Cytotoxic Necrotizing Factors (CNFs)−A Growing Toxin Family
Abstract
:1. Introduction
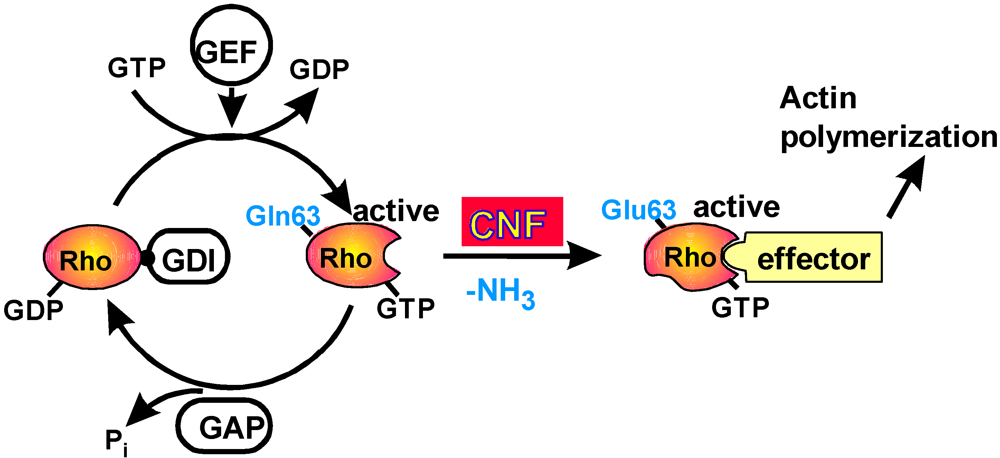
2. Mode of Action
3. Structure-Function Relationship

Cellular uptake of CNFs

Export of CNF
4. Conclusions
References and Notes
- Villalonga, P.; Ridley, A.J. Rho GTPases and cell cycle control. Growth Factors 2006, 24, 159–164. [Google Scholar] [PubMed]
- Aktories, K.; Schmidt, G.; Just, I. Rho GTPases as targets of bacterial protein toxins. Biol. Chem. 2000, 381, 421–426. [Google Scholar] [PubMed]
- Caprioli, A.; Falbo, V.; Roda, L.G.; Ruggeri, F.M.; Zona, C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 1983, 39, 1300–1306. [Google Scholar] [PubMed]
- Foxman, B.; Zhang, L.; Palin, K.; Tallman, P.; Marrs, C.F. Bacterial virulence characteristics of Escherichia coli isolates from first-time urinary tract infection. J. Infect. Dis. 1995, 171, 1514–1521. [Google Scholar] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 2002, 113Suppl 1A, 5–13. [Google Scholar] [CrossRef]
- Landraud, L.; Gauthier, M.; Boquet, P. Frequency of Escherichia coli strains producing the cytotoxic necrotizing factor (CNF1) in nosocomial urinary tract infections. Lett. Appl. Microbiol. 2000, 30, 213–216. [Google Scholar] [PubMed]
- Yamamoto, S.; Tsukamoto, T.; Terai, A.; Kurazono, H.; Takeda, Y.; Yoshida, O. Distribution of virulence factors in Escherichia coli isolated from urine of cystitis patients. Microbiol. Immunol. 1995, 39, 401–404. [Google Scholar] [PubMed]
- Rippere-Lampe, K.E.; O'Brien, A.D.; Conran, R.; Lockman, H.A. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 2001, 69, 3954–3964. [Google Scholar] [PubMed]
- Fournout, S.; Dozois, C.M.; Odin, M.; Desautels, C.; Pérès, S.; Hérault, F.; Daigle, F.; Segafredo, C.; Laffitte, J.; Oswald, E.; Fairbrother, J.M.; Oswald, I.P. Lack of a role of cytotoxic necrotizing factor 1 toxin from Escherichia coli in bacterial pathogenicity and host cytokine response in infected germfree piglets. Infect. Immun. 2000, 68, 839–847. [Google Scholar] [PubMed]
- Petkovsek, Z.; Elersic, K.; Gubina, M.; Zgur-Bertok, D.; Starcic, E.M. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J. Clin. Microbiol. 2009, 47, 1811–1817. [Google Scholar] [PubMed]
- Khan, N.A.; Wang, Y.; Kim, K.J.; Chung, J.W.; Wass, C.A.; Kim, K.S. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 2002, 277, 15607–15612. [Google Scholar] [PubMed]
- Ananias, M.; Yano, T. Serogroups and virulence genotypes of Escherichia coli isolated from patients with sepsis. Braz. J. Med. Biol. Res. 2008, 41, 877–883. [Google Scholar] [PubMed]
- Oswald, E.; de Rycke, J.; Guillot, J.F.; Boivin, R. Cytotoxic effect of multinucleation in HeLa cell cultures associated with the presence of Vir plasmid in Escherichia coli strains. FEMS Microbiol. Lett. 1989, 58, 95–100. [Google Scholar]
- Orden, J.A.; Dominguez-Bernal, G.; Martinez-Pulgarin, S.; Blanco, M.; Blanco, J.E.; Mora, A.; Blanco, J.; Blanco, J.; de la, F.R. Necrotoxigenic Escherichia coli from sheep and goats produce a new type of cytotoxic necrotizing factor (CNF3) associated with the eae and ehxA genes. Int. Microbiol. 2007, 10, 47–55. [Google Scholar] [PubMed]
- Oswald, E.; de Rycke, J. A single protein of 110 kDa is associated with the multinucleating and necrotizing activity coded by the Vir plasmid of Escherichia coli. FEMS Microbiol. Lett. 1990, 68, 279–284. [Google Scholar]
