Marine Toxins: Chemistry, Toxicity, Occurrence and Detection, with Special Reference to the Dutch Situation
Abstract
:1. Introduction

2. Poisoning Syndromes and Corresponding Toxins
2.1. Hydrophilic toxins
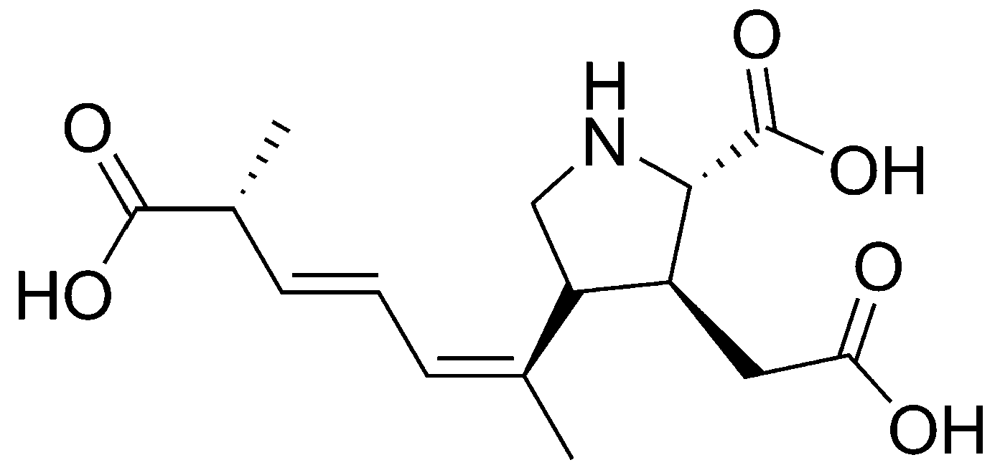
2.1.1. Amnesic shellfish poisoning (ASP)
| Toxin group | Syndrome | Genus | Species | Reference | |
|---|---|---|---|---|---|
| Hydrophilic toxins | Domoic acid | ASP | Pseudo-nitzschia | australis, calliantha, cuspidata, delicatissima, fraudulenta, galaxiae, multiseries, multistriata, pseudodelicatissima, pungens, seriata, turgidula | [25] |
| Saxitoxins | PSP | Alexandrium | angustitabulatum, catenella, fundyense, lusitanicum, minutum, tamarense, tamiyavanichii | [26,27,28] | |
| Gymnodinium | catenatum | [26] | |||
| Pyrodinium | bahamense | [26] | |||
| Lipophilic toxins | Brevetoxins | NSP | Karenia | brevis, brevisulcata, mikimotoi, selliformis, papilionacea | [8,29] [30] |
| Chatonella | cf. verruculosa | [30] | |||
| Okadaic acid and dinophysistoxins and pectenotoxins 1 | DSP | Phalacroma | rotundatum | [31] | |
| Prorocentrum | arenarium, belizeanum, concavem, lima | [32] | |||
| Dinophysis | acuminata, acuta, arenarium, caudate, fortii, mitra, norvegica, ovum, rotundata, sacculus, tripos | [33,34,35,36,37,38] | |||
| Yessotoxins | Protoceratium | reticulatum | [29,39] | ||
| Lingulodinium | polyedrum | [29] | |||
| Gonyaulax | polyhedra | [29] | |||
| Azaspiracids | AZP | Azadinium | spinosum | [40] | |
| Spirolides | – | Alexandrium | ostenfeldii, peruvianum | [41,42] | |
| Gymnodimines | – | Karenia | selliforme | [43] | |
| Gymnodium | mikimotoi | [44] |
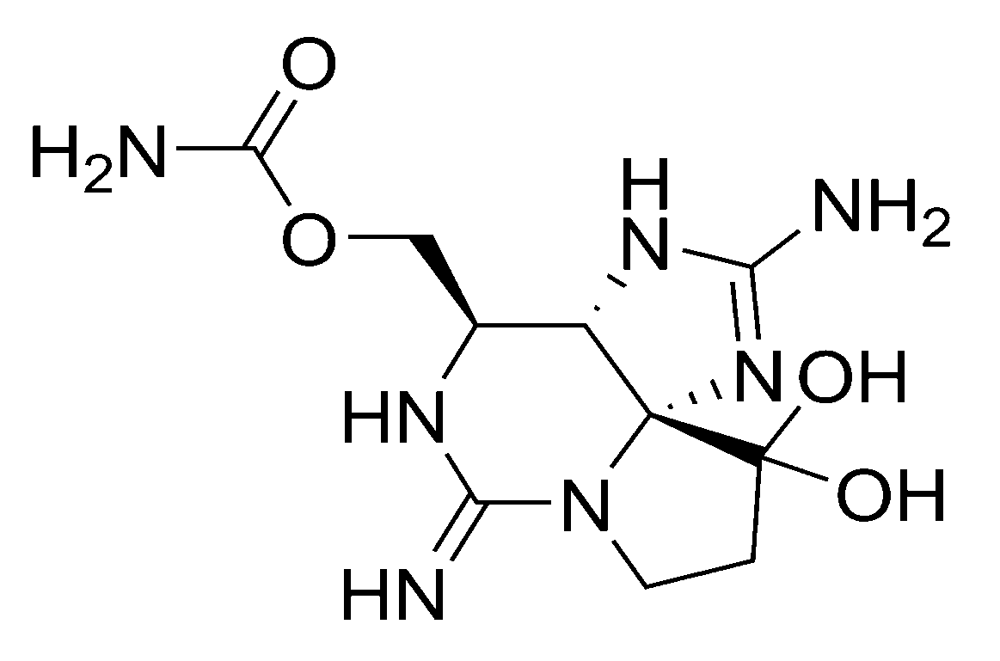
2.1.2. Paralytic shellfish poisoning (PSP)
2.2. Lipophilic toxins
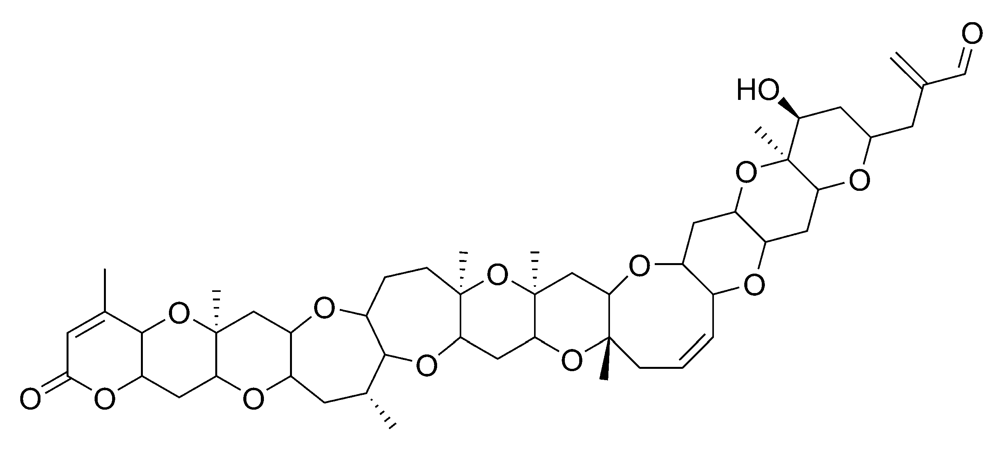
2.2.1. Neurologic shellfish poisoning (NSP)
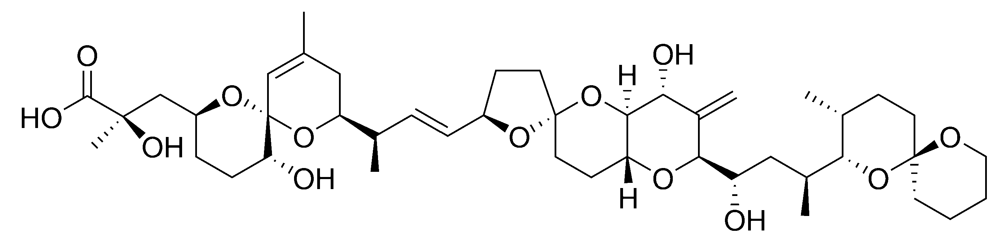
2.2.2. Diarrhetic shellfish poisoning (DSP)
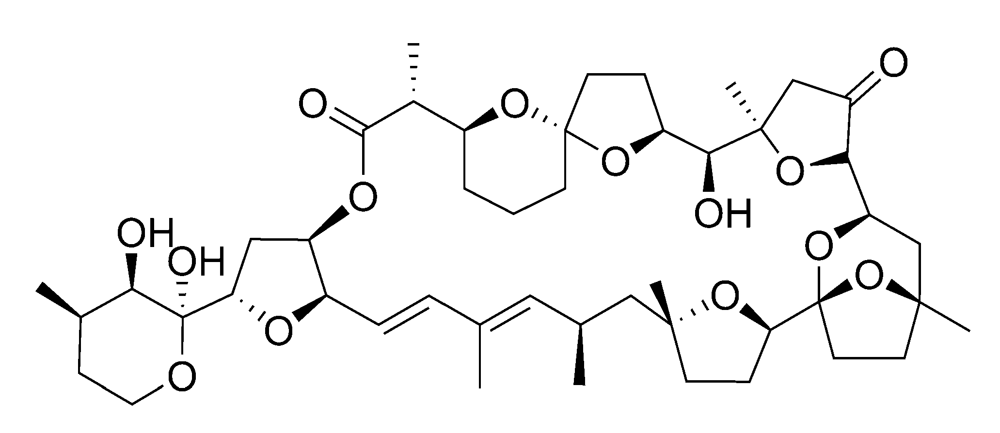
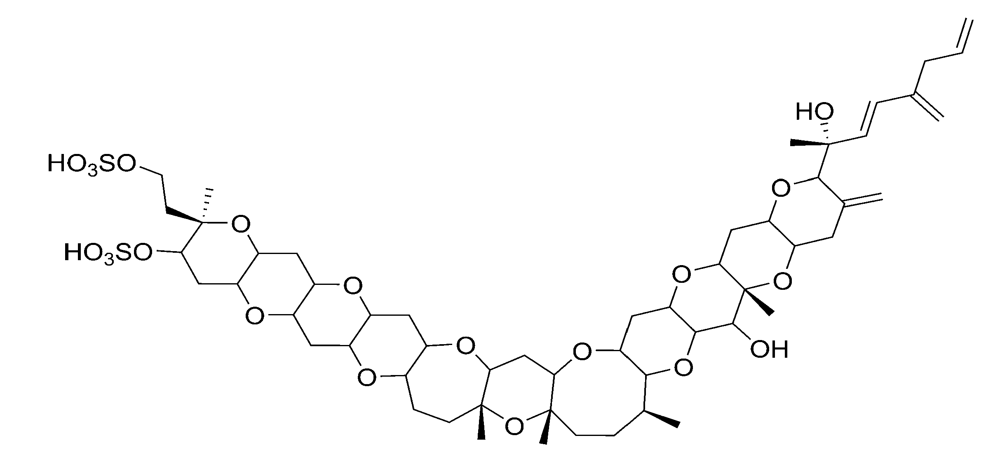
2.2.3. Azaspiracid shellfish poisoning (AZP)
