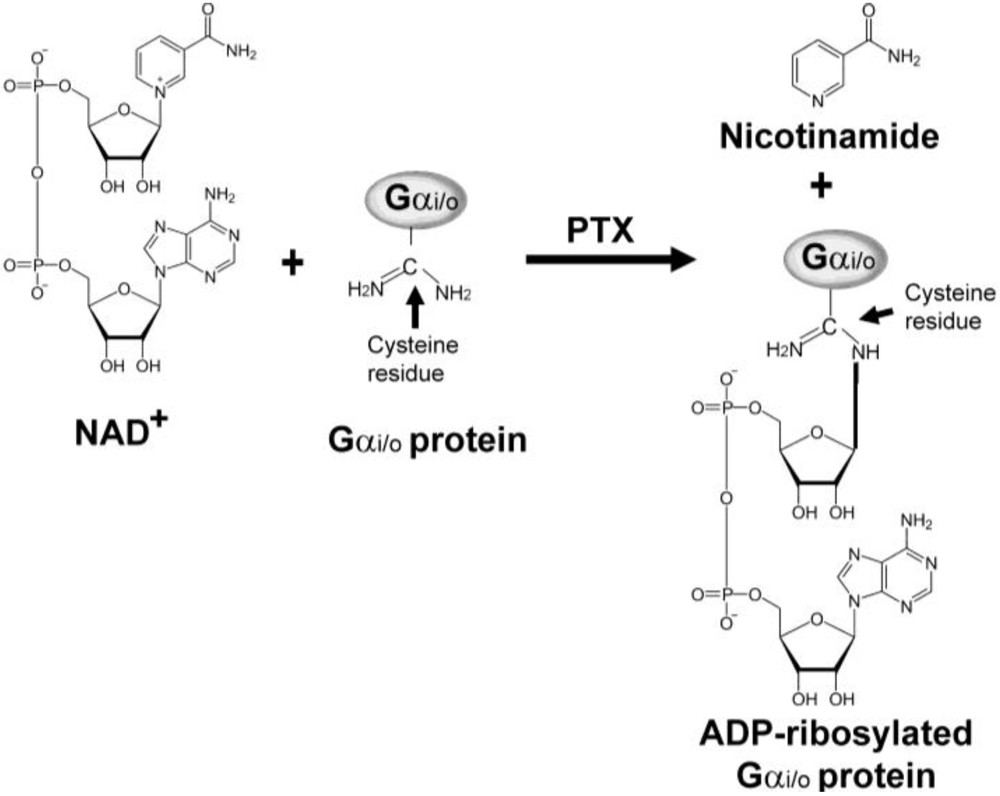
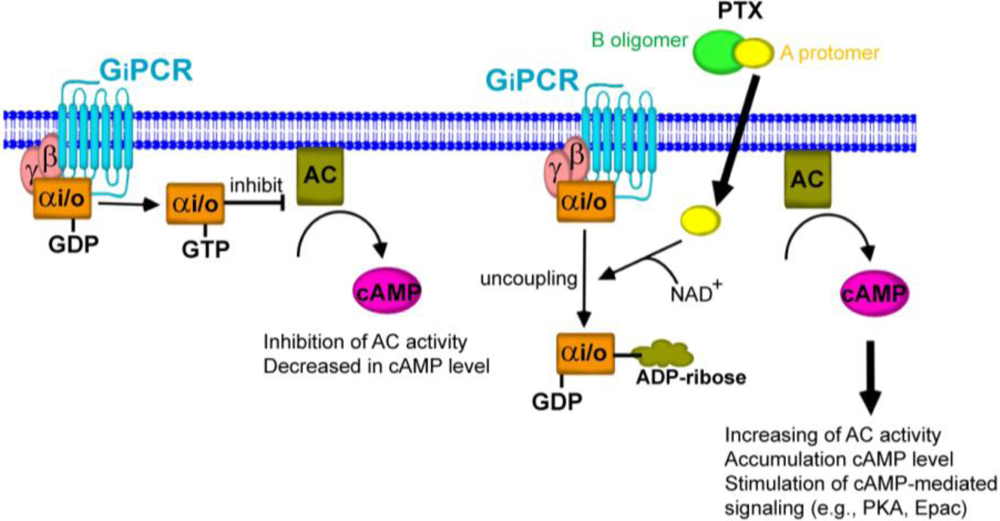
4. Gi/o Protein-Dependent Actions of PTX
| α Subunit | Amino Acid | Expression [34,35] | Effect on Effectors | Toxin (Site of Action) |
|---|---|---|---|---|
| αo | 354 | Heart, neurons, neuroendocrine cells | Inhibition of AC activity [36] | PTX at cysteine 351 [1,37,38] |
| Inhibition of Ca2+ channel [39] | ||||
| Activation of K+ channel [40] | ||||
| αi1-αi3 | 354 | Neurons and ubiquitous | Inhibition of AC activity [41] | PTX at cysteine 351 [1,37,38] |
| Inhibition of Ca2+ channel [39,42] | ||||
| Activation of K+ channel [40,43] | ||||
| αz | 355 | Platelets | Inhibition of AC activity [44] | -(cyteine modified by PTX does not present) [46] |
| Inhibition of Ca2+ channel [45] | ||||
| Activation of K+ channel [40] | ||||
| αt | 350 | Rod/cone outer segments | Activation of cGMP-PDE [47] | PTX at cysteine 351 [37,38] |
| CTX at arginine147 [37] | ||||
| αgust | 353 | Taste buds; sweet and/or bitter | Activation of cGMP-PDE [48] | PTX at cysteine 350 [37,38] |
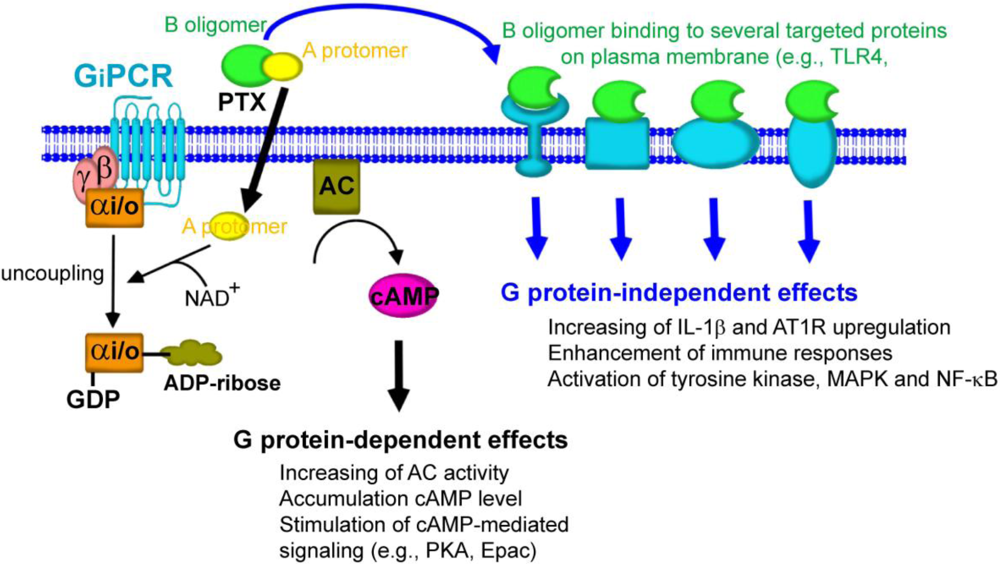
5. Gi/o Protein-Independent Actions of PTX
| Gi/o Protein-Dependent Effects of PTX | Gi/o Protein-Independent Effects of PTX | |
|---|---|---|
| Subunit | A-protomer (S1 subunit) | B-oligomer (S2-S3 dimer, S2-S5 dimer, and S5 monomer) |
| Onset of action | Slow | Rapid |
| Concentration of toxin to induce the effects | Low | High |
| Biological effects | Enhance insulin secretion [49] | Induce dendritic cell maturation [14] |
| Inhibit lymphocyte and neutrophil migration [52,76] | Inhibit growth cone guidance [78] | |
| Inhibit enkephalin stimulation of GTPase [77] | Induce myelomonocytic cell adhesion [79] | |
| Inhibit autophosphorylation and activation of insulin receptor kinase [50] | Induce ERK1/2 activation in endothelial cell [59] | |
| Activate platelet aggregation [62,69] | ||
| Activate T lymphocyte [66,80] | ||
| Induce Th1/Th17 immune response through MAPK and IL-10 [74] | ||
| Activate tyrosine kinase signaling [12] | ||
| Inhibit Tat-induced TGF- β production [81] | ||
| Inhibit HIV type 1 replication [82] |
6. Conclusions
References
- Holbourn, K.P.; Shone, C.C.; Acharya, K.R. A family of killer toxins. Exploring the mechanism of ADP-ribosylating toxins. FEBS J. 2006, 273, 4579–4593. [Google Scholar]
- Locht, C.; Antoine, R. A proposed mechanism of ADP-ribosylation catalyzed by the pertussis toxin S1 subunit. Biochimie 1995, 77, 333–340. [Google Scholar]
- Pittman, M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev. Infect. Dis. 1979, 1, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.L. Subunit structure and enzymic activity of pertussis toxin. Microbiol. Sci. 1988, 5, 285–287. [Google Scholar]