- Kadhum, H.J.; Finlay, D.; Rowe, M.T.; Wilson, I.G.; Ball, H.J. Occurrence and characteristics of cytotoxic necrotizing factors, cytolethal distending toxins and other virulence factors in Escherichia coli from human blood and faecal samples. Epidemiol. Infect. 2008, 136, 752–760. [Google Scholar] [PubMed]
- Falzano, L.; Fiorentini, C.; Donelli, G.; Michel, E.; Kocks, C.; Cossart, P.; Cabanié, L.; Oswald, E.; Boquet, P. Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol. Microbiol. 1993, 9, 1247–1254. [Google Scholar] [PubMed]
- Rippere-Lampe, K.E.; Lang, M.; Ceri, H.; Olson, M.; Lockman, H.A.; O'Brien, A.D. Cytotoxic necrotizing factor type 1-positive Escherichia coli causes increased inflammation and tissue damage to the prostate in a rat prostatitis model. Infect. Immun. 2001, 69, 6515–6519. [Google Scholar] [PubMed]
- Hopkins, A.M.; Walsh, S.V.; Verkade, P.; Boquet, P.; Nusrat, A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 2003, 116, 725–742. [Google Scholar] [PubMed]
- Hofmann, P.; Le Negrate, G.; Mograbi, B.; Hofmann, V.; Brest, P.; Alliana-Schmid, A.; Flatau, G.; Boquet, P.; Rossi, B. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 2000, 68, 522–528. [Google Scholar] [PubMed]
- Doye, A.; Mettouchi, A.; Bossis, G.; Clément, R.; Buisson-Touati, C.; Flatau, G.; Gagnoux, L.; Piechaczyk, M.; Boquet, P.; Lemichez, E. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 2002, 111, 553–564. [Google Scholar] [PubMed]
- Lax, A.J. Bacterial toxins and cancer - a case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [PubMed]
- Miraglia, A.G.; Travaglione, S.; Meschini, S.; Falzano, L.; Matarrese, P.; Quaranta, M.G.; Viora, M.; Fiorentini, C.; Fabbri, A. Cytotoxic necrotizing factor 1 prevents apoptosis via the Akt/IkappaB kinase pathway: role of nuclear factor-kappaB and Bcl-2. Mol. Biol. Cell 2007, 18, 2735–2744. [Google Scholar] [PubMed]
- Schmidt, G.; Sehr, P.; Wilm, M.; Selzer, J.; Mann, M.; Aktories, K. Gln63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature 1997, 387, 725–729. [Google Scholar] [PubMed]
- Flatau, G.; Lemichez, E.; Gauthier, M.; Chardin, P.; Paris, S.; Fiorentini, C.; Boquet, P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 1997, 387, 729–733. [Google Scholar] [PubMed]
- Horiguchi, Y.; Nakai, T.; Kume, K. Purification and characterization of Bordetella bronchiseptica dermonecrotic toxin. Microb. Pathogen. 1989, 6, 361–368. [Google Scholar]
- Masuda, M.; Betancourt, L.; Matsuzawa, T.; Kashimoto, T.; Takao, T.; Shimonishi, Y.; Horiguchi, Y. Activation of Rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 2000, 19, 521–530. [Google Scholar] [PubMed]
- Schmidt, G.; Goehring, U.-M.; Schirmer, J.; Uttenweiler-Joseph, S.; Wilm, M.; Lohmann, M.; Giese, A.; Schmalzing, G.; Aktories, K. Lysine and polyamines are substrates for transglutamination of Rho by the Bordetella dermonecrotic toxin. Infect. Immun. 2001, 69, 7663–7670. [Google Scholar] [PubMed]
- McNichol, B.A.; Rasmussen, S.B.; Meysick, K.C.; O'Brien, A.D. A single amino acid substitution in the enzymatic domain of cytotoxic necrotizing factor type 1 of Escherichia coli alters the tissue culture phenotype to that of the dermonecrotic toxin of Bordetella spp. Mol. Microbiol. 2006, 60, 939–950. [Google Scholar] [PubMed]
- Schmidt, G.; Goehring, U.-M.; Schirmer, J.; Lerm, M.; Aktories, K. Identification of the C-terminal part of Bordetella dermonecrotic toxin as a transglutaminase for Rho GTPases. J. Biol. Chem. 1999, 274, 31875–31881. [Google Scholar] [PubMed]
