AIP56: A Novel Bacterial Apoptogenic Toxin
Abstract
:1. The Apoptogenic Exotoxin AIP56 Is the Key Virulence Factor of Photobacterium damselae subsp. piscicida
| Accession | Description | Max score | Total score | Query coverage | E value |
|---|---|---|---|---|---|
| YP_003422532.1 | Aip56 [Photobacterium damselae subsp. piscicida] | 1064 | 1064 | 98% | 0.0 |
| BAF99004.1 | apoptosis inducing protein [Photobacterium damselae subsp. piscicida] | 1046 | 1046 | 98% | 0.0 |
| ZP_02194626.1 | hypothetical protein 1103602000593_AND4_00648 [Vibrio sp. AND4] | 597 | 597 | 99% | 9.00E-169 |
| CBA72068.1 | non-LEE encoded type III effector C [Arsenophonus nasoniae] | 306 | 306 | 94% | 3.00E-81 |
| CBA72300.1 | apoptosis inducing protein [Arsenophonus nasoniae] | 281 | 281 | 93% | 1.00E-73 |
| CBA76058.1 | non-LEE encoded type III effector C [Arsenophonus nasoniae] | 275 | 275 | 93% | 7.00E-72 |
| YP_002308522.1 | hypothetical protein D [Bacteriophage APSE-2] | 194 | 194 | 36% | 2.00E-47 |
| CBA74519.1 | non-LEE encoded type III effector C [Arsenophonus nasoniae] | 192 | 192 | 94% | 8.00E-47 |
| ZP_04620510.1 | Non-LEE encoded type III effector C [Yersinia aldovae ATCC 35236] | 178 | 178 | 48% | 2.00E-42 |
| NP_286533.1 | hypothetical protein Z0986 [Escherichia coli O157:H7 EDL933] | 178 | 178 | 50% | 2.00E-42 |
| YP_003229213.1 | T3SS secreted effector NleC-like protein [Escherichia coli O26:H11 str. 11368] | 178 | 178 | 50% | 2.00E-42 |
| YP_002328603.1 | T3SS secreted effector NleC homolog [Escherichia coli O127:H6 str. E2348/69] | 178 | 178 | 50% | 2.00E-42 |
| YP_003234807.1 | T3SS secreted effector NleC-like protein [Escherichia coli O111:H- str. 11128] | 177 | 177 | 50% | 4.00E-42 |
| YP_003234967.1 | T3SS secreted effector NleC-like protein [Escherichia coli O111:H- str. 11128] | 174 | 174 | 50% | 2.00E-41 |
| YP_003365223.1 | T3SS effector protein NleC [Citrobacter rodentium ICC168] | 173 | 173 | 48% | 6.00E-41 |
| ZP_03043710.1 | non-LEE encoded type III effector C [Escherichia coli E22] | 172 | 172 | 50% | 7.00E-41 |
| ZP_02576318.1 | non-LEE encoded type III effector C [Salmonella enterica subsp. enterica serovar 4,[5],12:i:- str. CVM23701] | 169 | 169 | 48% | 9.00E-40 |
| YP_001762636.1 | hypothetical protein Swoo_4286 [Shewanella woodyi ATCC 51908] | 83.2 | 83.2 | 28% | 6.00E-14 |
| ZP_03318593.1 | hypothetical protein PROVALCAL_01527 [Providencia alcalifaciens DSM 30120] | 38.9 | 38.9 | 25% | 1.7 |

2. AIP56 Induces a Typical Apoptotic Death in Fish Macrophages and Neutrophils
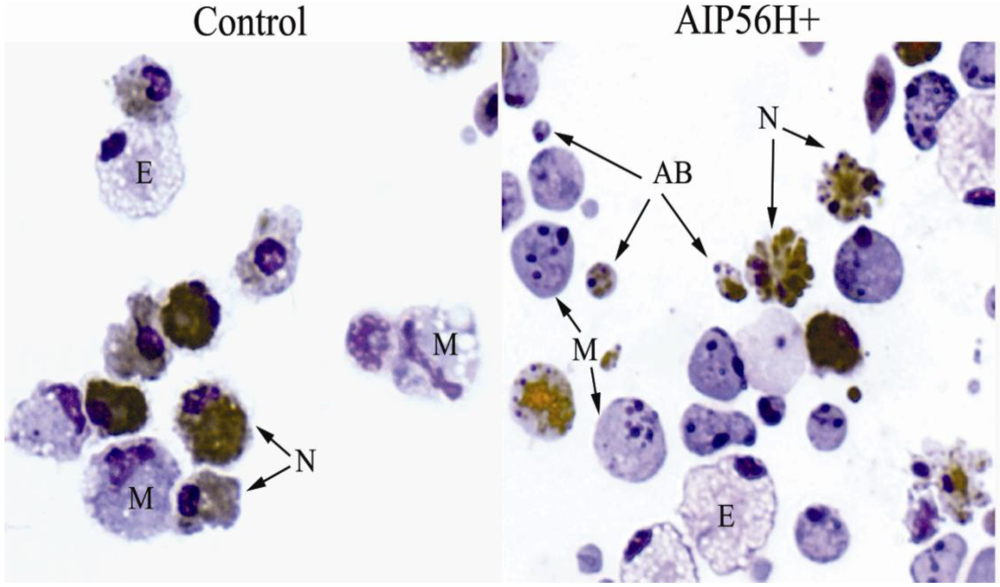
3. Pathogenetic Consequences of the in Vivo Cytopathological Effects of AIP56 Toxin

Acknowledgements
References and Notes
- Snieszko, S.F.; Bullock, G.L.; Hollis, E.; Boone, J.G. Pasteurella Sp. from an Epizootic of White Perch (Roccus Americanus) in Chesapeake Bay Tidewater Areas. J. Bacteriol. 1964, 88, 1814–1815. [Google Scholar] [PubMed]
- Janssen, W.A.; Surgalla, M.J. Morphology, physiology, and serology of a Pasteurella species pathogenic for white perch. (Roccus americanus). J. Bacteriol. 1968, 96, 1606–1610. [Google Scholar] [PubMed]
- Gauthier, G.; Lafay, B.; Ruimy, R.; Breittmayer, V.; Nicolas, J.L.; Gauthier, M.; Christen, R. Small-subunit rRNA sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (Snieszko et al.) Janssen and Surgalla to the genus Photobacterium as Photobacterium damsela subsp. piscicida comb. nov. Int. J. Syst. Bacteriol. 1995, 45, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Trüper, H.G.; de’Clari, L. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) ‘in apposition’. Int. J. Syst. Bacteriol. 1997, 47, 908–909. [Google Scholar]