- Katada, T.; Ui, M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J. Biol. Chem. 1982, 257, 7210–7216. [Google Scholar]
- Kurose, H.; Katada, T.; Amano, T.; Ui, M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J. Biol. Chem. 1983, 258, 4870–4875. [Google Scholar] [PubMed]
- Tamura, M.; Nogimori, K.; Yajima, M.; Ase, K.; Ui, M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J. Biol. Chem. 1983, 258, 6756–6761. [Google Scholar]
- Lando, Z.; Teitelbaum, D.; Arnon, R. Induction of experimental allergic encephalomyelitis in genetically resistant strains of mice. Nature 1980, 287, 551–552. [Google Scholar]
- Linthicum, D.S.; Munoz, J.J.; Blaskett, A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell. Immunol. 1982, 73, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Racke, M.K.; Hu, W.; Lovett-Racke, A.E. PTX cruiser: driving autoimmunity via TLR4. Trends Immunol. 2005, 26, 289–291. [Google Scholar]
- Jajoo, S.; Mukherjea, D.; Pingle, S.; Sekino, Y.; Ramkumar, V. Induction of adenosine A1 receptor expression by pertussis toxin via an adenosine 5'-diphosphate ribosylation-independent pathway. J. Pharmacol. Exp. Ther. 2006, 317, 1–10. [Google Scholar]
- Li, H.; Wong, W.S. Pertussis toxin activates tyrosine kinase signaling cascade in myelomonocytic cells: a mechanism for cell adhesion. Biochem. Biophys. Res. Commun. 2001, 283, 1077–1082. [Google Scholar]
- Melien, O.; Sandnes, D.; Johansen, E.J.; Christoffersen, T. Effects of pertussis toxin on extracellular signal-regulated kinase activation in hepatocytes by hormones and receptor-independent agents: evidence suggesting a stimulatory role of G(i) proteins at a level distal to receptor coupling. J. Cell. Physiol. 2000, 184, 27–36. [Google Scholar]
- Wang, Z.Y.; Yang, D.; Chen, Q.; Leifer, C.A.; Segal, D.M.; Su, S.B.; Caspi, R.R.; Howard, Z.O.; Oppenheim, J.J. Induction of dendritic cell maturation by pertussis toxin and its B subunit differentially initiate Toll-like receptor 4-dependent signal transduction pathways. Exp. Hematol. 2006, 34, 1115–1124. [Google Scholar]
- Nishida, M.; Suda, R.; Nagamatsu, Y.; Tanabe, S.; Onohara, N.; Nakaya, M.; Kanaho, Y.; Shibata, T.; Uchida, K.; Sumimoto, H.; et al. Pertussis toxin up-regulates angiotensin type 1 receptors through Toll-like receptor 4-mediated Rac activation. J. Biol. Chem. 2010, 285, 15268–15277. [Google Scholar] [PubMed]
- Tamura, M.; Nogimori, K.; Murai, S.; Yajima, M.; Ito, K.; Katada, T.; Ui, M.; Ishii, S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 1982, 21, 5516–5522. [Google Scholar] [PubMed]
