Molecular Conversion of Muscarinic Acetylcholine Receptor M5 to Muscarinic Toxin 7 (MT7)-Binding Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Receptor Constructs and Mutagenesis
2.3. Cell Culture and Receptor Expression
2.4. Intracellular Ca2+ Concentration Measurement
2.5. Radioligand Binding
2.6. Data Analysis
3. Results and Discussion
3.1. Mutagenesis of the M5 Receptor Subtype
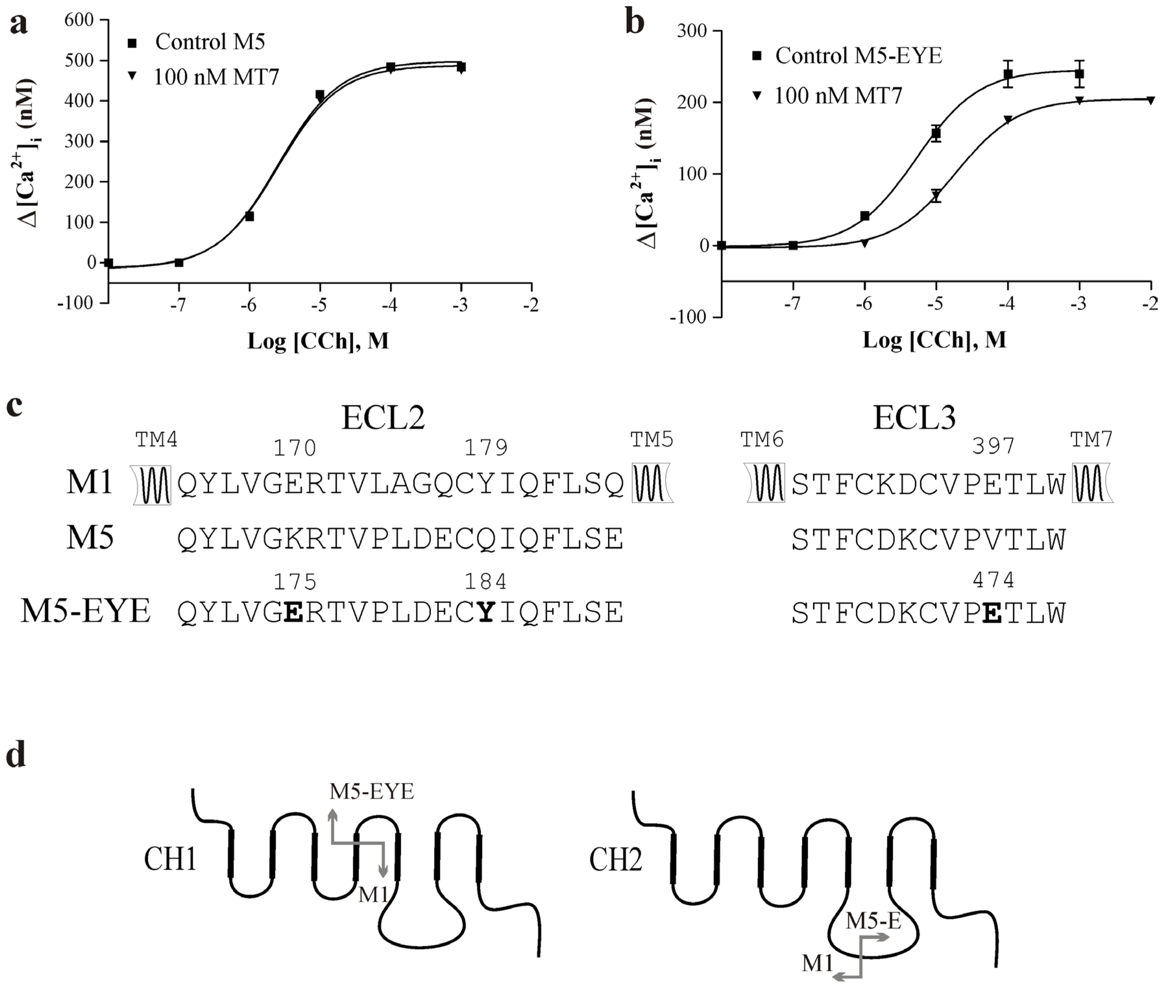
| Construct | EC50 for CCh, μM | Max. Δ[Ca2+]i, nM | App. Ki for MT7, nM |
|---|---|---|---|
| M5 | 2.56 ± 0.34 | 498 ± 10 | − |
| M5-EYE | 5.29 ± 0.03 | 246 ± 33 | 24.06 ± 4.44 |
| CH1 | 4.60 ± 1.61 | 236 ± 10 | 11.54 ± 2.56 |
| CH2 | 6.14 ± 1.10 | 293 ± 17 | 1.85 ± 0.61 |
| Construct | Kd for [3H]NMS, nM | pIC50 for MT7 | p Kx for MT7 | Cooperativity factor α |
|---|---|---|---|---|
| M5 | 0.79 ± 0.16 | − | − | − |
| M5-ELYE | 0.54 ± 0.09 | 9.22 ± 0.11 | 9.48 ± 0.10 | 0.08 ± 0.02 |
| M5-EAYE | 0.49 ± 0.08 | 8.89 ± 0.04 | 9.16 ± 0.04 | 0.08 ± 0.01 |
| M5-ELY | 0.73 ± 0.09 | 8.21 ± 0.05 | 8.38 ± 0.04 | 0.21 ± 0.01 |
3.2. The Role of the ECL3 Glutamate
3.3. Allosteric Interactions and Irreversibility of Binding
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar] [PubMed]
- Wess, J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 423–450. [Google Scholar]
- Eglen, R.M.; Choppin, A.; Dillon, M.P.; Hegde, S. Muscarinic receptor ligands and their therapeutic potential. Curr. Opin. Chem. Biol. 1999, 3, 426–432. [Google Scholar]
- Eglen, R.M.; Choppin, A.; Watson, N. Therapeutic opportunities from muscarinic receptor research. Trends Pharmacol. Sci. 2001, 22, 409–414. [Google Scholar]
- Heinrich, J.N.; Butera, J.A.; Carrick, T.; Kramer, A.; Kowal, D.; Lock, T.; Marquis, K.L.; Pausch, M.H.; Popiolek, M.; Sun, S.C.; Tseng, E.; Uveges, A.J.; Mayer, S.C. Pharmacological comparison of muscarinic ligands: Historical versus more recent muscarinic M1-preferring receptor agonists. Eur. J. Pharmacol. 2009, 605, 53–56. [Google Scholar]
- Adem, A.; Åsblom, A.; Johansson, G.; Mbugua, P.M.; Karlsson, E. Toxins from the venom of the green mamba Dendroaspis angusticeps that inhibit the binding of quinuclidinyl benzilate to muscarinic acetylcholine receptors. Biochim. Biophys. Acta 1988, 968, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Max, S.I.; Liang, J.S.; Potter, L.T. Purification and properties of m1-toxin, a specific antagonist of m1 muscarinic receptors. J. Neurosci. 1993, 13, 4293–4300. [Google Scholar]
- Tsetlin, V. Snake venom alpha-neurotoxins and other “three-finger” proteins. Eur. J. Biochem. 1999, 264, 281–286. [Google Scholar]
