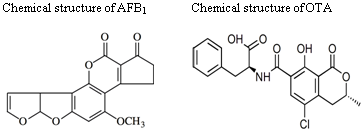
Antigenotoxic Studies of Different Substances to Reduce the DNA Damage Induced by Aflatoxin B1 and Ochratoxin A
Abstract
:1. Introduction
2. Generalities of Aflatoxins
3. DNA Damage by Aflatoxin B1 (AFB1)
3.1. Antimutagenic Strategies Used to Control the AFB1 Damage
| Methods | Technique | Example | Reference |
|---|---|---|---|
| Physical | Inactivation by heat | Vapor pressure | [38] |
| Microwave treatment | [39] | ||
| Nixtamalization | [40] | ||
| Inactivation by radiation | Ultraviolet light | [39] | |
| Radiation gamma | [1,41] | ||
| Elimination by adsorbent substances | Zeolites | [1] | |
| Bentonites | [39] | ||
| Aluminosilicates | [42] | ||
| Chemical | Extraction by solvents organics | Ethanol 95% | [43] |
| Acetone 90% | [39] | ||
| Chemical destruction | Hexane-ethanol | [43] | |
| Hydrogen peroxide | [44] | ||
| Ammonium hydroxyde | [45,46] | ||
| Methylamine | [39] | ||
| Sodium hypochlorite | [44] |
3.2. In Vitro Studies
3.3. In Vivo Studies
| Year | Biological model | Antimutagen | Type of study | Observation | Inhibition (%) | Reference |
|---|---|---|---|---|---|---|
| 1991 | Mice | Coffe | Acute | Micronucleus | 60 | [53] |
| 1992 | Rat | Vitamin A | Subchronic | Single cell electrophoresis | 50 | [50] |
| 1992 | Mice | Vitamins: thiamine, riboflavin, niacin, and folic acid | Subchronic | Micronucleus | 60 | [54] |
| 1993 | Mice | Ammonium hydroxyde | Subchronic | Micronucleus and Sister chromatid exchanges (SCEs) | 60 | [55] |
| 1998 | Rat | Carotenoids | Quantification of adducts | 65 | [52] | |
| 1998 | Mice | S. cerevisiae | Subchronic | Micronucleus | 70 | [22] |
| 2007 | Mice | Mannan | Subchronic | Micronucleus | 50 | [56] |
| 2009 | Mice | Mannan | Acute | single cell electrophoresis | 60 | [25] |
3.4. Antimutagenic Effect of Yeast
3.5. Protective Effect Caused by Constituents of Yeast Cell Wall against the DNA Damage Induced by AFB1
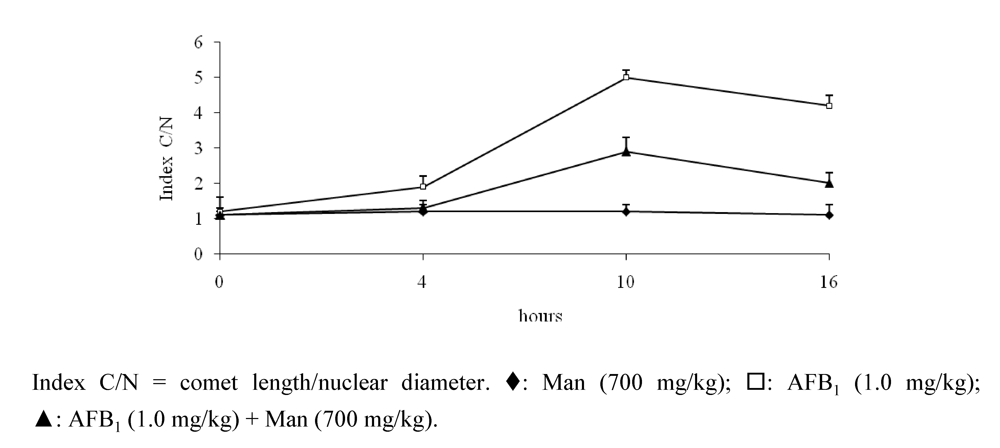
4. Generalities about the Ochratoxins
5. DNA Damage Caused by OTA
6. Ochratoxin A Decontamination Methods
| Methods | Technique | Example | Reference |
|---|---|---|---|
| Physical | Mechanical removal | Milling | [1] |
| Washing and brushing | [73] | ||
| Chemical | Pesticides | Dinocap, Penconazole | [1] |
| Fungicides | Iproidine, Azoxystrobin | [1] | |
| Essential oils | Oregano, Mint, Basil, Sage | [1] | |
| Gas treatment | Ozone | [74] | |
| Antifungals | Fusapyrone | [1] | |
| Biological | Spores | Conidia | [75] |
| Biodegradation | Acinetobacter, Lactobacillus, Streptococcus thermophilus, and Bifidobacterium | [1,76,77] |
| Food | Permissible limit (μg/kg) |
|---|---|
| Coffee beans | 1.0–5.0 |
| Instant coffee | 0.8–10 |
| Cereals | 4.0–5.0 |
| Table wine | 2.0 |
| Grape juice | 2.0 |
| Food for infants and childrens | 0.5 |
| Sausages | 3.0 |
| Dried fruits | 0.2 |
| Beer | 3.0 |
6.1. Antimutagenic Strategies for the Control of Ochratoxin A Damage
6.2. In Vitro Studies
6.3. In Vivo Studies