- Katada, T.; Tamura, M.; Ui, M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch. Biochem. Biophys. 1983, 224, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.E.; Boodhoo, A.; Armstrong, G.D.; Cockle, S.A.; Klein, M.H.; Read, R.J. The crystal structure of pertussis toxin. Structure 1994, 2, 45–57. [Google Scholar]
- Locht, C.; Keith, J.M. Pertussis toxin gene: Nucleotide sequence and genetic organization. Science 1986, 232, 1258–1264. [Google Scholar]
- Nicosia, A.; Perugini, M.; Franzini, C.; Casagli, M.C.; Borri, M.G.; Antoni, G.; Almoni, M.; Neri, P.; Ratti, G.; Rappuoli, R. Cloning and sequencing of the pertussis toxin genes: Operon structure and gene duplication. Proc. Natl. Acad. Sci. USA 1986, 83, 4631–4635. [Google Scholar]
- Saukkonen, K.; Burnette, W.N.; Mar, V.L.; Masure, H.R.; Tuomanen, E.I. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc. Natl. Acad. Sci. USA 1992, 89, 118–122. [Google Scholar]
- van’t Wout, J.; Burnette, W.N.; Mar, V.L.; Rozdzinski, E.; Wright, S.D.; Tuomanen, E.I. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect. Immun. 1992, 60, 3303–3308. [Google Scholar]
- Krueger, K.M.; Barbieri, J.T. Assignment of functional domains involved in ADP-ribosylation and B-oligomer binding within the carboxyl terminus of the S1 subunit of pertussis toxin. Infect. Immun. 1994, 62, 2071–2078. [Google Scholar]
- Antoine, R.; Locht, C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect. Immun. 1990, 58, 1518–1526. [Google Scholar]
- Burns, D.L.; Hausman, S.Z.; Lindner, W.; Robey, F.A.; Manclark, C.R. Structural characterization of pertussis toxin A subunit. J. Biol. Chem. 1987, 262, 17677–17682. [Google Scholar]
- Sekura, R.D.; Fish, F.; Manclark, C.R.; Meade, B.; Zhang, Y.L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J. Biol. Chem. 1983, 258, 14647–14651. [Google Scholar] [PubMed]
- Burns, D.L.; Manclark, C.R. Role of cysteine 41 of the A subunit of pertussis toxin. J. Biol. Chem. 1989, 264, 564–568. [Google Scholar]
- Krueger, K.M.; Barbieri, J.T. Molecular characterization of the in vitro activation of pertussis toxin by ATP. J. Biol. Chem. 1993, 268, 12570–12578. [Google Scholar] [PubMed]
- Van Ness, B.G.; Howard, J.B.; Bodley, J.W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J. Biol. Chem. 1980, 255, 10710–10716. [Google Scholar] [PubMed]
- Gilman, A.G. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987, 56, 615–649. [Google Scholar]
- Katada, T.I.T.; Takahashi, K.; Nishina, H.; Kanaho, Y. Bacterial Toxins and Virulence Factors in Disease; Dekker, Inc.: New York, NY, USA, 1995; Volume 8, pp. 459–489. [Google Scholar]