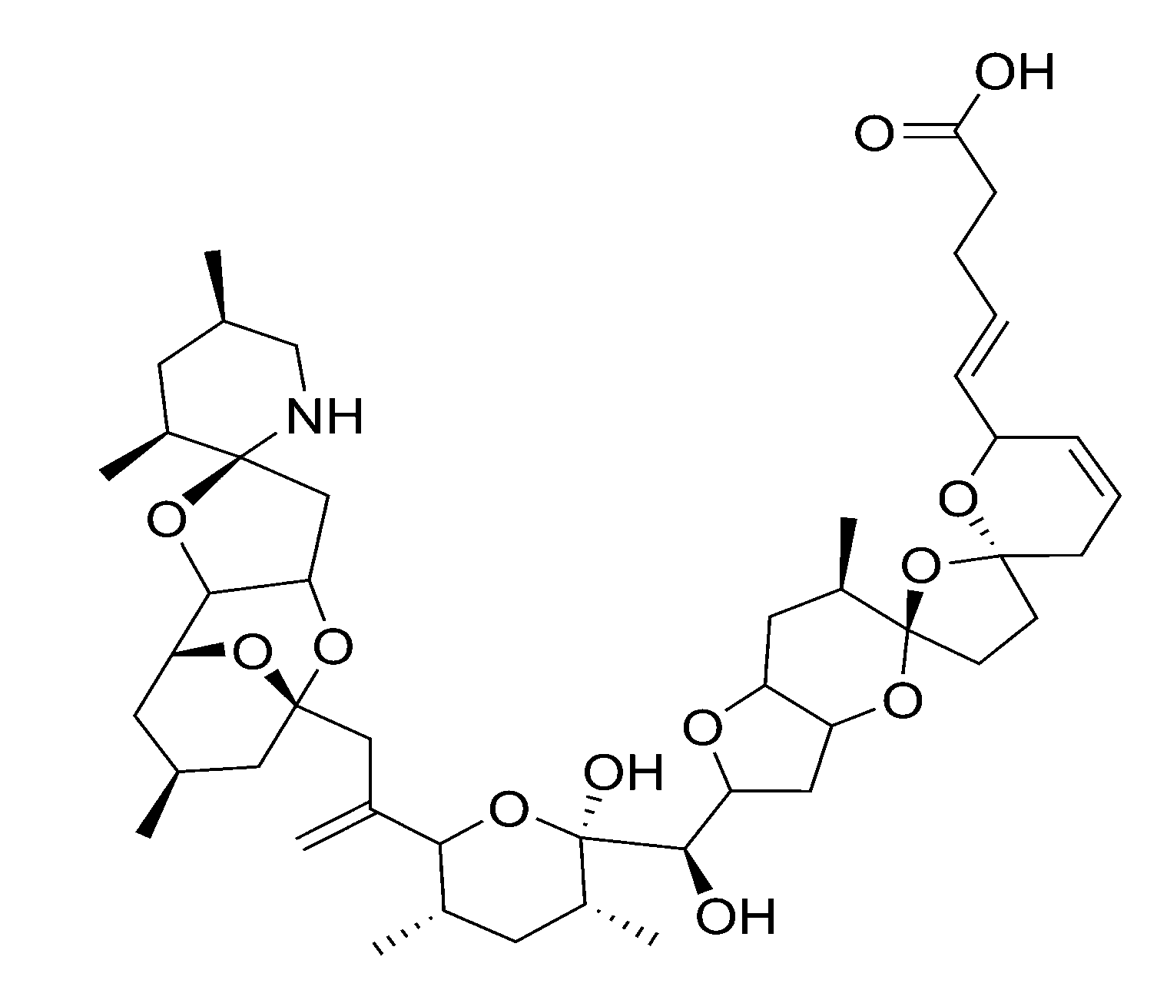
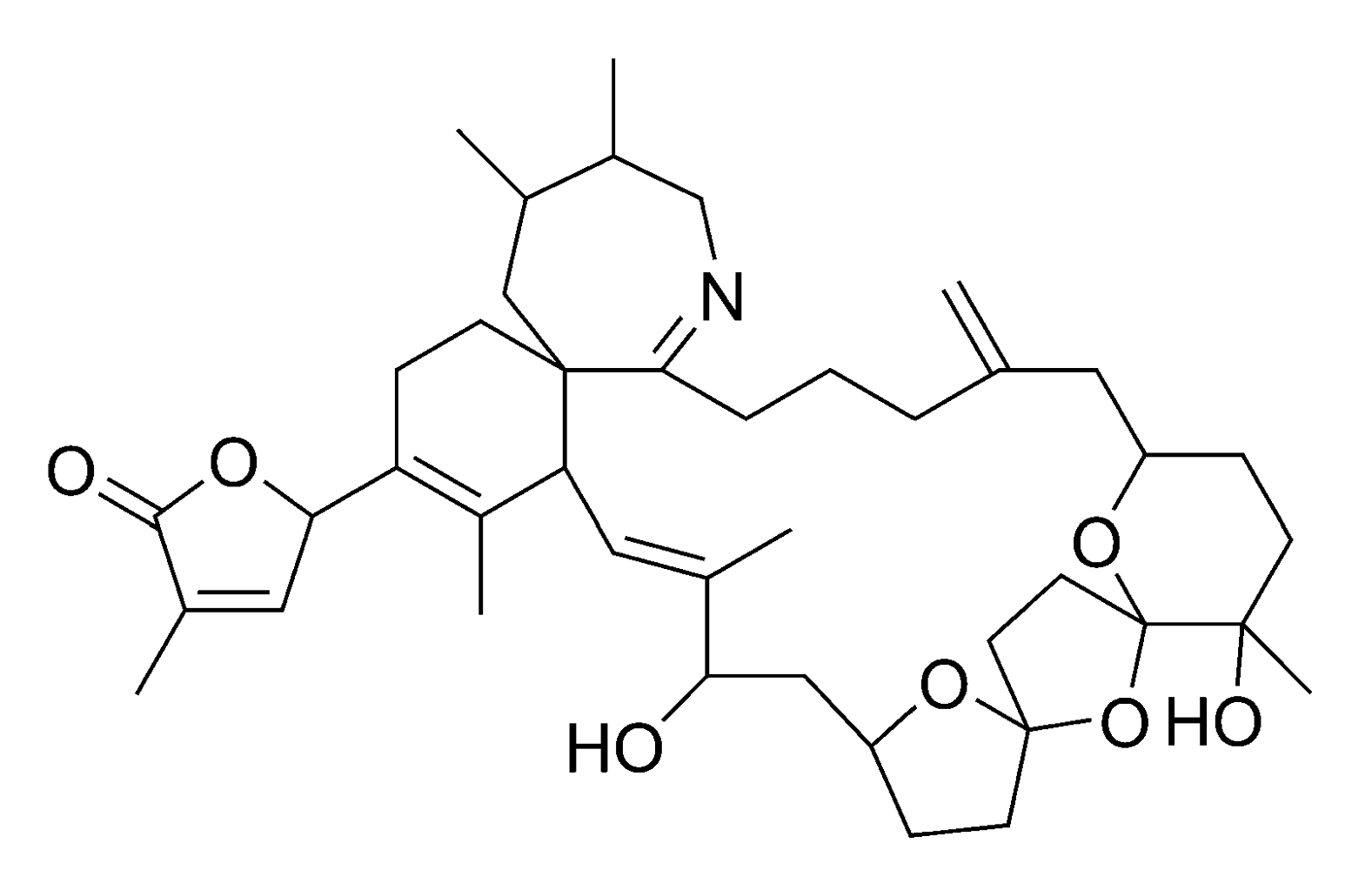
2.2.4. Spirolides and gymnodimines (cyclic imines)
3. Methods of Analysis
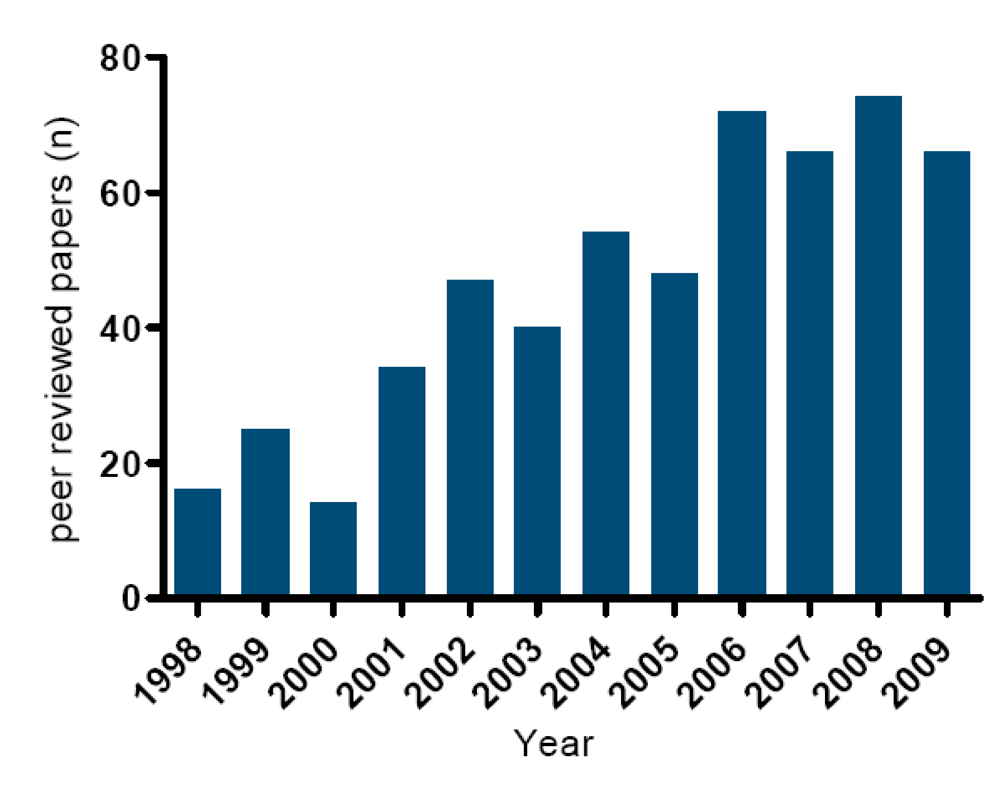
3.1. Current official methods described in legislation and their limitations
| Country | OA and DTXs | AZAs | PTXs | YTXs | Reference |
|---|---|---|---|---|---|
| Norway | MBA Chemical | Chemical | Chemical | MBA Chemical | [113] |
| Sweden 1 | MBA Chemical | MBA Chemical | MBA Chemical | MBA Chemical | [113] |
| Finland 2 | [113] | ||||
| Denmark | MBA Chemical | Chemical | Chemical | Chemical | [113] |
| Ireland | MBA Chemical | MBA Chemical | MBA Chemical | MBA Chemical | [113] |
| United Kingdom | MBA | MBA | MBA | MBA | [113] |
| Germany | Chemical | Chemical | Chemical | Chemical | [113] |
| The Netherlands | RBA Chemical | RBA | 113] | ||
| Belgium | MBA | MBA | MBA | MBA | [113] |
| France | MBA | MBA | MBA | MBA | [113] |
| Austria | MBA Chemical | MBA Chemical | MBA Chemical | MBA Chemical | [113] |
| Portugal | MBA Chemical Biochemical | Chemical | Chemical | MBA | [113] |
| Spain | MBA | MBA | MBA | MBA | [113] |
| Italy | MBA Chemical | MBA | MBA | MBA Chemical | [113] |
| Greece | MBA Chemical | MBA | [113] | ||
| Turkey | MBA | MBA | MBA | MBA | [114] |
| Canada | MBA | MBA | MBA | MBA | [114] |
| United States 3 | [114] | ||||
| Venezuela | MBA | MBA | MBA | MBA | [114] |
| Brazil 3 | [114] | ||||
| Chili | MBA | MBA | MBA | MBA | [114] |
| Uruguay | MBA | MBA | MBA | MBA | [114] |
| Republic of Korea | MBA Chemical | MBA Chemical | MBA Chemical | MBA Chemical | [114] |
| Japan | MBA | MBA | MBA | MBA | [114] |
| Thailand | MBA | MBA | MBA | MBA | [114] |
| New Zealand | Chemical | Chemical | Chemical | Chemical | [113] |
| Country or Continent | OA, DTXs (µg/kg) | PTXs (µg/kg) | AZAs (µg/kg) | YTXs (µg/kg) | MBA (MU/kg) | Reference |
|---|---|---|---|---|---|---|
| EU | 160 WF | Included in OA | 160 | 1000 | [12] | |
| United States | 200 | NR | NR | NR | [60] | |
| Canada | 1000 DG | NR | NR | NR | [115] | |