| Antimutagen | Cell line or strains of study | Inhibition% | Reference |
|---|---|---|---|
| Aspartame, phenylalanine, and piroxicam | Vero cells | 50 | [79] |
| Lactobacillus rhamnosus | 76 | [81] | |
| Rosmarinic acid | Human hepatoma cells (Hep G2) | 40 | [84] |
| Chrysin, quercetin genistein, and biochanin A | Caco-2-cells | 70 | [83] |
| Epigallocathechin gallate, and epicatechin gallate | Pig kidney cells (LLC-PK1) | 80 | [79] |
| Gallic acid, vanillic acid, protocatechuic acid, caffeic acid, chlorogenic acid, and 4-hydroxybenzoic acid | Ochratoxigenic Aspergilli strains | 50–60 | [82] |
| Year | Biological model | Evaluation parameters | Chemopreventive substance | Conclusion | Reference |
|---|---|---|---|---|---|
| 1996 | Mice | Quantification of aducts | Indomethacin and aspirin | These substances reduce the amounts of DNA adducts, particularly in the urinary bladder and kidney. This suggests a role of protaglandin H synthase in the metabolism of OTA leading to active metabolites which react with DNA | [85] |
| 1998 | Rats | Quantification of aducts | Aspartame | The molecular mechanism mediating the preventive effect of Aspartame is the delivery of phenylalanine by cleavage of the peptide and also the direct effect of the peptide on the bending capacity and transport of the toxin | [90] |
| 2001 | Rats | LPO, GSH, GR, GSPx, SOD, CAT, and GST | Melatonin (Mel) | Mel has a protective effect against OTA toxicity through an inhibition of the oxidative damage and stimulation of GST activities | [88] |
| 2004 | Rats | LPO, GSPx, CAT, and SOD | Melatonin (Mel) | Mel decreased the OTA-induced damage to support the antioxidant defense system and/or with free radical scavenger action | [87] |
| 2006 | Mice | Colonic probiotic bacteria, colon enzyme glucuronidases, and chromosomal aberrations | Dietary honey | Substituting sugars with honey in processed food can inhibit the harmful and genotoxic effects of mycotoxins, and improve the gut microflora | [86] |
| 2008 | Rats | Micronucleus | Inula crithmoides extract | The extract alone was successful in counteracting the oxidative stress and protect against the cytotoxicity produced by OTA | [89] |
7. Conclusions
References
- Kabak, B.; Dobson, A.D.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol Rev. 2003, 16, 497–516. [Google Scholar]
- Murphy, P.A.; Hendrich, S.; Landgren, C. Scientific status summary: Food mycotoxins-an update. J. Food Sci. 2006, 71, R51–R65. [Google Scholar]
- Girish, C.K.; Smith, T.K. Impact of feed-borne mycotoxins on avian cell-mediated and humoral immune responses. World Mycotoxin J. 2008, 1, 105–121. [Google Scholar]
- Santacroce, M.P.; Conversano, M.C.; Casalino, E.; Lai, O.; Zizzadoro, C.; Centoducati, G.; Crescenzo, G. Aflatoxins in aquatic species: metabolism, toxicity and perspectives. Rev. Fish Biol. Fish. 2008, 18, 99–130. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T. Risk assessment to humans of mycotoxins in animal-derived food products. Vet. Hum. Toxicol. 1991, 33, 325–333. [Google Scholar]