- Ui, M. Islet-activating protein, pertussis toxin: A probe for functions of the inhibitory guanine nucleotide regulatory component of adenylate cyclase. Trends Pharmacol. Sci. 1984, 5, 277–279. [Google Scholar]
- West, R.E., Jr.; Moss, J.; Vaughan, M.; Liu, T.; Liu, T.Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J. Biol. Chem. 1985, 260, 14428–14430. [Google Scholar] [PubMed]
- Fields, T.A.; Casey, P.J. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem. J. 1997, 321, 561–571. [Google Scholar]
- Cabrera-Vera, T.M.; Vanhauwe, J.; Thomas, T.O.; Medkova, M.; Preininger, A.; Mazzoni, M.R.; Hamm, H.E. Insights into G protein structure, function, and regulation. Endocr. Rev. 2003, 24, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.D.; Medzihradsky, F. Go mediates the coupling of the mu opioid receptor to adenylyl cyclase in cloned neural cells and brain. Proc. Natl. Acad. Sci. USA 1993, 90, 4062–6406. [Google Scholar]
- Katada, T.; Ui, M. Slow interaction of islet-activating protein with pancreatic islets during primary culture to cause reversal of alpha-adrenergic inhibition of insulin secretion. J. Biol. Chem. 1980, 255, 9580–9588. [Google Scholar]
- Milligan, G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem. J. 1988, 255, 1–13. [Google Scholar]
- Chen, H.; Lambert, N.A. Inhibition of dendritic calcium influx by activation of G-protein-coupled receptors in the hippocampus. J. Neurophysiol. 1997, 78, 3484–3488. [Google Scholar]
- Yamada, M.; Inanobe, A.; Kurachi, Y. G protein regulation of potassium ion channels. Pharmacol. Rev. 1998, 50, 723–760. [Google Scholar]
- Wittpoth, C.; Scholich, K.; Yigzaw, Y.; Stringfield, T.M.; Patel, T.B. Regions on adenylyl cyclase that are necessary for inhibition of activity by beta gamma and G(ialpha) subunits of heterotrimeric G proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 9551–9556. [Google Scholar]
- Jeong, S.W.; Ikeda, S.R. Effect of G protein heterotrimer composition on coupling of neurotransmitter receptors to N-type Ca(2+) channel modulation in sympathetic neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 907–912. [Google Scholar]
- Chen, C. Gi-3 protein mediates the increase in voltage-gated K+ currents by somatostatin on cultured ovine somatotrophs. Am. J. Physiol. 1998, 275, E278–E284. [Google Scholar]
- Obadiah, J.; Avidor-Reiss, T.; Fishburn, C.S.; Carmon, S.; Bayewitch, M.; Vogel, Z.; Fuchs, S.; Levavi-Sivan, B. Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell. Mol. Neurobiol. 1999, 19, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Ikeda, S.R. G protein alpha subunit G alpha z couples neurotransmitter receptors to ion channels in sympathetic neurons. Neuron 1998, 21, 1201–1212. [Google Scholar]
