Anthrax Lethal Toxin-Mediated Disruption of Endothelial VE-Cadherin Is Attenuated by Inhibition of the Rho-Associated Kinase Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Antibodies
2.3. Endothelial Cell Culture and Treatment
2.4. Albumin Permeability Assay
2.5. Immunocytochemistry
2.6. Real-Time PCR
2.7. Preparation of Whole Cell Extracts
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
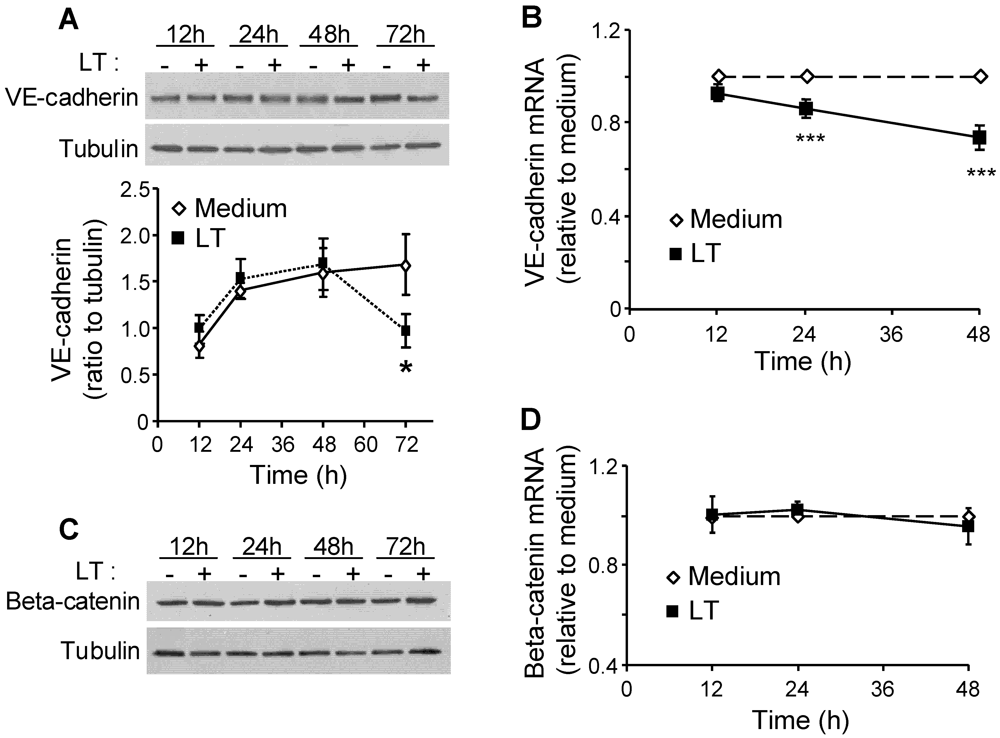
3.1. LT Reduces VE-Cadherin Protein and mRNA Expression
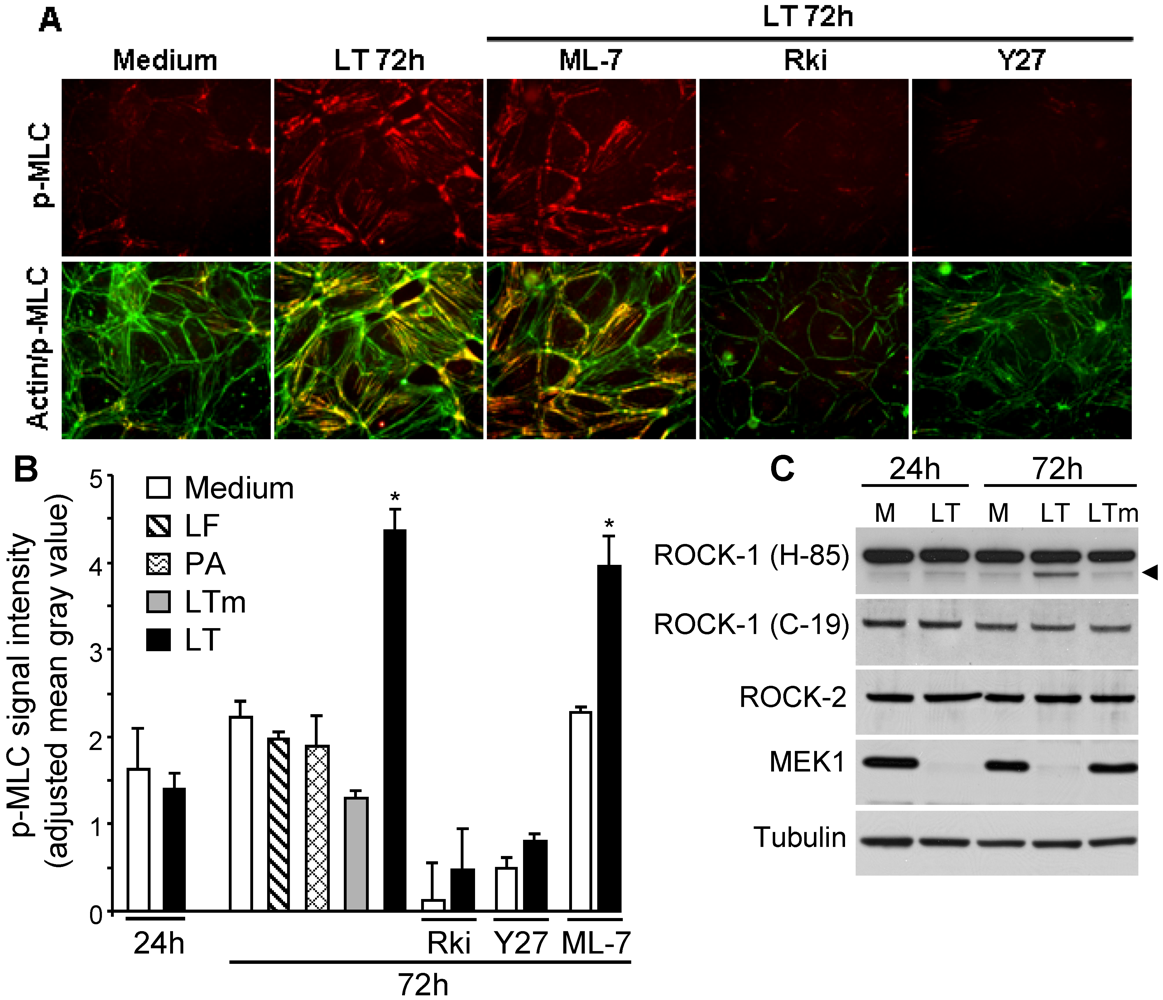
3.2. LT Induces MLC Phosphorylation that Is Attenuated by ROCK Inhibitors
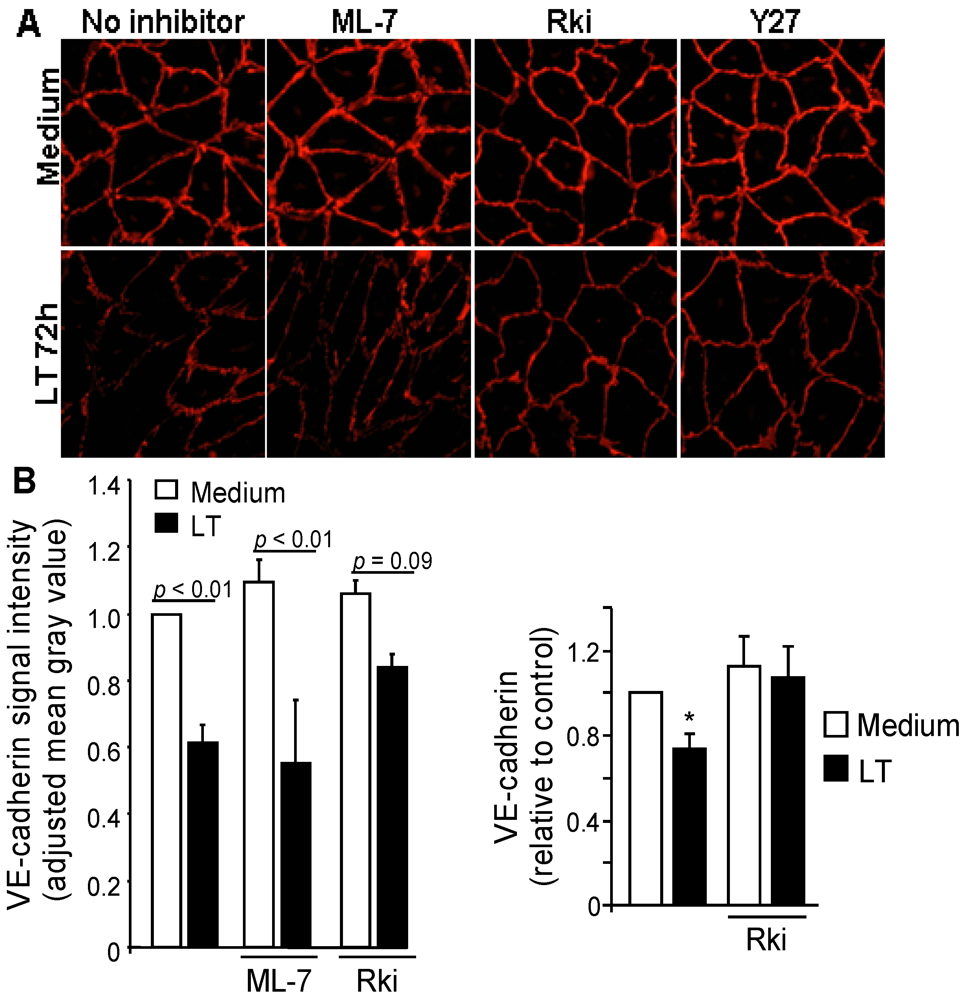
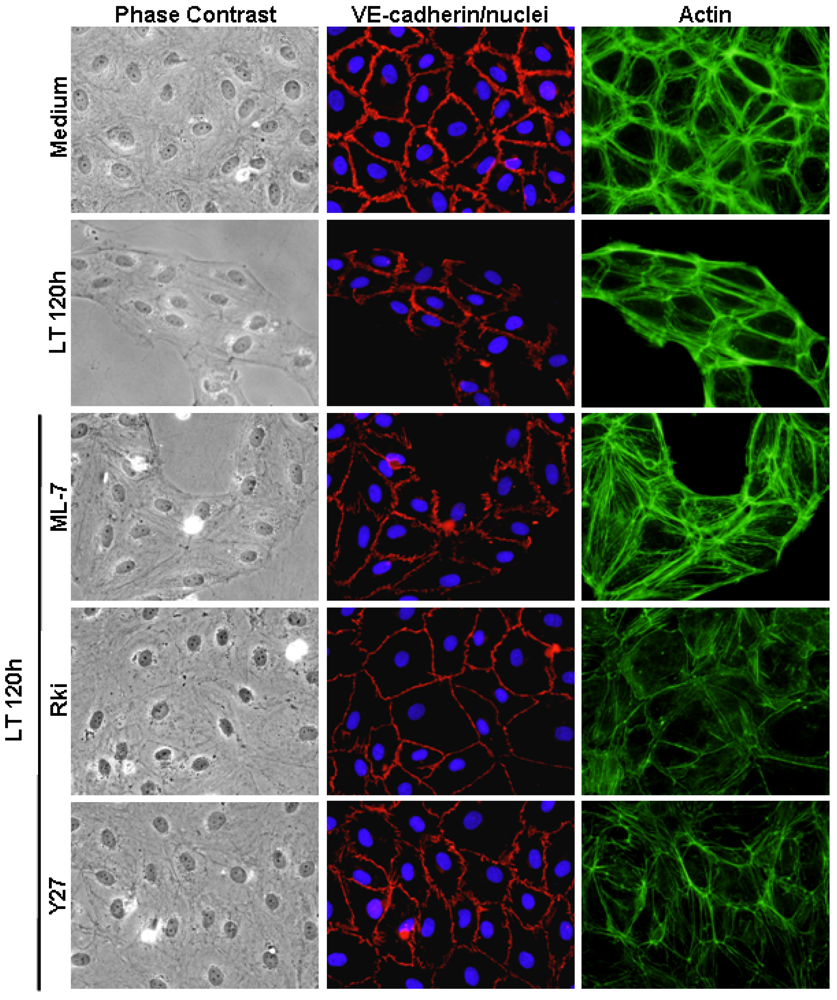
3.3. ROCK Inhibition Protects AJ Integrity
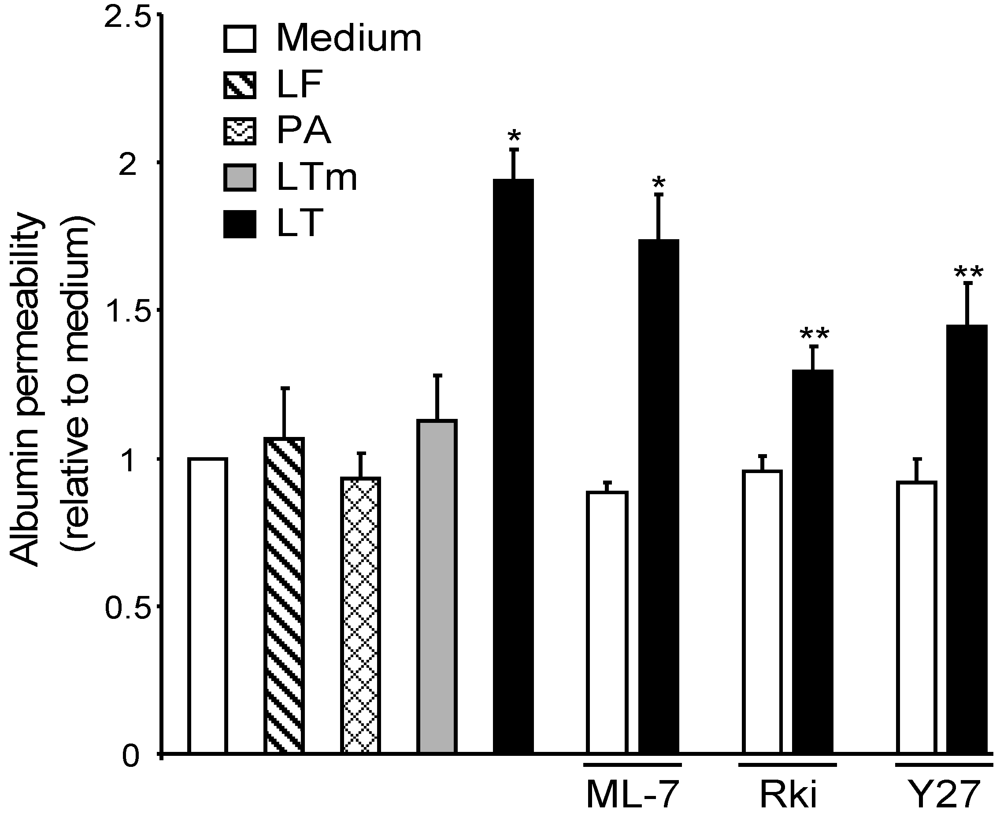
3.4. ROCK Inhibition Attenuates LT-Induced Albumin Leakage
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Moayeri, M.; Leppla, S. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 2004, 7, 19–24. [Google Scholar]
- Mourez, M. Anthrax toxins. Rev. Physiol. Biochem. Pharmacol. 2004, 152, 135–164. [Google Scholar]
- Scobie, H.; Young, J. Interactions between anthrax toxin receptors and protective antigen. Curr. Opin. Microbiol. 2005, 8, 106–112. [Google Scholar]
- Leppla, S. Anthrax toxin edema factor: A bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1982, 79, 3162–3166. [Google Scholar]
- Turk, B. Manipulation of host signalling pathways by anthrax toxins. Biochem. J. 2007, 402, 405–417. [Google Scholar]
- Duesbery, N.; Webb, C.; Leppla, S.; Gordon, V.; Klimpel, K.; Copeland, T.; Ahn, N.; Oskarsson, M.; Fukasawa, K.; Paull, K.; et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 1998, 280, 734–737. [Google Scholar] [PubMed]