| Japan | 50 (~200 µg/kg OA-eq) | 114] | ||||
| Australia and New Zealand | 200 WF | NR | NR | NR | [116] |
3.2. Development of alternative methods
3.2.1. In-vitro assays
3.2.2. Biochemical methods
3.2.3. Chemical methods
4. Occurrence of Toxic Events in the Netherlands (1960–2009)

5. Conclusions
Acknowledgements
References
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health. 2008, 7, S4. [Google Scholar]
- Peperzak, L. Future increase in harmful algal blooms in the North Sea due to climate change. Water Sci. Technol. 2005, 51, 31–36. [Google Scholar]
- Benemann, J.R. Microalgae aquaculture feeds. J. Appl. Phycol. 1992, 4, 233–245. [Google Scholar]
- Daranas, A.H.; Norte, M.; Fernandez, J.J. Toxic marine microalgae. Toxicon 2001, 39, 1101–1132. [Google Scholar]
- Botana, A.M.; Rodriguez Veijtes, M.; Alfonso, A.; Louzao, M. Phycotoxins: Paralytic shellfish poisoning and diarrhetic shellfish poisoning. In Handbook of Food Analysis—Residues and Other Food Component Analysis; Nollet, L.M.L., Ed.; Dekker: New York, NY, USA, 1996; pp. 1147–1169. [Google Scholar]
- Van Dolah, F.M.; Ramsdell, J.S. Review and assessment of in vitro detection methods for algal toxins. J. AOAC Int. 2001, 84, 1617–1625. [Google Scholar]
- Landsberg, J.H.; Flewelling, L.J.; Naar, J. Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: Decadal advancements. Harmful Algae 2009, 8, 598–607. [Google Scholar]
- Miller, G. Wildlife biology—confused pelicans may have lingered too long up north. Science 2009, 323, 449–449. [Google Scholar]
- Morgan, K.L.; Larkin, S.L.; Adams, C.M. Firm-level economic effects of HABS: A tool for business loss assessment. Harmful Algae 2009, 8, 212–218. [Google Scholar]
- Hoagland, P.; Scatasta, S. The economic effect of harmful algal blooms. In Ecology on Harmful Algae; Graneli, E., Turner, T., Eds.; Springer: Berlin, Germany, 2006; Volume 189, pp. 391–402. [Google Scholar]
- EC. Commission directive 2004/853/EC specific hygiene rules for food of animal origin. Off. J. Eur. Commun. 2004, 22–82.