- Abdel-Wahhab, M.A.; Kholif, A.M. Mycotoxins in animal feeds: prevention strategies. Asian J. Anim. Sci. 2008, 2, 7–25. [Google Scholar]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar]
- Wang, J.S.; Groopman, J.D. DNA damage by mycotoxins. Mutat. Res. 1999, 424, 167–181. [Google Scholar]
- Gelderblom, W.C.A.; Snyman, S.D.; Abel, S.; Lebepe-Mazur, S.; Smuts, C.M.; van der Westhuitzen, L.; Marasas, W.F.O.; Victor, T.C.; Knasmuller, S.; Huber, W. Hepatotoxicity andcarcinogenicity of fumonisins in rats: a review regarding mechanistic implications for establising risk in humans. In Fumonisins in Food. Advances in Experimental Medicine and Biology; Jackson, L.S., DeVries, J.W., Bullerman, L.B., Eds.; Plenum: New York, NY, USA, 1995; Volume 392, pp. 279–296. [Google Scholar]
- WHO. Evaluation of certain mycotoxins in food. Fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives, Technical Report Series 906. World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Steyn, P.S. Mycotoxins, general view, chemistry and structure. Toxicol. Lett. 1995, 82-83, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar]
- Cleveland, T.E.; Dowd, P.E.; Desjardins, A.E.; Bhatnagar, D.; Coty, P.J. United States Department of Agriculture-Agricultural Research Service. Research on Preharvest Prevention of Mycotoxins and Mycotoxigenic Fungi in US Crops. Pest. Manag. Sci. 2003, 59, 629–642. [Google Scholar] [PubMed]
- Kumagai, S.; Nakajima, M.; Tabata, S.; Ishikuro, E.; Tanaka, T.; Norizuki, H.; Itoh, Y.; Aoyama, K.; Fujita, K.; Kai, S.; Sato, T.; Yoshiike, N.; Sugita-Konishi, Y. Aflatoxin and ochratoxin. A contamination of retail foods and intake of these mycotoxins in Japan. Food Add. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2008, 25, 1101–1106. [Google Scholar]
- Wang, J.; Liu, X.M. Contamination of aflatoxins in different kinds of foods in China. Biomed. Environ. Sci. 2007, 20, 483–487. [Google Scholar]
- Caldas, E.D.; Silva, S.C.; Oliveira, J.N. Aflatoxins and ochratoxin A in food and risks to human health. Rev. Saude Publ. 2002, 36, 319–323. [Google Scholar] [CrossRef]
- El-Zawahri, M.M.; Morad, M.M.; Khishin, A.F. Mutagenic effect of aflatoxin G1 in comparison with B1. J. Environ. Pathol. Toxicol. Oncol. 1990, 10, 45–51. [Google Scholar]
- Anwar, W.A.; Khalil, M.M.; Wild, C.P. Micronuclei, chromosomal aberrations and aflatoxin-albumin adducts in experimental animals after exposure to aflatoxin B1. Mutat. Res. 1994, 322, 61–67. [Google Scholar]
- Neal, G.E. Genetic implications in the metabolism and toxicity of mycotoxins. Toxicol. Lett. 1995, 82-83, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.L.; Knapton, A.; Wehr, C.M.; Hook, G.J.; Mirsalis, J.; Shelby, M.D.; MacGregor, J.T. Micronucleated erythrocyte frequency in peripheral blood of B6C3F(1) mice from short-term, prechronic, and chronic studies of the ntp carcinogenesis bioassay program. Environ. Mol. Mutagen. 2000, 36, 163–194. [Google Scholar]