- Thune, R.L.; Stanley, L.A.; Cooper, R.K. Pathogenesis of Gram-negative bacterial infections in warmwater fish. Annu. Rev. Fish Dis. 1993, 3, 337–368. [Google Scholar]
- Magariños, B.; Toranzo, A.E.; Romalde, J.L. Phenotypic and pathobiological characteristics of Pasteurella piscicida. Ann. Rev. Fish Dis. 1996, 6, 41–64. [Google Scholar]
- Romalde, J.L. Photobacterium damselae subsp. piscicida: an integrated view of a bacterial fish pathogen. Int. Microbiol. 2002, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.C.; dos Santos, N.M.S.; Ellis, A.E. Update on bacterial vaccines: Photobacterium damselae subsp. piscicida. In Progress in Fish Immunology; Mydtlyng, P.J., Ed.; Karger: Basel, Switzerland, 2005; Volume 121, pp. 75–84. [Google Scholar]
- Hawke, J.P.; Plakas, S.M.; Vernon Minton, R.; McPhearson, R.M.; Snider, T.G.; Guarino, A.M. Fish pasteurellosis of cultured striped bass (Morone saxatilis) in coastal Alabama. Aquaculture 1987, 65, 193–204. [Google Scholar]
- Toranzo, A.E.; Barreiro, S.; Casal, J.F.; Figueras, A.; Magarinos, B.; Barja, J.L. Pasteurellosis in cultured gilthead seabream (Sparus aurata): first report in Spain. Aquaculture 1991, 99, 1–15. [Google Scholar]
- Magariños, B.; Santos, Y.; Romalde, J.L.; Rivas, C.; Barja, J.L.; Toranzo, A.E. Pathogenic activities of live cells and extracellular products of the fish pathogen Pasteurella piscicida. J. Gen. Microbiol. 1992, 138, 2491–2498. [Google Scholar]
- Kusuda, R.; Salati, F. Major bacterial diseases affecting mariculture in Japan. Annu. Rev. Fish Dis. 1993, 3, 69–85. [Google Scholar]
- Noya, M.; Magarinos, B.; Toranzo, A.E.; Lamas, J. Sequential pathology of experimental pasteurellosis in gilthead seabream Sparus aurata. A light- and electron-microscopic study. Dis. Aquat. Org. 1995, 21, 177–186. [Google Scholar] [CrossRef]
- Bakopoulos, V.; Peric, Z.; Rodger, H.; Adams, A.; Richards, R. First report of fish pasteurellosis from Malta. J. Aquat. Anim. Health 1997, 9, 26–33. [Google Scholar]
- Poulos, C.; Bakopoulos, V.; Zolota, V.; Dimitriadis, G.J. Histopathological findings after sea bass (Dicentrarhus labrax L.) exposure to extracellular products of Photobacterium damselae subsp. piscicida produced in vivo. Aquaculture Res. 2004, 35, 931–936. [Google Scholar] [CrossRef]
- Bakopoulos, V.; Hanif, A.; Poulos, K.; Galeotti, M.; Adams, A.; Dimitriadis, G.J. The effect of in vivo growth on the cellular and extracellular components of the marine bacterial pathogen Photobacterium damsela subsp. piscicida. J. Fish Dis. 2004, 27, 1–13. [Google Scholar]
- do Vale, A.; Marques, F.; Silva, M.T. Apoptosis of sea bass (Dicentrarchus labrax L.) neutrophils and macrophages induced by experimental infection with Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 2003, 15, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Romalde, J.L.; Magariños, B. Immunization with bacterial antigens: pasteurellosis. In Fish Vaccinology; Gudding, R., Lillehaug, A., Midtlyng, P.J., Brown, F., Eds.; Karger: Basel, Switzerland, 1997; pp. 167–177. [Google Scholar]