- Tsu, R.C.; Ho, M.K.; Yung, L.Y.; Joshi, S.; Wong, Y.H. Role of amino- and carboxyl-terminal regions of G(alphaZ) in the recognition of Gi-coupled receptors. Mol. Pharmacol. 1997, 52, 38–45. [Google Scholar]
- Rarick, H.M.; Artemyev, N.O.; Hamm, H.E. A site on rod G protein alpha subunit that mediates effector activation. Science 1992, 256, 1031–1033. [Google Scholar]
- Yan, W.; Sunavala, G.; Rosenzweig, S.; Dasso, M.; Brand, J.G.; Spielman, A.I. Bitter taste transduced by PLC-beta(2)-dependent rise in IP(3) and alpha-gustducin-dependent fall in cyclic nucleotides. Am. J. Physiol. Cell Physiol. 2001, 280, C742–C751. [Google Scholar]
- Muller-Wieland, D.; White, M.F.; Behnke, B.; Gebhardt, A.; Neumann, S.; Krone, W.; Kahn, C.R. Pertussis toxin inhibits autophosphorylation and activation of the insulin receptor kinase. Biochem. Biophys. Res. Commun. 1991, 181, 1479–1485. [Google Scholar]
- Ui, M. The Multiple Biological Activities of Pertussis Toxin; John Wiley & Sons, Inc.: London, UK, 1988; pp. 121–145. [Google Scholar]
- Brito, G.A.; Souza, M.H.; Melo-Filho, A.A.; Hewlett, E.L.; Lima, A.A.; Flores, C.A.; Ribeiro, R.A. Role of pertussis toxin A subunit in neutrophil migration and vascular permeability. Infect. Immun. 1997, 65, 1114–1118. [Google Scholar]
- Houslay, M.D.; Milligan, G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem. Sci. 1997, 22, 217–224. [Google Scholar]
- Montminy, M. Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 1997, 66, 807–822. [Google Scholar]
- Bos, J.L. Epac proteins: Multi-purpose cAMP targets. Trends Biochem. Sci. 2006, 31, 680–686. [Google Scholar]
- de Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar]
- Pereira, L.; Metrich, M.; Fernandez-Velasco, M.; Lucas, A.; Leroy, J.; Perrier, R.; Morel, E.; Fischmeister, R.; Richard, S.; Benitah, J.P.; et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J. Physiol. 2007, 583, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Mangmool, S.; Shukla, A.K.; Rockman, H.A. beta-Arrestin-dependent activation of Ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J. Cell Biol. 2010, 189, 573–587. [Google Scholar]
- Garcia, J.G.; Wang, P.; Liu, F.; Hershenson, M.B.; Borbiev, T.; Verin, A.D. Pertussis toxin directly activates endothelial cell p42/p44 MAP kinases via a novel signaling pathway. Am. J. Physiol. Cell Physiol. 2001, 280, C1233–C1241. [Google Scholar]
- Lee, H.Y.; Kim, S.D.; Shim, J.W.; Kim, H.J.; Yun, J.; Baek, S.H.; Kim, K.; Bae, Y.S. A pertussis toxin sensitive G-protein-independent pathway is involved in serum amyloid A-induced formyl peptide receptor 2-mediated CCL2 production. Exp. Mol. Med. 2010, 42, 302–309. [Google Scholar]