- Liu, S.; Crown, D.; Miller-Randolph, S.; Moayeri, M.; Wang, H.; Hu, H.; Morley, T.; Leppla, S. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 12424–12429. [Google Scholar]
- Grinberg, L.; Abramova, F.; Yampolskaya, O.; Walker, D.; Smith, J. Quantitative pathology of inhalational anthrax I: Quantitative microscopic findings. Mod. Pathol. 2001, 14, 482–495. [Google Scholar]
- Twenhafel, N.; Leffel, E.; Pitt, M. Pathology of inhalational anthrax infection in the african green monkey. Vet. Pathol. 2007, 44, 716–721. [Google Scholar]
- Stearns-Kurosawa, D.; Lupu, F.; Taylor, F.J.; Kinasewitz, G.; Kurosawa, S. Sepsis and pathophysiology of anthrax in a nonhuman primate model. Am. J. Pathol. 2006, 169, 433–444. [Google Scholar]
- Guarner, J.; Jernigan, J.; Shieh, W.; Tatti, K.; Flannagan, L.; Stephens, D.; Popovic, T.; Ashford, D.; Perkins, B.; Zaki, S. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 2003, 163, 701–709. [Google Scholar]
- Moayeri, M.; Haines, D.; Young, H.; Leppla, S. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J. Clin. Invest. 2003, 112, 670–682. [Google Scholar] [PubMed]
- Kuo, S.; Willingham, M.; Bour, S.; Andreas, E.; Park, S.; Jackson, C.; Duesbery, N.; Leppla, S.; Tang, W.; Frankel, A. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb. Pathog. 2008, 44, 467–472. [Google Scholar]
- Moayeri, M.; Crown, D.; Dorward, D.; Gardner, D.; Ward, J.; Li, Y.; Cui, X.; Eichacker, P.; Leppla, S. The heart is an early target of anthrax lethal toxin in mice: A protective role for neuronal nitric oxide synthase (nNOS). PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef]