- EC. Laying down implementing measures for certain products under Regulation (EC) No. 853/2004 of the European Parliament and of the Council and for the organisation of official controls under Regulation (EC) No. 854/2004 of the European Parliament and of the Council and Regulation (EC) No. 882/2004 of the European Parliament and of the Council, derogating from Regulation (EC) No. 852/2004 of the European Parliament and of the Council and amending Regulations (EC) No. 853/2004 and (EC) No. 854/2004. Off. J. Eur. Commun. 2005, 40–41.
- Clayden, J.; Read, B.; Hebditch, K.R. Chemistry of domoic acid, isodomoic acids, and their analogues. Tetrahedron 2005, 61, 5713–5724. [Google Scholar]
- Adams, A.L.; Doucette, T.A.; James, R.; Ryan, C.L. Persistent changes in learning and memory in rats following neonatal treatment with domoic acid. Physiol. Behav. 2009, 96, 505–512. [Google Scholar]
- Kumar, K.P.; Kumar, S.P.; Nair, G.A. Risk assessment of the amnesic shellfish poison, domoic acid, on animals and humans. J. Environ. Biol. 2009, 30, 319–325. [Google Scholar]
- Jeffery, B.; Barlow, T.; Moizer, K.; Paul, S.; Boyle, C. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar]
- Perl, T.M.; Bedard, L.; Kosatsky, T.; Hockin, J.C.; Todd, E.C.; Remis, R.S. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N. Engl. J. Med. 1990, 322, 1775–1780. [Google Scholar]
- Bill, B.D.; Cox, F.H.; Horner, R.A.; Borchert, J.A.; Trainer, V.L. The first closure of shellfish harvesting due to domoic acid in Puget Sound, Washington, USA. Afr. J. Mar. Sci. 2006, 28, 435–440. [Google Scholar]
- Campbell, D.A.; Kelly, M.S.; Busman, M.; Bolch, C.J.; Wiggins, E.; Moeller, P.D.R.; Morton, S.L.; Hess, P.; Shumway, S.E. Amnesic shellfish poisoning in the king scallop, Pecten maximus, from the west coast of Scotland. J. Shellfish Res. 2001, 20, 75–84. [Google Scholar]
- Blanco, J.; Cano, J.; Marino, M.D.C.; Campos, M.J. Effect of phytoplankton containing paralytic shellfish and amnesic shellfish toxins on the culture of the king scallop Pecten maximus in Malaga (SE Spain). Aquat. Living Resour. 2006, 19, 267–273. [Google Scholar]
- James, K.J.; Gillman, M.; Amandi, M.F.; Lopez-Rivera, A.; Puente, P.F.; Lehane, M.; Mitrovic, S.; Furey, A. Amnesic shellfish poisoning toxins in bivalve molluscs in Ireland. Toxicon 2005, 46, 852–858. [Google Scholar]
- Vale, P.; Sampayo, M.A. Domoic acid in Portuguese shellfish and fish. Toxicon 2001, 39, 893–904. [Google Scholar]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.-P.; Domenico, A.D.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; Filipic, M.; Fink-Gremmels, J.; Furst, P.; Guerin, T.; Knutsen, H.K.; Livesey, C.; Machala, M.; Mutti, A.; Schlatter, J.; van Leeuwen, R.; Verger, P. Marine biotoxins in shellfish—Domoic acid. EFSA J. 2009, 1181, 1–61. [Google Scholar]
- Bates, S.; Trainer, V.L. The ecology of harmful diatoms. In Ecology on Harmful Algae; Graneli, E., Turner, T., Eds.; Springer: Berlin, Germany, 2006; Volume 189, pp. 81–93. [Google Scholar]
- Beppu, R.; Nojima, K.; Tsuruda, S.; Gomez-Delan, G.; Barte-Quilantang, M.; Taniyama, S.; Sagara, T.; Nishio, S.; Takayama, H.; Miyazawa, K.; Asakawa, M. Occurrence of PSP-producing dinoflagellate Alexandrium tamiyavanichii in Bingo-Nada, the central coastal water of the Seto Inland Sea, Hiroshima Prefecture, Japan. Mar. Pollut. Bull. 2008, 56, 758–763. [Google Scholar]
- Martin, J.L.; Hanke, A.R.; LeGresley, M.M. Long term phytoplankton monitoring, including harmful algal blooms, in the Bay of Fundy, eastern Canada. J. Sea Res. 2009, 61, 76–83. [Google Scholar]
- MacKenzie, L.; de Salas, M.; Adamson, J.; Beuzenberg, V. The dinoflagellate genus Alexandrium (Halim) in New Zealand coastal waters: Comparative morphology, toxicity and molecular genetics. Harmful Algae 2004, 3, 71–92. [Google Scholar]
- Wang, D.Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic shellfish poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar]
- Caroppo, C.; Congestri, R.; Bruno, M. On the presence of Phalacroma rotundatum in the southern Adriatic Sea (Italy). Aquat. Microb. Ecol. 1999, 17, 301–310. [Google Scholar]
- Hu, T.M.; Curtis, J.M.; Walter, J.A.; Wright, J.L.C. Identification of Dtx-4, a new water-soluble phosphatase inhibitor from the toxic dinoflagellate Prorocentrum-Lima. J. Chem. Soc. Chem. Comm. 1995, 597–599. [Google Scholar]
- Draisci, R.; Lucentini, L.; Giannetti, L.; Boria, P.; Poletti, R. First report of pectenotoxin-2 (PTX-2) in algae (Dinophysis fortii) related to seafood poisoning in Europe. Toxicon 1996, 34, 923–935. [Google Scholar]
- Jorgensen, K.; Andersen, P. Relation between the concentration of Dinophysis acuminata and diarrheic shellfish poisoning toxins in blue mussels (Mytilus edulis) during a toxic episode in the Limfjord (Denmark), 2006. J. Shellfish Res. 2007, 26, 1081–1087. [Google Scholar]
- MacKenzie, A.L.; Beuzenberg, V.; Holland, P.T.; McNabb, P.; Suzuki, T.; Selwood, A. Pectenotoxin and okadaic acid-based toxin profiles in Dinophysis acuta and Dinophysis acuminata from New Zealand. Harmful Algae 2005, 4, 75–85. [Google Scholar]
- Ten-Hage, L.; Delaunay, N.; Pichon, V.; Coute, A.; Puiseux-Dao, S.; Turquet, J. Okadaic acid production from the marine benthic dinoflagellate Prorocentrum arenarium Faust (Dinophyceae) isolated from Europa Island coral reef ecosystem (SW Indian Ocean). Toxicon 2000, 38, 1043–1054. [Google Scholar]
- Raho, N.; Pizarro, G.; Escalera, L.; Reguera, B.; Marin, I. Morphology, toxin composition and molecular analysis of Dinophysis ovum Schutt, a dinoflagellate of the "Dinophysis acuminata complex". Harmful Algae 2008, 7, 839–848. [Google Scholar]
- Spatharis, S.; Dolapsakis, N.P.; Economou-Amilli, A.; Tsirtsis, G.; Danielidis, D.B. Dynamics of potentially harmful microalgae in a confined Mediterranean Gulf—Assessing the risk of bloom formation. Harmful Algae 2009, 8, 736–743. [Google Scholar]
- Loader, J.I.; Hawkes, A.D.; Beuzenberg, V.; Jensen, D.J.; Cooney, J.M.; Wilkins, A.L.; Fitzgerald, J.M.; Briggs, L.R.; Miles, C.O. Convenient large-scale purification of yessotoxin from Protoceratium reticulatum culture and isolation of a novel furanoyessotoxin. J. Agric. Food Chem. 2007, 55, 11093–11100. [Google Scholar]
- Tillmann, U.; Elbrachter, M.; Krock, B.; John, U.; Cembella, A. Azadinium spinosum gen. et sp nov (Dinophyceae) identified as a primary producer of azaspiracid toxins. Eur. J. Phycol. 2009, 44, 63–79. [Google Scholar] [CrossRef]
- Cembella, A.D.; Lewis, N.I.; Quilliam, M.A. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia 2000, 39, 67–74. [Google Scholar]
- Touzet, N.; Franco, J.M.; Raine, R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae 2008, 7, 782–797. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar]
- Seki, T.; Satake, M.; Mackenzie, A.L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New-Zealand oysters and the dinoflagellate, Gymnodinium Sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar]
- Dell'Aversano, C.; Walter, J.A.; Burton, I.W.; Stirling, D.J.; Fattorusso, E.; Quilliam, M.A. Isolation and structure elucidation of new and unusual saxitoxin analogues from mussels. J. Nat. Prod. 2008, 71, 1518–1523. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cradevi, J.-P.; Dogliotti, E.; Domenico, A.D.; Fernandez-Cruz, M.L.; Fink-Gremmels, J.; Furst, P.; Galli, C.; Grandjean, P.; Gzyl, J.; Heinemeyer, G.; Johansson, N.; Mutti, A.; Schlatter, J.; van Leeuwen, R.; van Peteghem, C.; Verger, P. Marine biotoxins in shellfish - saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- Lagos, N.; Andrinolo, D. Paralytic shellfish poisoning (PSP): toxicology and kinetics. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; Botana, L.M., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 203–215. [Google Scholar]
- IOC; FAO; WHO, Report of the joint FAO/IOC/WHO ad hoc expert consulation on biotoxins in bivalve mollucs; SC.2005/WS/24; IOC/INF-1215. FAO/IOC/WHO: Oslo, Norway, 2004.