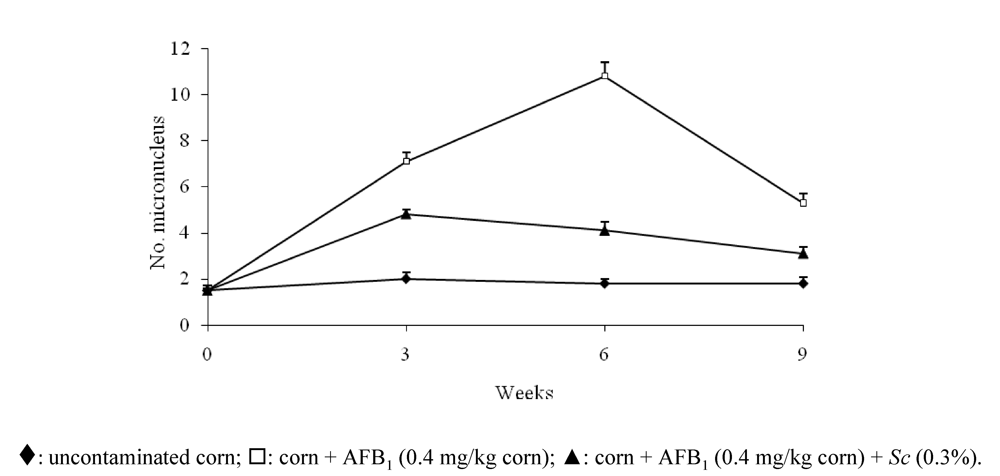
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Márquez-Márquez, R.; Reyes, A. Antigenotoxic effect of Saccharomyces cerevisiae on the damage produced in mice fed with aflatoxin B(1) contaminated corn. Food Chem. Toxicol. 2006, 44, 2058–2063. [Google Scholar]
- Kimura, M.; Lehmann, K.; Gopalan-Kriczky, P.; Lotlikar, P.D. Effect of diet on aflatoxin B1-DNA binding and aflatoxin B1-induced glutathione S-transferase placental form positive hepatic foci in the rat. Exp. Mol. Med. 2004, 36, 351–357. [Google Scholar]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Ann. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar]
- Madrigal-Santillán, E.; Morales-González, J.A.; Sánchez-Gutiérrez, M.; Reyes-Arellano, A.; Madrigal-Bujaidar, E. Investigation on the Protective Effect of alpha-Mannan against the DNA Damage Induced by Aflatoxin B(1) in Mouse Hepatocytes. Int. J. Mol. Sci. 2009, 10, 395–406. [Google Scholar]
- Raj, H.G.; Prasanna, H.R.; Mage, P.; Lotlikar, P.D. Effect of purified rat and hamster hepatic glutathione S-transferases on the microsome mediated binding of aflatoxin B1 to DNA. Cancer Lett. 1986, 33, 1–9. [Google Scholar]
- Eaton, D.L.; Ramsdel, H.S. Species and diet related differences in aflatoxin biotransformation. In Handbook of Applied Mycology, Mycotoxins in Ecological Systems; Bhatnagar, D., Lillehoj, E.B., Arora, D.K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1992; Volume 5, pp. 157–182. [Google Scholar]
- IARC. Evaluation of carcinogen risks to humans. Some naturally occurring substances: food, items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 56, 489–521. [Google Scholar] [PubMed]
- Labuza, T.P. Regulation of Mycotoxins. J. Food Prot. 1983, 46, 260–265. [Google Scholar]
- Guzmán-de-Peña, D.; Peña-Cabriales, J.J. Regulatory considerations of aflatoxin contamination of food in Mexico. Rev. Latinoam. Microbiol. 2005, 47, 160–164. [Google Scholar]
- Díaz, G.J.; Perilla, N.S.; Rojas, Y. Ocurrence of aflatoxins in selected colombians foods. Mycotoxin Res. 2001, 17, 15–20. [Google Scholar]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Philpott, M. Nutrition and mutagenesis. Annu. Rev. Nutr. 2008, 28, 313–329. [Google Scholar]
- Ellis, W.; Smith, J.; Simpson, B.; Oldham, J. Aflatoxin in food: Ocurrence, biosynthesis, effects on organism, detection and methods of control. Crit. Rev. Food Sci. Nutr. 1991, 30, 403–439. [Google Scholar]
- Robb, J. Mycotoxins: Contamination and descontamination. Feed Mix. 1993, 1, 18–23. [Google Scholar]
- Cazzaniga, D.; Basilico, J.C.; González, R.J.; Torres, R.L.; de Greef, D.M. Mycotoxins inactivation by extrusion cooking of corn fluor. Lett. Appl. Microbiol. 2001, 33, 144–147. [Google Scholar]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar]
- Park, D.L. Perspectives on mycotoxin decontamination procedures. Food Addit. Contam. 1993, 10, 49–60. [Google Scholar]