- Deshpande, A.; Hammon, R.; Sanders, C.; Graves, S. Quantitative analysis of the effect of cell type and cellular differentiation on protective antigen binding to human target cells. FEBS Lett. 2006, 580, 4172–4175. [Google Scholar]
- Walsh, J.; Pesik, N.; Quinn, C.; Urdaneta, V.; Dykewicz, C.; Boyer, A.; Guarner, J.; Wilkins, P.; Norville, K.; Barr, J.; et al. A case of naturally acquired inhalation anthrax: Clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin. Infect. Dis. 2007, 44, 968–971. [Google Scholar] [PubMed]
- Shoop, W.; Xiong, Y.; Wiltsie, J.; Woods, A.; Guo, J.; Pivnichny, J.; Felcetto, T.; Michael, B.; Bansal, A.; Cummings, R.; et al. Anthrax lethal factor inhibition. Proc. Natl. Acad. Sci. USA 2005, 102, 7958–7963. [Google Scholar]
- Tournier, J.; Quesnel-Hellmann, A.; Cleret, A.; Vidal, D. Contribution of toxins to the pathogenesis of inhalational anthrax. Cell. Microbiol. 2007, 9, 555–565. [Google Scholar]
- Molin, F.; Fasanella, A.; Simonato, M.; Garofolo, G.; Montecucco, C.; Tonello, F. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon 2008, 52, 824–828. [Google Scholar]
- Bolcome, R.E.; Sullivan, S.; Zeller, R.; Barker, A.; Collier, R.; Chan, J. Anthrax lethal toxin induces cell death-independent permeability in zebrafish vasculature. Proc. Natl. Acad. Sci. USA 2008, 105, 2439–2444. [Google Scholar]
- Rolando, M.; Munro, P.; Stefani, C.; Auberger, P.; Flatau, G.; Lemichez, E. Injection of Staphylococcus aureus EDIN by the Bacillus anthracis protective antigen machinery induces vascular permeability. Infect. Immun. 2009, 77, 3596–3601. [Google Scholar] [PubMed]
- Warfel, J.; Steele, A.; D’Agnillo, F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am. J. Pathol. 2005, 166, 1871–1881. [Google Scholar]