- de Carvalho, M.; Jacinto, J.; Ramos, N.; de Oliveira, V.; Pinho e Melo, T.; de Sa, J. Paralytic shellfish poisoning: Clinical and electrophysiological observations. J. Neurol. 1998, 245, 551–554. [Google Scholar]
- Azanza, M.P.V. Philippine foodborne-disease outbreaks (1995–2004). J. Food Saf. 2006, 26, 92–102. [Google Scholar]
- Meyer, K.F.; Sommer, H.; Schoenholz, P. Mussel poisoining. Am. J. Prev. Med. 1928, 2, 365–394. [Google Scholar]
- Pitcher, G.C.; Cembella, A.D.; Joyce, L.B.; Larsen, J.; Probyn, T.A.; Sebastian, C.R. The dinoflagellate Alexandrium minutum in Cape Town harbour (South Africa): Bloom characteristics, phylogenetic analysis and toxin composition. Harmful Algae 2007, 6, 823–836. [Google Scholar]
- Krock, B.; Seguel, C.G.; Cembella, A.D. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 2007, 6, 734–744. [Google Scholar]
- Robertson, A.; Stirling, D.; Robillot, C.; Llewellyn, L.; Negri, A. First report of saxitoxin in octopi. Toxicon 2004, 44, 765–771. [Google Scholar]
- Al-Sabi, A.; McArthur, J.; Ostroumov, V.; French, R.J. Marine toxins that target voltage-gated sodium channels. Mar. Drugs 2006, 4, 157–192. [Google Scholar]
- Pierce, R.H.; Henry, M.S.; Blum, P.C.; Hamel, S.L.; Kirkpatrick, B.; Cheng, Y.S.; Zhou, Y.; Irvin, C.M.; Naar, J.; Weidner, A.; Fleming, L.E.; Backer, L.C.; Baden, D.G. Brevetoxin composition in water and marine aerosol along a Florida beach: Assessing potential human exposure to marine biotoxins. Harmful Algae 2005, 4, 965–972. [Google Scholar]
- Kirkpatrick, B.; Fleming, L.E.; Backer, L.C.; Bean, J.A.; Tamer, R.; Kirkpatrick, G.; Kane, T.; Wanner, A.; Dalpra, D.; Reich, A.; Baden, D.G. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae 2006, 5, 526–533. [Google Scholar]
- Heil, D.C. Karenia brevis monitoring, management, and mitigation for Florida molluscan shellfish harvesting areas. Harmful Algae 2009, 8, 608–610. [Google Scholar]
- Ishida, H.; Nozawa, A.; Nukaya, H.; Tsuji, K. Comparative concentrations of brevetoxins PbTx-2, PbTx-3, BTX-B1 and BTX-B5 in cockle, Austrovenus stutchburyi, greenshell mussel, Perna canaliculus, and Pacific oyster, Crassostrea gigas, involved neurotoxic shellfish poisoning in New Zealand. Toxicon 2004, 43, 779–789. [Google Scholar]
- FDA, Chapter 6: Natural Toxins (A chemical Hazard). In Fish and fisheries products hazards and controls guidance; Food and Drug Administration: Washington, DC, USA, 2001.
- Honkanen, R.E.; Codispoti, B.A.; Tse, K.; Boynton, A.L.; Honkanan, R.E. Characterization of natural toxins with inhibitory activity against serine/threonine protein phosphatases. Toxicon 1994, 32, 339–350. [Google Scholar]
- Vale, C.; Botana, L.M. Marine toxins and the cytoskeleton: Okadaic acid and dinophysistoxins. FEBS J. 2008, 275, 6060–6066. [Google Scholar]
- Garcia, C.; Truan, D.; Lagos, M.; Santelices, J.P.; Diaz, J.C.; Lagos, N. Metabolic transformation of dinophysistoxin-3 into dinophysistoxin-1 causes human intoxication by consumption of O-acyl-derivatives dinophysistoxins contaminated shellfish. J. Toxicol. Sci. 2005, 30, 287–296. [Google Scholar]
- Manerio, E.; Rodas, V.L.; Costas, E.; Hernandez, J.M. Shellfish consumption: A major risk factor for colorectal cancer. Med. Hypotheses 2008, 70, 409–412. [Google Scholar]
- Kat, M. The occurrence of prorocentrum species and coincidental gastrointestinal illness of mussel consumers. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier: New York, NY, USA, 1979; pp. 215–220. [Google Scholar]
- Scoging, A.; Bahl, M. Diarrhetic shellfish poisoning in the UK. Lancet 1998, 352, 117. [Google Scholar]
- Elgarch, A.; Vale, P.; Rifai, S.; Fassouane, A. Detection of diarrheic shellfish poisoning and azaspiracid toxins in Moroccan mussels: Comparison of the LC-MS method with the commercial immunoassay kit. Mar. Drugs 2008, 6, 587–594. [Google Scholar]
- Alexander, J.; Audunsson, G.A.; Benford, D.; Cockburn, A.; Cradevi, J.-P.; Dogliotti, E.; Domenico, A.D.; Fernandez-Cruz, M.L.; Fink-Gremmels, J.; Furst, P.; Galli, C.; Grandjean, P.; Gzyl, J.; Heinemeyer, G.; Johansson, N.; Mutti, A.; Schlatter, J.; van Leeuwen, R.; van Peteghem, C.; Verger, P. Marine biotoxins in shellfish - okadaic acid and analogues. EFSA J. 2008, 589, 1–62. [Google Scholar]
- Aune, T.; Larsen, S.; Aasen, J.A.B.; Rehmann, N.; Satake, M.; Hess, P. Relative toxicity of dinophysistoxin-2 (DTX-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon 2007, 49, 1–7. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cradevi, J.-P.; Dogliotti, E.; Domenico, A.D.; Fernandez-Cruz, M.L.; Fink-Gremmels, J.; Furst, P.; Galli, C.; Grandjean, P.; Gzyl, J.; Heinemeyer, G.; Johansson, N.; Mutti, A.; Schlatter, J.; van Leeuwen, R.; van Peteghem, C.; Verger, P. Marine biotoxins in shellfish - pectenotoxin group. EFSA J. 2009, 1109, 1–47. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Hawkes, A.D.; Jensen, D.J.; Selwood, A.I.; Beuzenberg, V.; Mackenzie, A.L.; Cooney, J.M.; Holland, P.T. Isolation and identification of pectenotoxins-13 and -14 from Dinophysis acuta in New Zealand. Toxicon 2006, 48, 152–159. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Samdal, I.A.; Sandvik, M.; Petersen, D.; Quilliam, M.A.; Naustvoll, L.J.; Rundberget, T.; Torgersen, T.; Hovgaard, P.; Jensen, D.J.; Cooney, J.M. A novel pectenotoxin, PTX-12, in Dinophysis spp. and shellfish from Norway. Chem. Res. Toxicol. 2004, 17, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Vale, P.; Sampayo, M.A.D. Pectenotoxin-2 seco acid, 7-epi-pectenotoxin-2 seco acid and pectenotoxin-2 in shellfish and plankton from Portugal. Toxicon 2002, 40, 979–987. [Google Scholar]
- Espina, B.; Rubiolo, J.A. Marine toxins and the cytoskeleton: pectenotoxins, unusual macrolides that disrupt actin. FEBS J. 2008, 275, 6082–6088. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Munday, R.; Dines, M.H.; Hawkes, A.D.; Briggs, L.R.; Sandvik, M.; Jensen, D.J.; Cooney, J.M.; Holland, P.T.; Quilliam, M.A.; MacKenzie, A.L.; Beuzenberg, V.; Towers, N.R. Isolation of pectenotoxin-2 from Dinophysis acuta and its conversion to pectenotoxin-2 seco acid, and preliminary assessment of their acute toxicities. Toxicon 2004, 43, 1–9. [Google Scholar]
- Bowden, B.F. Yessotoxins-polycyclic ethers from dinoflagellates: Relationships to diarrhetic shellfish toxins. Toxin. Rev. 2006, 25, 137–157. [Google Scholar]