- Lee, H.Y.; Lee, S.Y.; Kim, S.D.; Shim, J.W.; Kim, H.J.; Jung, Y.S.; Kwon, J.Y.; Baek, S.H.; Chung, J.; Bae, Y.S. Sphingosylphosphorylcholine stimulates CCL2 production from human umbilical vein endothelial cells. J. Immunol. 2011, 186, 4347–4353. [Google Scholar]
- Sindt, K.A.; Hewlett, E.L.; Redpath, G.T.; Rappuoli, R.; Gray, L.S.; Vandenberg, S.R. Pertussis toxin activates platelets through an interaction with platelet glycoprotein Ib. Infect. Immun. 1994, 62, 3108–3114. [Google Scholar]
- Strnad, C.F.; Carchman, R.A. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 1987, 225, 16–20. [Google Scholar]
- Thom, R.E.; Casnellie, J.E. Pertussis toxin activates protein kinase C and a tyrosine protein kinase in the human T cell line Jurkat. FEBS Lett. 1989, 244, 181–184. [Google Scholar]
- Wong, W.S.; Rosoff, P.M. Pharmacology of pertussis toxin B-oligomer. Can. J. Physiol. Pharmacol. 1996, 74, 559–564. [Google Scholar]
- Schneider, O.D.; Weiss, A.A.; Miller, W.E. Pertussis toxin utilizes proximal components of the T-cell receptor complex to initiate signal transduction events in T cells. Infect. Immun. 2007, 75, 4040–4049. [Google Scholar]
- Raze, D.; Veithen, A.; Sato, H.; Antoine, R.; Menozzi, F.D.; Locht, C. Genetic exchange of the S2 and S3 subunits in pertussis toxin. Mol. Microbiol. 2006, 60, 1241–1250. [Google Scholar]
- Gierschik, P. ADP-ribosylation of signal-transducing guanine nucleotide-binding proteins by pertussis toxin. Curr. Top. Microbiol. Immunol. 1992, 175, 69–96. [Google Scholar]
- Banga, H.S.; Walker, R.K.; Winberry, L.K.; Rittenhouse, S.E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J. Biol. Chem. 1987, 262, 14871–14874. [Google Scholar]
- Alfano, M.; Grivel, J.C.; Ghezzi, S.; Corti, D.; Trimarchi, M.; Poli, G.; Margolis, L. Pertussis toxin B-oligomer dissociates T cell activation and HIV replication in CD4 T cells released from infected lymphoid tissue. AIDS 2005, 19, 1007–1014. [Google Scholar]
- Moss, J.; Bruni, P.; Hsia, J.A.; Tsai, S.C.; Watkins, P.A.; Halpern, J.L.; Burns, D.L.; Kanaho, Y.; Chang, P.P.; Hewlett, E.L.; et al. Pertussis toxin-catalyzed ADP-ribosylation: effects on the coupling of inhibitory receptors to the adenylate cyclase system. J. Recept. Res. 1984, 4, 459–474. [Google Scholar]
- Nencioni, L.; Volpini, G.; Peppoloni, S.; Bugnoli, M.; de Magistris, T.; Marsili, I.; Rappuoli, R. Properties of pertussis toxin mutant PT-9K/129G after formaldehyde treatment. Infect. Immun. 1991, 59, 625–630. [Google Scholar]