- Fiorentini, C.; Fabbri, A.; Flatau, G.; Donelli, G.; Matarrese, P.; Lemichez, E.; Falzano, L.; Boquet, P. Escherichia coli cytotoxic necrotizing factor 1 (CNF1), a toxin that activates the Rho GTPase. J. Biol. Chem. 1997, 272, 19532–19537. [Google Scholar] [PubMed]
- Denko, N.; Langland, R.; Barton, M.; Lieberman, M.A. Uncoupling of S-phase and mitosis by recombinant cytotoxic necrotizing factor 2 (CNF2). Exp. Cell Res. 1997, 234, 132–138. [Google Scholar] [PubMed]
- Malorni, W.; Fiorentini, C. Is the Rac GTPase-activating toxin CNF1 a smart hijacker of host cell fate? FASEB J. 2006, 20, 606–609. [Google Scholar] [PubMed]
- Hoffmann, C.; Pop, M.; Leemhuis, J.; Schirmer, J.; Aktories, K.; Schmidt, G. The Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFY) selectively activates RhoA. J. Biol. Chem. 2004, 279, 16026–16032. [Google Scholar] [PubMed]
- Sugai, M.; Hatazaki, K.; Mogami, A.; Ohta, H.; Pérès, S.Y.; Hérault, F.; Horiguchi, Y.; Masuda, M.; Ueno, Y.; Komatsuzawa, H.; Suginaka, H.; Oswald, E. Cytotoxic necrotizing factor type 2 produced by pathogenic Escherichia coli deamidates a Gln residue in the conserved G-3 domain of the Rho family and preferentially inhibits the GTPase activity of RhoA and Rac1. Infect. Immun. 1999, 67, 6550–6557. [Google Scholar] [PubMed]
- Stoll, T.; Markwirth, G.; Reipschlager, S.; Schmidt, G. A new member of a growing toxin family - Escherichia coli cytotoxic necrotizing factor 3 (CNF3). Toxicon 2009, 54, 745–753. [Google Scholar] [PubMed]
- Lerm, M.; Pop, M.; Fritz, G.; Aktories, K.; Schmidt, G. Proteasomal degradation of cytotoxic necrotizing factor 1-activated Rac. Infect. Immun. 2002, 70, 4053–4058. [Google Scholar] [PubMed]
- Munro, P.; Flatau, G.; Doye, A.; Boyer, L.; Oregioni, O.; Mege, J.L.; Landraud, L.; Lemichez, E. Activation and proteasomal degradation of Rho GTPases by CNF1 elicit a controlled inflammatory response. J. Biol. Chem. 2004, 279, 35849–35857. [Google Scholar] [PubMed]
- Pop, M.; Aktories, K.; Schmidt, G. Isotype-specific degradation of Rac activated by the cytotoxic necrotizing factor 1 (CNF1). J. Biol. Chem. 2004, 279, 35840–35848. [Google Scholar] [PubMed]
- Lemichez, E.; Flatau, G.; Bruzzone, M.; Boquet, P.; Gauthier, M. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol. Microbiol. 1997, 24, 1061–1070. [Google Scholar] [PubMed]
- Schmidt, G.; Selzer, J.; Lerm, M.; Aktories, K. The Rho-deamidating cytotoxic-necrotizing factor CNF1 from Escherichia coli possesses transglutaminase activity - cysteine-866 and histidine-881 are essential for enzyme activity. J. Biol. Chem. 1998, 273, 13669–13674. [Google Scholar] [PubMed]
- Buetow, L.; Flatau, G.; Chiu, K.; Boquet, P.; Ghosh, P. Structure of the Rho-activating domain of Escherichia coli cytotoxic necrotizing factor 1. Nat. Struct. Biol. 2001, 8, 584–588. [Google Scholar] [PubMed]
- Hoffmann, C.; Aktories, K.; Schmidt, G. Change in substrate specificity of Cytotoxic Necrotizing Factor (CNF) unmasks proteasome-independent down-regulation of constitutively active RhoA. J. Biol. Chem. 2007, 282, 10826–10832. [Google Scholar] [PubMed]
- Buetow, L.; Ghosh, P. Structural elements required for deamidation of RhoA by cytotoxic necrotizing factor 1. Biochemistry 2003, 42, 12784–12791. [Google Scholar] [PubMed]
- Contamin, S.; Galmiche, A.; Doye, A.; Flatau, G.; Benmerah, A.; Boquet, P. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell 2000, 11, 1775–1787. [Google Scholar] [PubMed]
- Fabbri, A.; Gauthier, M.; Boquet, P. The 5' region of cnf1 harbours a translational regulatory mechanism for CNF1 synthesis and encodes the cell-binding domain of the toxin. Mol Microbiol 1999, 33, 108–118. [Google Scholar] [PubMed]