- Miles, C.O.; Samdal, I.A.; Aasen, J.A.B.; Jensen, D.J.; Quilliam, M.A.; Petersen, D.; Briggs, L.M.; Wilkins, A.L.; Rise, F.; Cooney, J.M.; MacKenzie, A.L. Evidence for numerous analogs of yessotoxin in Protoceratium reticulatum. Harmful Algae 2005, 4, 1075–1091. [Google Scholar]
- Aasen, J.A.B.; Samdal, I.A.; Miles, C.O.; Dahl, E.; Briggs, L.R.; Aune, T. Yessotoxins in Norwegian blue mussels (Mytilus edulis): Uptake from Protoceratium reticulatum, metabolism and depuration. Toxicon 2005, 45, 265–272. [Google Scholar]
- Ciminiello, P.; Fattorusso, E.; Forino, M.; Magno, S.; Poletti, R.; Viviani, R. Isolation of adriatoxin, a new analogue of yessotoxin from mussels of the Adriatic sea. Tetrahedron Lett. 1998, 39, 8897–8900. [Google Scholar]
- Aune, T.; Sorby, R.; Yasumoto, T.; Ramstad, H.; Landsverk, T. Comparison of oral and intraperitoneal toxicity of yessotoxin towards mice. Toxicon 2002, 40, 77–82. [Google Scholar]
- Draisci, R.; Ferretti, E.; Palleschi, L.; Marchiafava, C.; Poletti, R.; Milandri, A.; Ceredi, A.; Pompei, M. High levels of yessotoxin in mussels and precense of yessotoxin and homoyessotoxin in dinoflagellates of the Adriatic Sea. Toxicon 1999, 37, 1187–1193. [Google Scholar]
- Vale, P.; Botelho, M.J.; Rodrigues, S.M.; Gomes, S.S.; Sampayo, M.A.D. Two decades of marine biotoxin monitoring in bivalves from Portugal (1986–2006): A review of exposure assessment. Harmful Algae 2008, 7, 11–25. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cradevi, J.-P.; Dogliotti, E.; Domenico, A.D.; Fernandez-Cruz, M.L.; Fink-Gremmels, J.; Furst, P.; Galli, C.; Grandjean, P.; Gzyl, J.; Heinemeyer, G.; Johansson, N.; Mutti, A.; Schlatter, J.; van Leeuwen, R.; van Peteghem, C.; Verger, P. Marine biotoxins in shellfish—yessotoxin group. EFSA J. 2008, 907, 1–62. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cradevi, J.-P.; Dogliotti, E.; Domenico, A.D.; Fernandez-Cruz, M.L.; Fink-Gremmels, J.; Furst, P.; Galli, C.; Grandjean, P.; Gzyl, J.; Heinemeyer, G.; Johansson, N.; Mutti, A.; Schlatter, J.; van Leeuwen, R.; van Peteghem, C.; Verger, P. Marine biotoxins in shellfish—azaspiracid group. EFSA J. 2008, 723, 1–52. [Google Scholar]
- James, K.J.; Saez, M.J.F.; Furey, A.; Lehane, M. Azaspiracid poisoning, the food-borne illness associated with shellfish consumption. Food Addit. Contam. A 2004, 21, 879–892. [Google Scholar]
- Krock, B.; Tillmann, U.; John, U.; Cembella, A.D. Characterization of azaspiracids in plankton size-fractions and isolation of an azaspiracid-producing dinoflagellate from the North Sea. Harmful Algae 2009, 8, 254–263. [Google Scholar]
- Rehmann, N.; Hess, P.; Quilliam, M.A. Discovery of new analogs of the marine biotoxin azaspiracid in blue mussels (Mytilus edulis) by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 549–558. [Google Scholar]
- Ronzitti, G.; Hess, P.; Rehmann, N.; Rossini, G.P. Azaspiracid-1 alters the E-cadherin pool in epithelial cells. Toxicol. Sci. 2007, 95, 427–435. [Google Scholar]
- Twiner, M.J.; Hess, P.; Dechraoui, M.Y.B.; McMahon, T.; Samons, M.S.; Satake, M.; Yasumoto, T.; Ramsdell, J.S.; Doucette, G.J. Cytotoxic and cytoskeletal effects of azaspiracid-1 on mammalian cell lines. Toxicon 2005, 45, 891–900. [Google Scholar]
- Ito, E.; Satake, M.; Ofuji, K.; Kurita, N.; McMahon, T.; James, K.J.; Yasumoto, T. Multiple organ damage caused by a new toxin azaspiracid, isolated from mussels produced in Ireland. Toxicon 2000, 38, 917–930. [Google Scholar]
- James, K.J.; Lehane, M.; Moroney, C.; Fernandez-Puente, P.; Satake, M.; Yasumoto, T.; Furey, A. Azaspiracid shellfish poisoning: Unusual toxin dynamics in shellfish and the increased risk of acute human intoxications. Food Addit. Contam. A 2002, 19, 555–561. [Google Scholar]
- Torgersen, T.; Bremnes, N.B.; Rundberget, T.; Aune, T. Structural confirmation and occurrence of azaspiracids in Scandinavian brown crabs (Cancer pagurus). Toxicon 2008, 51, 93–101. [Google Scholar]
- Amzil, Z.; Sibat, M.; Royer, F.; Savar, V. First report on azaspiracid and yessotoxin groups detection in French shellfish. Toxicon 2008, 52, 39–48. [Google Scholar]
- Vale, P.; Bire, R.; Hess, P. Confirmation by LC-MS/MS of azaspiracids in shellfish from the Portuguese north-western coast. Toxicon 2008, 51, 1449–1456. [Google Scholar]
- Alvarez, G.; Uribe, E.; Avalos, P.; Marino, C.; Blanco, J. First identification of azaspiracid and spirolides in Mesodesma donacium and Mulinia edulis from Northern Chile. Toxicon 2010, 55, 638–641. [Google Scholar]
- Lopez-Rivera, A.; O'Callaghan, K.; Moriarty, M.; O'Driscoll, D.; Hamilton, B.; Lehane, M.; James, K.J.; Furey, A. First evidence of azaspiracids (AZAs): A family of lipophilic polyether marine toxins in scallops (Argopecten purpuratus) and mussels (Mytilus chilensis) collected in two regions of Chile. Toxicon 2009, 55, 692–701. [Google Scholar]
- Klontz, K.C.; Abraham, A.; Plakas, S.M.; Dickey, R.W. Mussel-associated azaspiracid intoxication in the United States. Ann. Intern. Med. 2009, 150, 361. [Google Scholar]
- Regel, E. Commission Directive 2004/853/EC Specific hygiene rules for food of animal origin. Off. J. Eur. Commun. 2004, 22–82. [Google Scholar]
- MacKinnon, S.L.; Walter, J.A.; Quilliam, M.A.; Cembella, A.D.; LeBlanc, P.; Burton, I.W.; Hardstaff, W.R.; Lewis, N.I. Spirolides isolated from Danish strains of the toxigenic dinoflagellate Alexandrium ostenfeldii. J. Nat. Prod. 2006, 69, 983–987. [Google Scholar]
- Aasen, J.A.B.; MacKinnon, S.L.; LeBlanc, P.; Walter, J.A.; Hovgaard, P.; Aune, T.; Quilliam, M.A. Detection and identification of spirolides in Norwegian shellfish and plankton. Chem. Res. Toxicol. 2005, 18, 509–515. [Google Scholar]
- Aasen, J.A.B.; Hardstaff, W.; Aune, T.; Quilliam, M.A. Discovery of fatty acid ester metabolites of spirolide toxins in mussels from Norway using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1531–1537. [Google Scholar]
- Ciminiello, P.; Dell'Aversano, C.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Spirolide toxin profile of Adriatic Alexandrium ostenfeldii cultures and structure elucidation of 27-hydroxy-13,19-didesmethyl spirolide C. J. Nat. Prod. 2007, 70, 1878–1883. [Google Scholar]
- Richard, D.J.A.; Arsenault, E.; Cembella, A.D.; Quilliam, M.A. Investigations into the toxicology and pharmacology of spirolides, a novel group of shellfish toxins. In Proceedings of the 9th Conference on Harmfull Algal Blooms, Hobart, Australia, 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; pp. 383–386.