- Samarajeewa, U.; Sen, A.C.; Cohen, M.D.; Wei, C.I. Detoxification of aflatoxin in foods and feeds by physical and chemical methods. J. Food Prot. 1990, 53, 489–501. [Google Scholar]
- Elias-Orozco, R.; Castellanos-Nava, A.; Gaytán-Martínez, M.; Figueroa-Cárdenas, J.D.; Loarca-Piña, G. Comparison of nixtamalization and extrusion processes for a reduction in aflatoxin content. Food Addit. Contam. 2002, 19, 878–885. [Google Scholar]
- Shahin, A.A.; Aziz, N.H. Influence of gamma rays and sodium chloride on aflatoxin production by Aspergillus flavus. Microbios 1997, 90, 163–175. [Google Scholar]
- Kubena, L.F.; Harvey, N.E.; Phillips, T.D.; Clement, A.B. Effect of hidrated sodium calcium aluminosilicate on aflatoxicosis in broiler chicks. Poult. Sci. 1993, 72, 651–653. [Google Scholar]
- Stahr, H.M.; Obioha, W.O. Detoxification of aflatoxin contaminated corn by methanol extraction. Vet. Hum. Toxicol. 1982, 24, 16–17. [Google Scholar]
- Mukendi, N.; Rollmann, B.; de Meester, C. Detoxification of aflatoxin B1 by different chemical methods and evaluation of the effectiveness of the treatments applied. J. Pharm. Belg. 1991, 46, 182–188. [Google Scholar]
- Dvorák, M. Possibilities of chemical detoxification of aflatoxin. Vet. Med. (Praha) 1990, 35, 37–42. [Google Scholar] [PubMed]
- Norred, W.P.; Voss, K.A.; Bacon, C.W.; Riley, R.T. Effectiveness of ammonia treatment in detoxification of fumonisin-contaminated corn. Food Chem. Toxicol. 1991, 29, 815–819. [Google Scholar]
- Singh, J.; Reen, R.K.; Wiebel, F.J. Piperine, a major ingredient of black and long peppers, protects against AFB1-induced cytotoxicity and micronuclei formation in H4IIEC3 rat hepatoma cells. Cancer Lett. 1994, 86, 195–200. [Google Scholar]
- Soni, K.B.; Lahiri, M.; Chackradeo, P.; Bhide, S.V.; Kuttan, R. Protective effect of food additives on aflatoxin-induced mutagenicity and hepatocarcinogenicity. Cancer Lett. 1997, 115, 129–133. [Google Scholar]
- Loarca-Piña, G.; Kuzmicky, P.A.; Gonzalez de Mejia, E.; Kado, N.Y.; Hsieh, D.P. Antimutagenicity of ellagic acid against aflatoxin B1 in the Salmonella microsuspencion assay. Mutat. Res. 1996, 17, 15–21. [Google Scholar]
- Decoudu, S.; Cassand, P.; Daubeze, M.; Frayssient, C.; Narbonne, J.F. Effect of vitamin A dietary intake on in vitro and in vivo activation of aflatoxin B1. Mutat. Res. 1992, 269, 269–278. [Google Scholar]
- Kusamran, W.R.; Tepsuwan, A.; Kupradinum, P. Antimutagenic and anticarcinogenic potentials of some Thai vegetables. Mutat. Res. 1998, 402, 247–258. [Google Scholar]
- Gradelet, S.; Le Bon, A.M.; Berges, R.; Suschetet, M.; Astorg, P. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis 1998, 19, 403–411. [Google Scholar]
- Abraham, S.K. Inhibitory effects of coffe on the genotoxicity of carcinogens in mice. Mutat. Res. 1991, 262, 109–114. [Google Scholar]
- Lim-Sylianco, C.Y.; Sylianco, W.L. Antigenotoxic effects of B vitamins. Asian Food J. 1992, 7, 100–104. [Google Scholar]