- Dejana, E.; Orsenigo, F.; Lampugnani, M. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008, 121, 2115–2122. [Google Scholar]
- Park, S.; Leppla, S. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr. Purif. 2000, 18, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Leppla, S.; Schneerson, R.; Shiloach, J. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J. Ind. Microbiol. Biotechnol. 2002, 28, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Elliott, J.; Nguyen, M.; Plant, A. Quantifying myosin light chain phosphorylation in single adherent cells with automated fluorescence microscopy. BMC Cell Biol. 2007, 8. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar]
- Warfel, J.; D’Agnillo, F. Anthrax lethal toxin enhances TNF-induced endothelial VCAM-1 expression via an IFN regulatory factor-1-dependent mechanism. J. Immunol. 2008, 180, 7516–7524. [Google Scholar]
- Bogatcheva, N.; Verin, A. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc. Res. 2008, 76, 202–207. [Google Scholar]
- Loirand, G.; Guérin, P.; Pacaud, P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 2006, 98, 322–334. [Google Scholar]
- Zhou, Q.; Gensch, C.; Liao, J.K. Rho-associated coiled-coil-forming kinases (ROCKs): Potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol. Sci. 2011, 32, 167–173. [Google Scholar]
- Sebbagh, M.; Renvoizé, C.; Hamelin, J.; Riché, N.; Bertoglio, J.; Bréard, J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 2001, 3, 346–352. [Google Scholar]
- Cui, X.; Moayeri, M.; Li, Y.; Li, X.; Haley, M.; Fitz, Y.; Correa-Araujo, R.; Banks, S.; Leppla, S.; Eichacker, P. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R699–R709. [Google Scholar]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar]