- Pizza, M.; Bartoloni, A.; Prugnola, A.; Silvestri, S.; Rappuoli, R. Subunit S1 of pertussis toxin: Mapping of the regions essential for ADP-ribosyltransferase activity. Proc. Natl. Acad. Sci. USA 1988, 85, 7521–7525. [Google Scholar]
- Nasso, M.; Fedele, G.; Spensieri, F.; Palazzo, R.; Costantino, P.; Rappuoli, R.; Ausiello, C.M. Genetically detoxified pertussis toxin induces Th1/Th17 immune response through MAPKs and IL-10-dependent mechanisms. J. Immunol. 2009, 183, 1892–1899. [Google Scholar]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [PubMed]
- Stewart, S.J.; Prpic, V.; Johns, J.A.; Powers, F.S.; Graber, S.E.; Forbes, J.T.; Exton, J.H. Bacterial toxins affect early events of T lymphocyte activation. J. Clin. Invest. 1989, 83, 234–242. [Google Scholar]
- Spangrude, G.J.; Sacchi, F.; Hill, H.R.; van Epps, D.E.; Daynes, R.A. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J. Immunol. 1985, 135, 4135–4143. [Google Scholar]
- Burns, D.L.; Hewlett, E.L.; Moss, J.; Vaughan, M. Pertussis toxin inhibits enkephalin stimulation of GTPase of NG108-15 cells. J. Biol. Chem. 1983, 258, 1435–1438. [Google Scholar]
- Kindt, R.M.; Lander, A.D. Pertussis toxin specifically inhibits growth cone guidance by a mechanism independent of direct G protein inactivation. Neuron 1995, 15, 79–88. [Google Scholar]
- Li, H.; Wong, W.S. Mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of CD14 and urokinase receptor. Immunology 2000, 100, 502–509. [Google Scholar]
- Gray, L.S.; Huber, K.S.; Gray, M.C.; Hewlett, E.L.; Engelhard, V.H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J. Immunol. 1989, 142, 1631–1638. [Google Scholar]
- Zocchi, M.R.; Contini, P.; Alfano, M.; Poggi, A. Pertussis toxin (PTX) B subunit and the nontoxic PTX mutant PT9K/129G inhibit Tat-induced TGF-beta production by NK cells and TGF-beta-mediated NK cell apoptosis. J. Immunol. 2005, 174, 6054–6061. [Google Scholar]
- Alfano, M.; Pushkarsky, T.; Poli, G.; Bukrinsky, M. The B-oligomer of pertussis toxin inhibits human immunodeficiency virus type 1 replication at multiple stages. J. Virol. 2000, 74, 8767–8770. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mangmool, S.; Kurose, H. Gi/o Protein-Dependent and -Independent Actions of Pertussis Toxin (PTX). Toxins 2011, 3, 884-899. https://doi.org/10.3390/toxins3070884
Mangmool S, Kurose H. Gi/o Protein-Dependent and -Independent Actions of Pertussis Toxin (PTX). Toxins. 2011; 3(7):884-899. https://doi.org/10.3390/toxins3070884
Chicago/Turabian StyleMangmool, Supachoke, and Hitoshi Kurose. 2011. "Gi/o Protein-Dependent and -Independent Actions of Pertussis Toxin (PTX)" Toxins 3, no. 7: 884-899. https://doi.org/10.3390/toxins3070884