- Márquez-Márquez, R.; Madrigal-Bujaidar, E.; Tejada de Hernández, I. Genotoxic evaluation of ammonium inactivated aflatoxin B1 in mice fed with contaminated corn. Mutat. Res. 1993, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
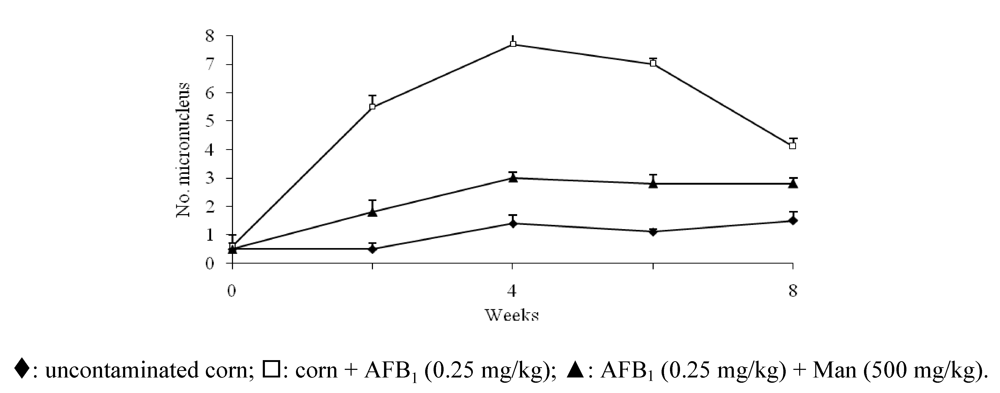
- Madrigal-Santillán, E.; Alvarez-González, I.; Márquez-Márquez, R.; Velázquez-Guadarrama, N.; Madrigal-Bujaidar, E. Inhibitory effect of mannan on the toxicity produced in mice fed aflatoxin B1 contaminated corn. Arch. Environ. Contam. Toxicol. 2007, 53, 466–472. [Google Scholar]
- Girard, I.D.; Dawson, K.A. Effect of yeast culture on the growth of representative ruminal bacteria. J. Anim. Sci. 1994, 77, 300–304. [Google Scholar]
- Madrigal-Bujaidar, E.; Madrigal-Santillán, E.; Pages, N.; Kogan, G.; Chamorro, C. Etude de L’antigenotoxicite de diferentes substances pour reduire la toxicite de L’aflatoxine B1 chez la souris. In Toxines et recherchers biomédicales; Francoise, G.-P., Cassian, B., Simone, P.-D., Sauviat, M.-P., Eds.; Reencontres en Toxinologie, Elsevier: Paris, France, 2002; pp. 123–132. [Google Scholar]
- Chorvatovicová, D.; Machova, E.; Sandula, J.; Kogan, G. Protective effect of the yeast glucomannan against cyclophosphamide-induced mutagenicity. Mutat. Res. 1999, 444, 117–122. [Google Scholar]
- Kogan, G.; Machova, E.; Chorvatovicová, D.; Slovakova, D.; Soltes, L.; Sandula, J. Chitin-glucan complex of Aspergillus niger and its derivates: antimutagenic, antiinfective and antiviral activity. In Advances in Chitin Science; Domard, A., Roberts, G.A., Eds.; Jackes André Publisher V II: Lyon, France, 1998; pp. 640–647. [Google Scholar]
- Aizawa, K.; Matsumoto, T.; Tsukadi, K.; Ito, A.; Satu, H.; Suzuki, S.; Suzuki, M. Antitumor effect of a Baker´s yeast mannan-mitomycin C conjugate against mouse hepatoma, MHI34, in vivo and in vitro. Int. J. Immunopharmacol. 1989, 11, 191–195. [Google Scholar]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaky, Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen 2000, 35, 206–221. [Google Scholar]
- Pohland, A.C. Mycotoxins in review. Food Addit. Contam. 1993, 10, 17–28. [Google Scholar]
- Favilla, M.; Pascale, M.; Ricelli, A.; Evidente, A.; Amalfitano, C.; Altomare, C. Inhibition of species of the Aspergillus section nigri and Ochratoxin A production in grapes by fusapyrone. Appl. Environ. Microbiol. 2008, 74, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Vogel, S.; Villamil-Jimenez, L.C. Micotoxinas en la salud pública. Rev. Salud Pública. 2006, 8, 129–135. [Google Scholar] [CrossRef]
- Bragulat, M.R.; Martínez, E.; Castellá, G.; Cabañes, F.J. Ochratoxin A and citrinin producing species of the genus Penicillium from feedstuffs. Int. J. Food Microbiol. 2008, 15, 43–48. [Google Scholar]
- Visconti, A.; Perrone, G.; Cozzi, G.; Solfrizzo, M. Managing ochratoxin A risk in the grape-wine food chain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 193–202. [Google Scholar]