- Villar Gonzalez, A.; Rodriguez-Velasco, M.L.; Ben-Gigirey, B.; Botana, L.M. First evidence of spirolides in Spanish shellfish. Toxicon 2006, 48, 1068–1074. [Google Scholar]
- Nicolaou, K.C.; Chen, J.S. Total synthesis of complex heterocyclic natural products. Pure Appl. Chem. 2008, 80, 727–742. [Google Scholar]
- Alfonso, C.; Alfonso, A.; Otero, P.; Rodriguez, P.; Vieytes, M.R.; Elliot, C.; Higgins, C.; Botana, L.M. Purification of five azaspiracids from mussel samples contaminated with DSP toxins and azaspiracids. J. Chromatogr. B 2008, 865, 133–140. [Google Scholar]
- Rundberget, T.; Sandvik, M.; Larsen, K.; Pizarro, G.M.; Reguera, B.; Castberg, T.; Gustad, E.; Loader, J.I.; Rise, F.; Wilkins, A.L.; Miles, C.O. Extraction of microalgal toxins by large-scale pumping of seawater in Spain and Norway, and isolation of okadaic acid and dinophysistoxin-2. Toxicon 2007, 50, 960–970. [Google Scholar]
- Yasumoto, T.; Hashimot, Y.; Bagnis, R.; Randall, J.E.; Banner, A.H. Toxicity of surgeonfishes. Bull. Jpn. Soc. Sci. Fish. 1971, 37, 724–734. [Google Scholar]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, K. Occurrence of new type of toxic shellfish in Japan and chemical properties of the toxin. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier: New York, NY, USA, 1979; pp. 395–398. [Google Scholar]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Matsumoto, G.K.; Clardy, J. Diarrhetic Shellfish Poisoning. In Seafood Toxins. Based on a Symposium at the 186th Meeting of the American Chemical Society, Washington, DC, USA, 28 August–2 September, 1983; Ragelis, E.P., Ed.; ACS: Washington, DC, USA, 1984; pp. 207–214. [Google Scholar]
- EU harmonised standard operating procedure for detection of lipophilic toxins by mouse bioassay, version 5 June 2009. Available online: http://www.aesan.msps.es/en/CRLMB/web/home.shtml (access on 15 July 2009).
- Hess, P.; Nguyen, L.; Aasen, J.A.B.; Keogh, M.; Kilcoyne, J.; McCarron, P.; Aune, T. Tissue distribution, effects of cooking and parameters affecting the extraction of azaspiracids from mussels, Mytilus edulis, prior to analysis by liquid chromatography coupled to mass spectrometry. Toxicon 2005, 46, 62–71. [Google Scholar]
- Anonymous. Approaches to Validation and Regulatory Acceptance of Alternative Methods to the Mouse Bioassay as Method of Reference; Bunderinstitut fur risikobewertung: Berlin, Germany, 2005. [Google Scholar]
- Van Egmond, H.P.; van Apeldoorn, M.E.; Speijers, G.J.A. Marine Biotoxins; Food and Agriculture Organization of the united nations: Rome, Italy, 2004; Volume 80. [Google Scholar]
- Anonymous. Fish Products Standars and Methods Manual—Canadian Guidelines for Chemical Contaminants and Toxins in Fish and Fish Products. Canadian Food Inspection Agency: Ottawa, ON, Canada, 2007; Appendix 3. [Google Scholar]
- Anonymous. Standard 1.4.1. Contaminants and natural toxicants. Food Standards Australia New Zealand, 2009. [Google Scholar]
- Gonzalez, J.C.; Leira, F.; Fontal, O.I.; Vieytes, M.R.; Arevalo, F.F.; Vieites, J.M.; Bermudez-Puente, M.; Muniz, S.; Salgado, C.; Yasumoto, T.; Botana, L.M. Inter-laboratory validation of the fluorescent protein phosphatase inhibition assay to determine diarrhetic shellfish-toxins: Intercomparison with liquid chromatography and mouse bioassay. Anal. Chim. Acta 2002, 466, 233–246. [Google Scholar]
- Vieytes, M.R.; Fontal, O.I.; Leira, F.; deSousa, J.M.V.B.; Botana, L.M. A fluorescent microplate assay for diarrheic shellfish toxins. Anal. Biochem. 1997, 248, 258–264. [Google Scholar]
- Honkanen, R.E.; Stapleton, J.D.; Bryan, D.E.; Abercrombie, J. Development of a protein phosphatase-based assay for the detection of phosphatase inhibitors in crude whole cell and animal extracts. Toxicon 1996, 34, 1385–1392. [Google Scholar]
- Hummert, C.; Reichelt, M.; Luckas, B. New strategy for the determination of microcystins and diarrhetic shellfish poisoning (DSP) toxins, two potent phosphatases 1 and 2A inhibitors and tumor promoters. Fresenius J. Anal. Chem. 2000, 366, 508–513. [Google Scholar]
- Leira, F.; Alvarez, C.; Cabado, A.G.; Vieites, J.M.; Vieytes, M.R.; Botana, L.M. Development of a F actin-based live-cell fluorimetric microplate assay for diarrhetic shellfish toxins. Anal. Biochem. 2003, 317, 129–135. [Google Scholar]
- Rossini, G.P. Functional assays in marine biotoxin detection. Toxicology 2005, 207, 451–462. [Google Scholar]
- Pierotti, S.; Albano, C.; Milandri, A.; Callegari, F.; Poletti, R.; Rossini, G.P. A slot blot procedure for the measurement of yessotoxins by a functional assay. Toxicon 2007, 49, 36–45. [Google Scholar]
- Vilarino, N.; Fonfria, E.S.; Molgo, J.; Araoz, R.; Botana, L.M. Detection of gymnodimine-A and 13-desmethyl C spirolide phycotoxins by fluorescence polarization. Anal. Chem. 2009, 81, 2708–2714. [Google Scholar]
- Laycock, M.V.; Jellett, J.F.; Easy, D.J.; Donovan, M.A. First report of a new rapid assay for diarrhetic shellfish poisoning toxins. Harmful Algae 2006, 5, 74–78. [Google Scholar]
- Vale, P.; Gomes, S.S.; Lameiras, J.; Rodrigues, S.M.; Botelho, M.J.; Laycock, M.V. Assessment of a new lateral flow immunochromatographic (LFIC) assay for the okadaic acid group of toxins using naturally contaminated bivalve shellfish from the Portuguese coast. Food Addit. Contam. A 2009, 26, 214–220. [Google Scholar]
- Campas, M.; de la Iglesia, P.; Le Berre, M.; Kane, M.; Diogene, J.; Marty, J.L. Enzymatic recycling-based amperometric immunosensor for the ultrasensitive detection of okadaic acid in shellfish. Biosens. Bioelectron. 2008, 24, 716–722. [Google Scholar]
- Llamas, N.M.; Stewart, L.; Fodey, T.; Higgins, H.C.; Velasco, M.L.R.; Botana, L.M.; Elliott, C.T. Development of a novel immunobiosensor method for the rapid detection of okadaic acid contamination in shellfish extracts. Anal. Bioanal. Chem. 2007, 389, 581–587. [Google Scholar]
- Briggs, L.R.; Miles, C.O.; Fitzgerald, J.M.; Ross, K.M.; Garthwaite, I.; Towers, N.R. Enzyme-linked immunosorbent assay for the detection of yessotoxin and its analogues. J. Agric. Food Chem. 2004, 52, 5836–5842. [Google Scholar]
- Samdal, I.A.; Aasen, J.A.B.; Briggs, L.R.; Dahl, E.; Miles, C.O. Comparison of ELISA and LC-MS analyses for yessotoxins in blue mussels (Mytilus edulis). Toxicon 2005, 46, 7–15. [Google Scholar]
- Alfonso, C.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Quantification of yessotoxin using the fluorescence polarization technique and study of the adequate extraction procedure. Anal. Biochem. 2005, 344, 266–274. [Google Scholar]
- Fonfria, E.S.; Vilarino, N.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Feasibility of using a surface plasmon resonance-based biosensor to detect and quantify yessotoxin. Anal. Chim. Acta 2008, 617, 167–170. [Google Scholar]
- Pazos, M.J.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Vieites, J.M.; Botana, L.M. Resonant mirror biosensor detection method based on yessotoxin-phosphodiesterase interactions. Anal. Biochem. 2004, 335, 112–118. [Google Scholar]