- do Vale, A.; Silva, M.T.; dos Santos, N.M.; Nascimento, D.S.; Reis-Rodrigues, P.; Costa-Ramos, C.; Ellis, A.E.; Azevedo, J.E. AIP56, a novel plasmid-encoded virulence factor of Photobacterium damselae subsp. piscicida with apoptogenic activity against sea bass macrophages and neutrophils. Mol. Microbiol. 2005, 58, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulos, V.; Adams, A.; Richards, R.H. The effect of iron limitation growth conditions on the cell and extracellular components of the fish pathogen Pasteurella piscicida. J. Fish Dis. 1997, 20, 297–305. [Google Scholar]
- San Luis, B.B.; Hedreyda, C.T. Analysis of a gene (vch) encoding hemolysin isolated and sequenced from Vibrio campbellii. J. Gen. Appl. Microbiol. 2006, 52, 303–313. [Google Scholar]
- Darby, A.C.; Choi, J.H.; Wilkes, T.; Hughes, M.A.; Werren, J.H.; Hurst, G.D.; Colbourne, J.K. Characteristics of the genome of Arsenophonus nasoniae, son-killer bacterium of the wasp Nasonia. Insect. Mol. Biol. 2010, 19 (Suppl 1), 75–89. [Google Scholar]
- Degnan, P.H.; Yu, Y.; Sisneros, N.; Wing, R.A.; Moran, N.A. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc. Natl. Acad. Sci. USA 2009, 106, 9063–9068. [Google Scholar]
- Schiavo, G.; Benfenati, F.; Poulain, B.; Rossetto, O.; Polverino de Laureto, P.; DasGupta, B.R.; Montecucco, C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 1992, 359, 832–835. [Google Scholar]
- Rossetto, O.; Seveso, M.; Caccin, P.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: turning bad guys into good by research. Toxicon 2001, 39, 27–41. [Google Scholar]
- Nielsen, H.; Engelbrecht, J.; Brunak, S.; von Heijne, G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997, 10, 1–6. [Google Scholar]
- Bendtsen, J.D.; Nielsen, H.; von Heijne, G.; Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004, 340, 783–795. [Google Scholar]
- Hacker, J.; Blum-Oehler, G.; Muhldorfer, I.; Tschape, H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 1997, 23, 1089–1097. [Google Scholar]
- do Vale, A.; Costa-Ramos, C.; Silva, A.; Silva, D.S.; Gartner, F.; dos Santos, N.M.; Silva, M.T. Systemic macrophage and neutrophil destruction by secondary necrosis induced by a bacterial exotoxin in a Gram-negative septicaemia. Cell. Microbiol. 2007, 9, 988–1003. [Google Scholar]
- Wyllie, A.H.; Kerr, J.F.; Currie, A.R. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar]
- Pallardy, M.; Biola, A.; Lebrec, H.; Breard, J. Assessment of apoptosis in xenobiotic-induced immunotoxicity. Methods 1999, 19, 36–47. [Google Scholar]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar]
- Reis, M.I.; do Vale, A.; Pinto, C.; Nascimento, D.S.; Costa-Ramos, C.; Silva, D.S.; Silva, M.T.; dos Santos, N.M. First molecular cloning and characterisation of caspase-9 gene in fish and its involvement in a gram negative septicaemia. Mol. Immunol. 2007, 44, 1754–1764. [Google Scholar]
- Reis, M.I.; Nascimento, D.S.; do Vale, A.; Silva, M.T.; dos Santos, N.M. Molecular cloning and characterisation of sea bass (Dicentrarchus labrax L.) caspase-3 gene. Mol. Immunol. 2007, 44, 774–783. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar]
- Henson, P.M.; Hume, D.A. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006, 27, 244–250. [Google Scholar]
- Domingos, P.M.; Steller, H. Pathways regulating apoptosis during patterning and development. Curr. Opin. Genet. Dev. 2007, 17, 294–299. [Google Scholar]