- Chung, J.W.; Hong, S.J.; Kim, K.J.; Goti, D.; Stins, M.F.; Shin, S.; Dawson, V.L.; Dawson, T.M.; Kim, K.S. 37 kDa laminin receptor precusor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 2003, 278, 16857–16862. [Google Scholar] [PubMed]
- Kim, K.J.; Chung, J.W.; Kim, K.S. 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J. Biol. Chem. 2005, 280, 1360–1368. [Google Scholar] [PubMed]
- Blumenthal, B.; Hoffmann, C.; Aktories, K.; Backert, S.; Schmidt, G. The Cytotoxic Necrotizing Factors from Yersinia pseudotuberculosis and from Escherichia coli Bind to Different Cellular Receptors but Take the Same Route to the Cytosol. Infect. Immun. 2007, 75, 3344–3353. [Google Scholar] [PubMed]
- McNichol, B.A.; Rasmussen, S.B.; Carvalho, H.M.; Meysick, K.C.; O'Brien, A.D. Two domains of cytotoxic necrotizing factor type 1 bind the cellular receptor, laminin receptor precursor protein. Infect. Immun. 2007, 75, 5095–5104. [Google Scholar] [PubMed]
- Werner, G.; Hagenmaier, H.; Drautz, H.; Baumgartner, A.; Zahner, H. Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity. J. Antibiotics 1984, 37, 110–117. [Google Scholar]
- Pei, S.; Doye, A.; Boquet, P. Mutation of specific acidic residues of the CNF1 T domain into lysine alters cell membrane translocation of the toxin. Mol. Microbiol. 2001, 41, 1237–1247. [Google Scholar] [PubMed]
- O'Keefe, D.O.; Cabiaux, V.; Choe, S.; Eisenberg, D.; Collier, R.J. pH-dependent insertion of proteins into membranes: B-chain mutation of diphtheria toxin inhibits membrane translocation, Glu-349 » Lys. Proc. Natl. Acad. Sci. USA 1992, 89, 6202–6206. [Google Scholar]
- Knust, Z.; Blumenthal, B.; Aktories, K.; Schmidt, G. Cleavage of Escherichia coli cytotoxic necrotizing factor 1 is required for full biologic activity. Infect. Immun. 2009, 77, 1835–1841. [Google Scholar] [PubMed]
- Kouokam, J.C.; Wai, S.N.; Fallman, M.; Dobrindt, U.; Hacker, J.; Uhlin, B.E. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli. Infect. Immun. 2006, 74, 2022–2030. [Google Scholar] [PubMed]
- Davis, J.M.; Carvalho, H.M.; Rasmussen, S.B.; O'Brien, A.D. Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis. Infect. Immun. 2006, 74, 4401–4408. [Google Scholar] [PubMed]
- Yu, H.; Kim, K.S. Ferredoxin is involved in secretion of cytotoxic necrotizing factor 1 across the cytoplasmic membrane in Escherichia coli K1. Infect. Immun. 2010, 78, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Diana, G.; Valentini, G.; Travaglione, S.; Falzano, L.; Pieri, M.; Zona, C.; Meschini, S.; Fabbri, A.; Fiorentini, C. Enhancement of learning and memory after activation of cerebral Rho GTPases. Proc. Natl. Acad. Sci. USA 2007, 104, 636–641. [Google Scholar]
- Pavone, F.; Luvisetto, S.; Marinelli, S.; Straface, E.; Fabbri, A.; Falzano, L.; Fiorentini, C.; Malorni, W. The Rac GTPase-activating bacterial protein toxin CNF1 induces analgesia up-regulating mu-opioid receptors. Pain 2009, 145, 219–229. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Knust, Z.; Schmidt, G. Cytotoxic Necrotizing Factors (CNFs)−A Growing Toxin Family. Toxins 2010, 2, 116-127. https://doi.org/10.3390/toxins2010116
Knust Z, Schmidt G. Cytotoxic Necrotizing Factors (CNFs)−A Growing Toxin Family. Toxins. 2010; 2(1):116-127. https://doi.org/10.3390/toxins2010116
Chicago/Turabian StyleKnust, Zeynep, and Gudula Schmidt. 2010. "Cytotoxic Necrotizing Factors (CNFs)−A Growing Toxin Family" Toxins 2, no. 1: 116-127. https://doi.org/10.3390/toxins2010116