- Magnoli, C.E.; Astoreca, A.L.; Chiacchiera, S.M.; Dalcero, A.M. Occurrence of ochratoxin A and ochratoxigenic mycoflora in corn and corn based foods and feeds in some South American countries. Mycopathologia 2007, 163, 249–260. [Google Scholar]
- Serra Bonvehí, J. Occurrence of ochratoxin A in cocoa products and chocolate. J. Agric. Food Chem. 2004, 52, 6347–6352. [Google Scholar] [CrossRef] [PubMed]
- Walker, R. Risk assessment of ochratoxin: current views of the European Scientific Committee on Food, the JECFA and the Codex Committee on Food Additives and Contaminants. Adv. Exp. Med. Biol. 2002, 504, 249–255. [Google Scholar] [PubMed]
- Arbillaga, L.; Azqueta, A.; Expeleta, O.; Lopez de Cerfin, A. Oxidative DNA damage by ochratoxin A in the HK2 human kidney cell line: evidence of the relationship with cytotoxicity. Mutagenesis 2007, 22, 35–42. [Google Scholar]
- Marin-Kuan, M.; Cavin, C.; Delatour, T.; Schilter, B. Ochratoxin A carcinogenicity involves a complex network of epigenetic mechanisms. Toxicon 2008, 52, 195–202. [Google Scholar]
- Iacumin, L.; Chiesa, L.; Boscolo, D.; Manzano, M.; Cantoni, C.; Orlic, S.; Corni, G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009, 26, 65–70. [Google Scholar]
- McKenzie, K.S.; Sarr, A.B.; Mayura, K.; Bailey, R.H.; Miller, D.R.; Rogers, T.D.; Norred, W.P.; Voss, K.A.; Plattner, R.D.; Kubena, L.F.; Phillips, T.D. Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food Chem. Toxicol. 1997, 35, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, H.; Mathieu, F.; Taillander, P.; Lebrihi, A. Conidia of black aspergilla as new biological adsorbents for ochratoxin A in grapes juices and musts. J. Agric. Food Chem. 2005, 53, 8224–8229. [Google Scholar]
- Abrunhosa, L.; Serra, R.; Venancio, A. Biodegradation of ochratoxin A by fungi isolated from grapes. J. Agric. Food Chem. 2002, 50, 7493–7496. [Google Scholar]
- Varga, J.; Rigo, K.; Téren, J. Degradation of ochratoxin A by Aspergillus species. Int. J. Food Microbiol. 2000, 59, 1–7. [Google Scholar]
- Petchkongkaew, A.; Taillandier, P.; Gasaluck, P.; Lebrihi, A. Isolation of bacillus spp. From Thai fermented soybean (Thua-nao): screening for aflatoxin B1 and ochratoxin A detoxification. J. Appl. Microbiol. 2008, 104, 1495–1502. [Google Scholar] [PubMed]
- Costa, S.; Utan, A.; Cervellati, R.; Speroni, E.; Guerra, M.C. Catechins: natural free-radical scavengers against ochratoxin A-induced cell damage in a pig kidney cell line (LLC-PK1). Food Chem. Toxicol. 2007, 45, 1910–1917. [Google Scholar]
- Baudrimont, I.; Ahouandjivo, R.; Creppy, E.E. Prevention of lipid peroxidation induced by ochratoxin A in Vero cells in culture by several agents. Chem. Biol. Interact. 1997, 104, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Turbic, A.; Ahokas, J.T.; Haskard, C.A. Selective in vitro binding of dietary mutagens, individually or in combination, by lactic acid bacteria. Food Addit. Contam. 2002, 19, 144–152. [Google Scholar]
- Palumbo, J.D.; O´Keeffe, T.L. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia 2007, 164, 241–248. [Google Scholar]