- Don, M.M.; Ablett, G.; Bishop, C.J.; Bundesen, P.G.; Donald, K.J.; Searle, J.; Kerr, J.F. Death of cells by apoptosis following attachment of specifically allergized lymphocytes in vitro. Aust. J. Exp. Biol. Med. Sci. 1977, 55, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.M.; Bird, C.C.; Waddell, A.W.; Currie, A.R. Morphological aspects of glucocorticoid-induced cell death in human lymphoblastoid cells. J. Pathol. 1978, 126, 181–187. [Google Scholar]
- Wood, W.; Turmaine, M.; Weber, R.; Camp, V.; Maki, R.A.; McKercher, S.R.; Martin, P. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development 2000, 127, 5245–5252. [Google Scholar] [PubMed]
- Parnaik, R.; Raff, M.C.; Scholes, J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 2000, 10, 857–860. [Google Scholar]
- Silva, M.T.; do Vale, A.; dos Santos, N.M. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis 2008, 13, 463–482. [Google Scholar]
- Ogasawara, J.; Watanabe-Fukunaga, R.; Adachi, M.; Matsuzawa, A.; Kasugai, T.; Kitamura, Y.; Itoh, N.; Suda, T.; Nagata, S. Lethal effect of the anti-Fas antibody in mice. Nature 1993, 364, 806–809. [Google Scholar]
- Haslett, C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 1999, 160, S5–S11. [Google Scholar]
- Devitt, A.; Parker, K.G.; Ogden, C.A.; Oldreive, C.; Clay, M.F.; Melville, L.A.; Bellamy, C.O.; Lacy-Hulbert, A.; Gangloff, S.C.; Goyert, S.M.; Gregory, C.D. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14-/- mice. J. Cell Biol. 2004, 167, 1161–1170. [Google Scholar]
- Medan, D.; Wang, L.; Yang, X.; Dokka, S.; Castranova, V.; Rojanasakul, Y. Induction of neutrophil apoptosis and secondary necrosis during endotoxin-induced pulmonary inflammation in mice. J. Cell Physiol. 2002, 191, 320–326. [Google Scholar]
- Knapp, S.; Leemans, J.C.; Florquin, S.; Branger, J.; Maris, N.A.; Pater, J.; van Rooijen, N.; van der Poll, T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 2003, 167, 171–179. [Google Scholar]
- Li, M.O.; Sarkisian, M.R.; Mehal, W.Z.; Rakic, P.; Flavell, R.A. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science 2003, 302, 1560–1563. [Google Scholar]
- Bianchi, S.M.; Prince, L.R.; McPhillips, K.; Allen, L.; Marriott, H.M.; Taylor, G.W.; Hellewell, P.G.; Sabroe, I.; Dockrell, D.H.; Henson, P.W.; Whyte, M.K. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2008, 177, 35–43. [Google Scholar]
- Weinrauch, Y.; Zychlinsky, A. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 1999, 53, 155–187. [Google Scholar]
- Narayanan, S.K.; Nagaraja, T.G.; Chengappa, M.M.; Stewart, G.C. Leukotoxins of gram-negative bacteria. Vet. Microbiol. 2002, 84, 337–356. [Google Scholar]
- DeLeo, F.R. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 2004, 9, 399–413. [Google Scholar]
- Kennedy, A.D.; DeLeo, F.R. Neutrophil apoptosis and the resolution of infection. Immunol. Res. 2009, 43, 25–61. [Google Scholar]
- do Vale, A.; Costa-Ramos, C.; Silva, D.S.; Macedo, P.M.; Fernandes, R.; Sampaio, P.; dos Santos, N.M.; Silva, M.T. Cytochemical and ultrastructural study of anoikis and secondary necrosis in enterocytes detached in vivo. Apoptosis 2007, 12, 1069–1083. [Google Scholar]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell. Biol. 1994, 124, 619–626. [Google Scholar]
- Denecker, G.; Vercammen, D.; Steemans, M.; Vanden Berghe, T.; Brouckaert, G.; Van Loo, G.; Zhivotovsky, B.; Fiers, W.; Grooten, J.; Declercq, W.; Vandenabeele, P. Death receptor-induced apoptotic and necrotic cell death: differential role of caspases and mitochondria. Cell. Death Differ. 2001, 8, 829–840. [Google Scholar]