- Karlsson, E.; Jolkkonen, M.; Mulugeta, E.; Onali, P.; Adem, A. Snake toxins with high selectivity for subtypes of muscarinic acetylcholine receptors. Biochimie 2000, 82, 793–806. [Google Scholar]
- Ségalas, I.; Roumestand, C.; Zinn-Justin, S.; Gilquin, B.; Ménez, R.; Ménez, A.; Toma, F. Solution structure of a green mamba toxin that activates muscarinic acetylcholine receptors, as studied by nuclear magnetic resonance and molecular modeling. Biochemistry 1995, 34, 1248–1260. [Google Scholar]
- Kornisiuk, E.; Jerusalinsky, D.; Cervenansky, C.; Harvey, A.L. Binding of muscarinic toxins MTx1 and MTx2 from the venom of the green mamba Dendroaspis angusticeps to cloned human muscarinic cholinoceptors. Toxicon 1995, 33, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kornisiuk, E.; Jerusalinsky, D.; Cervenansky, C.; Harvey, A.L. Corrigendum. Toxicon 1995, 33, 1111. [Google Scholar]
- Jolkkonen, M.; van Giersbergen, P.L.; Hellman, U.; Wernstedt, C.; Karlsson, E. A toxin from the green mamba Dendroaspis angusticeps: Amino acid sequence and selectivity for muscarinic m4 receptors. FEBS Lett. 1994, 352, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.S.; Carsi-Gabrenas, J.; Krajewski, J.L.; McCafferty, J.M.; Purkerson, S.L.; Santiago, M.P.; Strauss, W.L.; Valentine, H.H.; Potter, L.T. Anti-muscarinic toxins from Dendroaspis angusticeps. Toxicon 1996, 34, 1257–1267. [Google Scholar] [PubMed]
- Näsman, J.; Jolkkonen, M.; Ammoun, S.; Karlsson, E.; Åkerman, K.E. Recombinant expression of a selective blocker of M(1) muscarinic receptors. Biochem. Biophys. Res. Commun. 2000, 271, 435–439. [Google Scholar] [PubMed]
- Mourier, G.; Dutertre, S.; Fruchart-Gaillard, C.; Menez, A.; Servent, D. Chemical synthesis of MT1 and MT7 muscarinic toxins: Critical role of Arg-34 in their interaction with M1 muscarinic receptor. Mol. Pharmacol. 2003, 63, 26–35. [Google Scholar]
- Fruchart-Gaillard, C.; Mourier, G.; Marquer, C.; Stura, E.; Birdsall, N.J.; Servent, D. Different interactions between MT7 toxin and the human muscarinic M1 receptor in its free and N-methylscopolamine-occupied states. Mol. Pharmacol. 2008, 74, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, A.; Peräkylä, M.; Åkerman, K.E.; Näsman, J. Muscarinic toxin 7 selectivity is dictated by extracellular receptor loops. J. Biol. Chem. 2004, 279, 50923–50929. [Google Scholar] [PubMed]