- Aktories, K.; Barbieri, J. Bacterial cytotoxins: Targeting eukaryotic switches. Nat. Rev. Microbiol. 2005, 3, 397–410. [Google Scholar]
- Rolando, M.; Stefani, C.; Flatau, G.; Auberger, P.; Mettouchi, A.; Mhlanga, M.; Rapp, U.; Galmiche, A.; Lemichez, E. Transcriptome dysregulation by anthrax lethal toxin plays a key role in induction of human endothelial cell cytotoxicity. Cell Microbiol. 2010, 12, 891–905. [Google Scholar]
- Yao, L.; Romero, M.J.; Toque, H.A.; Yang, G.; Caldwell, R.B.; Caldwell, R.W. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J. Cardiovasc. Dis. Res. 2010, 1, 165–170. [Google Scholar]
- Gong, P.; Angelini, D.; Yang, S.; Xia, G.; Cross, A.; Mann, D.; Bannerman, D.; Vogel, S.; Goldblum, S. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endotheli. J. Biol. Chem. 2008, 283, 13437–13449. [Google Scholar]
- Gillrie, M.; Krishnegowda, G.; Lee, K.; Buret, A.; Robbins, S.; Looareesuwan, S.; Gowda, D.C.; Ho, M. Src family kinase-dependent disruption of endothelial barrier function by Plasmodium falciparum merozoite proteins. Blood 2007, 110, 3426–3435. [Google Scholar] [PubMed]
- Farkas, A.; Szatmári, E.; Orbók, A.; Wilhelm, I.; Wejksza, K.; Nagyoszi, P.; Hutamekalin, P.; Bauer, H.; Bauer, H.; Traweger, A.; et al. Hyperosmotic mannitol induces Src kinase-dependent phosphorylation of beta-catenin in cerebral endothelial cells. J. Neurosci. Res. 2005, 80, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Barbero, S.; Cheresh, D. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J. Biol. Chem. 2005, 280, 31906–31912. [Google Scholar]