- Sergent, T.; Garsou, S.; Schaut, A.; De Saeger, S.; Pussemier, L.; Van Peteghem, C.; Larondelle, Y.; Schneider, Y.J. Differential modulation of ochratoxin A absorption across Caco-2-cells by dietary polyphenols, used at realistic intestinal concentrations. Toxicol. Lett. 2005, 159, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, C.; Galvano, F.; Pierdomenico, L.; Speroni, E.; Guerra, M.C. Effects of rosmarinic acid against aflatoxin B1 and ochratoxin A induced cell damage in a human hepatoma cell line (Hep G2). J. Appl. Toxicol. 2004, 24, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Obrecht-Pflumio, S.; Grosse, Y.; Pfohl-Leszkowicz, A.; Dirheimer, G. Protection by indomethacin and aspirin genotoxicity of ochratoxin A, particulary in the urinary bladder and kidney. Arch. Toxicol. 1996, 70, 244–248. [Google Scholar]
- Ezz El-Arab, A.M.; Girgis, S.M.; Hegazy, E.M.; Abd El-Khalek, A.B. Effect of dietary honey on intestinal microflora and toxicity of mycotoxins in mice. BMC Complement. Altern. Med. 2006, 50, 799–804. [Google Scholar]
- Soyoz, M.; Ozcelik, N.; Kilinc, I.; Altuntas, I. The effects of ochratoxin A on lipid peroxidation and antioxidant enzymes: a protective role of melatonin. Cell. Biol. Toxicol. 2004, 20, 213–219. [Google Scholar]
- Abdel-Raheim, M.A.; Hussein, A.A. Melatonin reduces oxidative stress induced by ochratoxin A in rat liver and kidney. Comp. Biochem. Physiol. Part C. 2001, 130, 305–313. [Google Scholar]
- Abdel-Wahhab, M.A.; Abdel-Azim, S.H.; El-Nekeety, A.A. Inula crithmoides extract protects against ochratoxin A-induced oxidative stress, clastogenic, and mutagenic alterations in male rats. Toxicon 2008, 52, 566–573. [Google Scholar]
- Creppy, E.E.; Baudrimont, I.; Anne-Marie. How aspartame prevents the toxicity of ochratoxin A. J. Toxicol Sci. 1998, 23, 165–172. [Google Scholar] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Madrigal-Santillán, E.; Morales-González, J.A.; Vargas-Mendoza, N.; Reyes-Ramírez, P.; Cruz-Jaime, S.; Sumaya-Martínez, T.; Pérez-Pastén, R.; Madrigal-Bujaidar, E. Antigenotoxic Studies of Different Substances to Reduce the DNA Damage Induced by Aflatoxin B1 and Ochratoxin A. Toxins 2010, 2, 738-757. https://doi.org/10.3390/toxins2040738
Madrigal-Santillán E, Morales-González JA, Vargas-Mendoza N, Reyes-Ramírez P, Cruz-Jaime S, Sumaya-Martínez T, Pérez-Pastén R, Madrigal-Bujaidar E. Antigenotoxic Studies of Different Substances to Reduce the DNA Damage Induced by Aflatoxin B1 and Ochratoxin A. Toxins. 2010; 2(4):738-757. https://doi.org/10.3390/toxins2040738
Chicago/Turabian StyleMadrigal-Santillán, Eduardo, José A. Morales-González, Nancy Vargas-Mendoza, Patricia Reyes-Ramírez, Sandra Cruz-Jaime, Teresa Sumaya-Martínez, Ricardo Pérez-Pastén, and Eduardo Madrigal-Bujaidar. 2010. "Antigenotoxic Studies of Different Substances to Reduce the DNA Damage Induced by Aflatoxin B1 and Ochratoxin A" Toxins 2, no. 4: 738-757. https://doi.org/10.3390/toxins2040738