- Sun, F.; Hamagawa, E.; Tsutsui, C.; Ono, Y.; Ogiri, Y.; Kojo, S. Evaluation of oxidative stress during apoptosis and necrosis caused by carbon tetrachloride in rat liver. Biochim. Biophys. Acta 2001, 1535, 186–191. [Google Scholar]
- Hentze, H.; Schwoebel, F.; Lund, S.; Keel, M.; Ertel, W.; Wendel, A.; Jaattela, M.; Leist, M. In vivo and in vitro evidence for extracellular caspase activity released from apoptotic cells. Biochem. Biophys. Res. Commun. 2001, 283, 1111–1117. [Google Scholar]
- Holdenrieder, S.; Eichhorn, P.; Beuers, U.; Samtleben, W.; Schoenermarck, U.; Zachoval, R.; Nagel, D.; Stieber, P. Nucleosomal DNA fragments in autoimmune diseases. Ann. N.Y. Acad. Sci. 2006, 1075, 318–327. [Google Scholar]
- Henson, P.M.; Johnston, R.B., Jr. Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J. Clin. Invest. 1987, 79, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar]
- Kawabata, K.; Hagio, T.; Matsuoka, S. The role of neutrophil elastase in acute lung injury. Eur. J. Pharmacol. 2002, 451, 1–10. [Google Scholar]
- Voynow, J.A.; Fischer, B.M.; Zheng, S. Proteases and cystic fibrosis. Int. J. Biochem. Cell Biol. 2008, 40, 1238–1245. [Google Scholar]
- Silva, M.T. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 2010, 87, 93–106. [Google Scholar]
- Silva, M.T. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J. Leukoc. Biol. 2010. [Google Scholar]
- Monack, D.; Falkow, S. Apoptosis as a common bacterial virulence strategy. Int. J. Med. Microbiol. 2000, 290, 7–13. [Google Scholar]
- Bhavsar, A.P.; Guttman, J.A.; Finlay, B.B. Manipulation of host-cell pathways by bacterial pathogens. Nature 2007, 449, 827–834. [Google Scholar]
- Cascales, E. The type VI secretion toolkit. EMBO Rep. 2008, 9, 735–741. [Google Scholar]
- Tateda, K.; Ishii, Y.; Horikawa, M.; Matsumoto, T.; Miyairi, S.; Pechere, J.C.; Standiford, T.J.; Ishiguro, M.; Yamaguchi, K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 2003, 71, 5785–5793. [Google Scholar]
- Allen, L.; Dockrell, D.H.; Pattery, T.; Lee, D.G.; Cornelis, P.; Hellewell, P.G.; Whyte, M.K. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J. Immunol. 2005, 174, 3643–3649. [Google Scholar]
- Lukaszewski, R.A.; Kenny, D.J.; Taylor, R.; Rees, D.G.; Hartley, M.G.; Oyston, P.C. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 2005, 73, 7142–7150. [Google Scholar]
- Wickstrum, J.R.; Bokhari, S.M.; Fischer, J.L.; Pinson, D.M.; Yeh, H.W.; Horvat, R.T.; Parmely, M.J. Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect. Immun. 2009, 77, 4827–4836. [Google Scholar]
- Parmely, M.J.; Fischer, J.L.; Pinson, D.M. Programmed cell death and the pathogenesis of tissue injury induced by type A Francisella tularensis. FEMS Microbiol. Lett. 2009, 301, 1–11. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Silva, M.T.; Dos Santos, N.M.S.; Do Vale, A. AIP56: A Novel Bacterial Apoptogenic Toxin. Toxins 2010, 2, 905-918. https://doi.org/10.3390/toxins2040905
Silva MT, Dos Santos NMS, Do Vale A. AIP56: A Novel Bacterial Apoptogenic Toxin. Toxins. 2010; 2(4):905-918. https://doi.org/10.3390/toxins2040905
Chicago/Turabian StyleSilva, Manuel T., Nuno M. S. Dos Santos, and Ana Do Vale. 2010. "AIP56: A Novel Bacterial Apoptogenic Toxin" Toxins 2, no. 4: 905-918. https://doi.org/10.3390/toxins2040905