- Lee, J.S.; Yanagi, T.; Kenma, R.; Yasumoto, T. Fluorometric-determination of diarrhetic shellfish toxins by high-performance liquid-chromatography. Agric. Biol. Chem. 1987, 51, 877–881. [Google Scholar]
- Sasaki, K.; Takizawa, A.; Tubaro, A.; Sidari, L.; Loggia, R.D.; Yasumoto, T. Fluorometric analysis of pectenotoxin-2 in microalgal samples by high performance liquid chromatography. Nat. Toxins 1999, 7, 241–246. [Google Scholar]
- Yasumoto, T.; Takizawa, A. Fluorometric measurement of yessotoxins in shellfish by high-pressure liquid chromatography. Biosci. Biotechnol. Biochem. 1997, 61, 1775–1777. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Hawkes, A.D.; Jensen, D.J.; Cooney, J.M.; Larsen, K.; Petersen, D.; Rise, F.; Beuzenberg, V.; Mackenzie, A.L. Isolation and identification of a cis-C(8)-diol-ester of okadaic acid from Dinophysis acuta in New Zealand. Toxicon 2006, 48, 195–203. [Google Scholar]
- Paz, B.; Daranas, A.H.; Cruz, P.G.; Franco, J.M.; Pizarro, G.; Souto, M.L.; Norte, M.; Fernandez, J.J. Characterisation of okadaic acid related toxins by liquid chromatography coupled with mass spectrometry. Toxicon 2007, 50, 225–235. [Google Scholar]
- Paz, B.; Daranas, A.H.; Cruz, P.G.; Franco, J.M.; Napolitano, J.G.; Norte, M.; Fernandez, J.J. Identification and characterization of DTX-5c and 7-hydroxymethyl-2-methylene-octa-4,7-dienyl okadaate from Prorocentrum belizeanum cultures by LC-MS. Toxicon 2007, 50, 470–478. [Google Scholar]
- Torgersen, T.; Wilkins, A.L.; Rundberget, T.; Miles, C.O. Characterization of fatty acid esters of okadaic acid and related toxins in blue mussels (Mytilus edulis) from Norway. Rapid Commun. Mass Spectrom. 2008, 22, 1127–1136. [Google Scholar]
- Torgersen, T.; Miles, C.O.; Rundberget, T.; Wilkins, A.L. New esters of okadaic acid in seawater and blue mussels (Mytilus edulis). J. Agric. Food Chem. 2008, 56, 9628–9635. [Google Scholar]
- Suzuki, T.; Beuzenberg, V.; Mackenzie, A.L.; Quilliam, M.A. Liquid chromatography—mass spectrometry of spiroketal stereoisomers of pectenotoxins and the analysis of novel pectenotoxin isomers in the toxic dinoflagellate Dinophysis acuta from New Zealand. J. Chromatogr. A 2003, 992, 141–150. [Google Scholar]
- Suzuki, T.; Horie, Y.; Koike, K.; Satake, M.; Oshima, Y.; Iwataki, M.; Yoshimatsu, S. Yessotoxin analogues in several strains of Protoceratium reticulatum in Japan determined by liquid chromatography-hybrid triple quadrupole/linear ion trap mass spectrometry. J. Chromatogr. A 2007, 1142, 172–177. [Google Scholar]
- Blanco, J.; Alvarez, G.; Uribe, E. Identification of pectenotoxins in plankton, filter feeders, and isolated cells of a Dinophysis acuminata with an atypical toxin profile, from Chile. Toxicon 2007, 49, 710–716. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Selwood, A.I.; Hawkes, A.D.; Jensen, D.J.; Cooney, J.M.; Beuzenberg, V.; MacKenzie, A.L. Isolation of yessotoxin 32-O-[beta-L-arabinofuranosyl-(5'-> 1'')-beta-L-arabinofuranoside] from Protoceratium reticulatum. Toxicon 2006, 47, 510–516. [Google Scholar]
- Suzuki, T.; Mackenzie, A.L.; Stirling, D.; Adamson, J. Pectenotoxin-2 seco acid: A toxin converted from pectenotoxin-2 by the New Zealand Greenshell mussel, Perna canaliculus. Toxicon 2001, 39, 507–514. [Google Scholar]
- McCarron, P.; Kilcoyne, J.; Miles, C.O.; Hess, P. Formation of azaspiracids-3, -4, -6, and -9 via decarboxylation of carboxyazaspiracid metabolites from shellfish. J. Agric. Food Chem. 2009, 57, 160–169. [Google Scholar]
- Quilliam, M.A.; Hess, P.; Dell' Aversano, C. Recent Developments in the Analysis of Phycotoxins by Liquid Chromatography-mass Spectrometry; de Koe, W.J., Samson, R.A., van Egmond, H.P., Gilbert, J., Sabino, M., Eds.; Mycotoxins and Phycotoxins in Perspective at the turn of the century: Wageningen, The Netherlands, 2001; pp. 383–391. [Google Scholar]
- Goto, H.; Igarashi, T.; Yamamoto, M.; Yasuda, M.; Sekiguchi, R.; Watai, M.; Tanno, K.; Yasumoto, T. Quantitative determination of marine toxins associated with diarrhetic shellfish poisoning by liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2001, 907, 181–189. [Google Scholar]
- McNabb, P.; Selwood, A.I.; Holland, P.T. Multiresidue method for determination of algal toxins in shellfish: Single-laboratory validation and interlaboratory study. J. AOAC Int. 2005, 88, 761–772. [Google Scholar]
- Stobo, L.A.; Lacaze, J.P.C.L.; Scott, A.C.; Gallacher, S.; Smith, E.A.; Quilliam, M.A. Liquid chromatography with mass spectrometry—detection of lipophilic shellfish toxins. J. AOAC Int. 2005, 88, 1371–1382. [Google Scholar]
- Fux, E.; McMillan, D.; Bire, R.; Hess, P. Development of an ultra-performance liquid chromatography-mass spectrometry method for the detection of lipophilic marine toxins. J. Chromatogr. A 2007, 1157, 273–280. [Google Scholar]
- Gerssen, A.; Mulder, P.P.J.; McElhinney, M.A.; De Boer, J. Liquid chromatography—tandem mass spectrometry method for the detection of marine lipophilic toxins under alkaline conditions. J. Chromatogr. A 2009, 1216, 1421–1430. [Google Scholar]
- Aune, T.; Yndestad, M. Diarrhetic shellfish poisoning. In Algal Toxins in Seafood and Drinking Water; Falconer, I.R., Ed.; Academic Press: London, UK, 1993; pp. 87–105. [Google Scholar]
- Murata, M.; Shimatani, M.; Sugitani, H.; Oshima, Y.; Yasumoto, T. Isolation and structural elucidation of the causative toxin of the diarrhetic shellfish poisoning. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 549–552. [Google Scholar]
- LNV, Jaarverslag Visserij. Ministry of Agriculture Nature Management and Fisheries: The Hague, The Netherland, 1971; pp. 394–397.
- LNV, Jaarverslag Visserij. Ministry of Agriculture Nature Management and Fisheries: The Hague, The Netherland, 1976; p. 292.
- LNV, Jaarverslag Visserij. Ministry of Agriculture Nature Management and Fisheries: The Hague, The Netherland, 1981; p. 170.
- Kat, M. Diarrhetic mussel poisoning in The Netherlands related to the dinoflagellate Dinophysis acuminata. Antonie Van Leeuwenhoek 1983, 49, 417–427. [Google Scholar]
- LNV, Jaarverslag Visserij. Ministry of Agriculture Nature Management and Fisheries: The Hague, The Netherland, 1986; pp. 64–65.
- LNV, Jaarverslag Visserij. Ministry of Agriculture Nature Management and Fisheries: The Hague, The Netherland, 1987; p. 163.
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.J.; Van den Top, H.J.; De Boer, J. Marine Toxins: Chemistry, Toxicity, Occurrence and Detection, with Special Reference to the Dutch Situation. Toxins 2010, 2, 878-904. https://doi.org/10.3390/toxins2040878
Gerssen A, Pol-Hofstad IE, Poelman M, Mulder PPJ, Van den Top HJ, De Boer J. Marine Toxins: Chemistry, Toxicity, Occurrence and Detection, with Special Reference to the Dutch Situation. Toxins. 2010; 2(4):878-904. https://doi.org/10.3390/toxins2040878
Chicago/Turabian StyleGerssen, Arjen, Irene E. Pol-Hofstad, Marnix Poelman, Patrick P.J. Mulder, Hester J. Van den Top, and Jacob De Boer. 2010. "Marine Toxins: Chemistry, Toxicity, Occurrence and Detection, with Special Reference to the Dutch Situation" Toxins 2, no. 4: 878-904. https://doi.org/10.3390/toxins2040878