- Kukkonen, J.P.; Näsman, J.; Ojala, P.; Oker-Blom, C.; Åkerman, K.E.O. Functional properties of muscarinic receptor subtypes Hm1, Hm3 and Hm5 expressed in Sf9 cells using the baculovirus expression system. J. Pharmacol. Exp. Ther. 1996, 279, 593–60. [Google Scholar] [PubMed]
- Kukkonen, J.; Åkerman, K.E. Apparent noncompetitive antagonism of muscarinic receptor mediated Ca2+ mobilization by some muscarinic antagonists. Biochem. Biophys. Res. Commun. 1992, 189, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Näreoja, K.; Kukkonen, J.; Rondinelli, S.; Toivola, D.; Meriluoto, J.; Näsman, J. Adrenoceptor activity of muscarinic toxins identified from mamba venoms. Br. J. Pharmacol. 2011, 164, 538–550. [Google Scholar]
- Max, S.I.; Liang, J.S.; Potter, L.T. Stable allosteric binding of m1-toxin to m1 muscarinic receptors. Mol. Pharmacol. 1993, 44, 1171–1175. [Google Scholar]
- Fruchart-Gaillard, C.; Mourier, G.; Marquer, C.; Menez, A.; Servent, D. Identification of various allosteric interaction sites on M1 muscarinic receptor using 125I-Met35-oxidized muscarinic toxin 7. Mol. Pharmacol. 2006, 69, 1641–1651. [Google Scholar]
- Olianas, M.C.; Maullu, C.; Adem, A.; Mulugeta, E.; Karlsson, E.; Onali, P. Inhibition of acetylcholine muscarinic M(1) receptor function by the M(1)-selective ligand muscarinic toxin 7 (MT-7). Br. J. Pharmacol. 2000, 131, 447–452. [Google Scholar]
- Koivula, K.; Rondinelli, S.; Näsman, J. The three-finger toxin MTalpha is a selective alpha(2B)-adrenoceptor antagonist. Toxicon 2010, 56, 440–447. [Google Scholar]
- Marquer, C.; Fruchart-Gaillard, C.; Letellier, G.; Marcon, E.; Mourier, G.; Zinn-Justin, S.; Menez, A.; Servent, D.; Gilquin, B. Structural model of ligand-G protein-coupled receptor (GPCR) complex based on experimental double mutant cycle data: MT7 snake toxin bound to dimeric hM1 muscarinic receptor. J. Biol. Chem. 2011, 286, 31661–31675. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rondinelli, S.; Näreoja, K.; Näsman, J. Molecular Conversion of Muscarinic Acetylcholine Receptor M5 to Muscarinic Toxin 7 (MT7)-Binding Protein. Toxins 2011, 3, 1393-1404. https://doi.org/10.3390/toxins3111393
Rondinelli S, Näreoja K, Näsman J. Molecular Conversion of Muscarinic Acetylcholine Receptor M5 to Muscarinic Toxin 7 (MT7)-Binding Protein. Toxins. 2011; 3(11):1393-1404. https://doi.org/10.3390/toxins3111393
Chicago/Turabian StyleRondinelli, Sergio, Katja Näreoja, and Johnny Näsman. 2011. "Molecular Conversion of Muscarinic Acetylcholine Receptor M5 to Muscarinic Toxin 7 (MT7)-Binding Protein" Toxins 3, no. 11: 1393-1404. https://doi.org/10.3390/toxins3111393