- Jernigan, J.; Stephens, D.; Ashford, D.; Omenaca, C.; Topiel, M.; Galbraith, M.; Tapper, M.; Fisk, T.; Zaki, S.; Popovic, T.; et al. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg. Infect. Dis. 2001, 7, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.; Brachman, P.; Utell, M.; Bumford, F.; Atchison, M. An epidemic of inhalation anthrax, the first in the twentieth century: I. Clinical features. 1960. Am. J. Med. 2002, 122, 4-12, discussion 12-13. [Google Scholar]
- Smith, H. Discovery of the anthrax toxin: The beginning of studies of virulence determinants regulated in vivo. Int. J. Med. Microbiol. 2002, 291, 411–417. [Google Scholar] [PubMed]
- Migone, T.; Subramanian, G.; Zhong, J.; Healey, L.; Corey, A.; Devalaraja, M.; Lo, L.; Ullrich, S.; Zimmerman, J.; Chen, A.; et al. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 2009, 361, 135–144. [Google Scholar] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Warfel, J.M.; D’Agnillo, F. Anthrax Lethal Toxin-Mediated Disruption of Endothelial VE-Cadherin Is Attenuated by Inhibition of the Rho-Associated Kinase Pathway. Toxins 2011, 3, 1278-1293. https://doi.org/10.3390/toxins3101278
Warfel JM, D’Agnillo F. Anthrax Lethal Toxin-Mediated Disruption of Endothelial VE-Cadherin Is Attenuated by Inhibition of the Rho-Associated Kinase Pathway. Toxins. 2011; 3(10):1278-1293. https://doi.org/10.3390/toxins3101278
Chicago/Turabian StyleWarfel, Jason M., and Felice D’Agnillo. 2011. "Anthrax Lethal Toxin-Mediated Disruption of Endothelial VE-Cadherin Is Attenuated by Inhibition of the Rho-Associated Kinase Pathway" Toxins 3, no. 10: 1278-1293. https://doi.org/10.3390/toxins3101278



