Analogs of the Scorpion Venom Peptide Stigmurin: Structural Assessment, Toxicity, and Increased Antimicrobial Activity
Abstract
:1. Introduction
2. Results
2.1. In Silico Evaluation
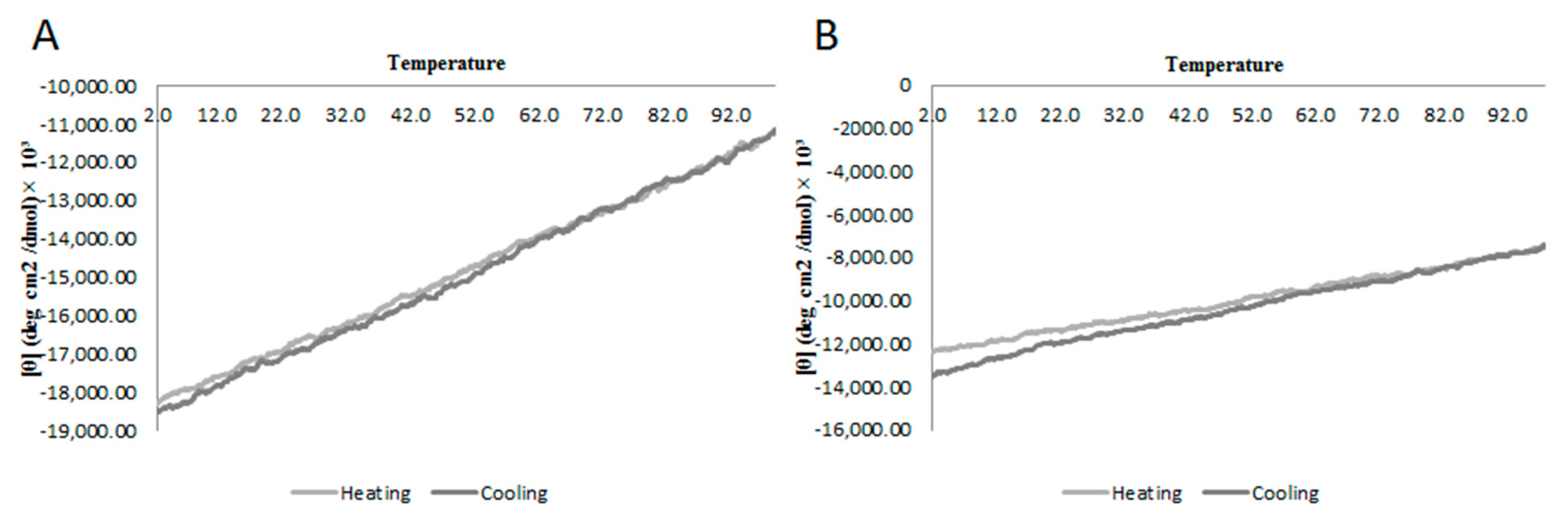
2.2. Circular Dichroism
2.3. Antimicrobial Activity
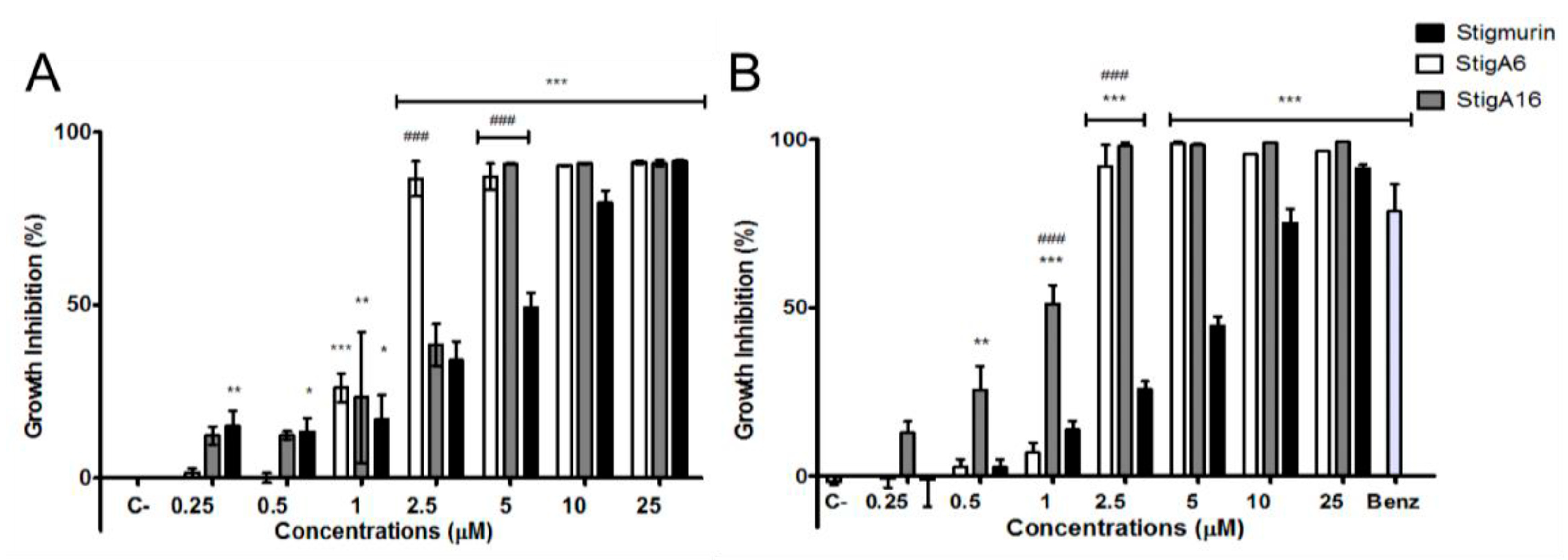
2.4. Antiparasitic Activity
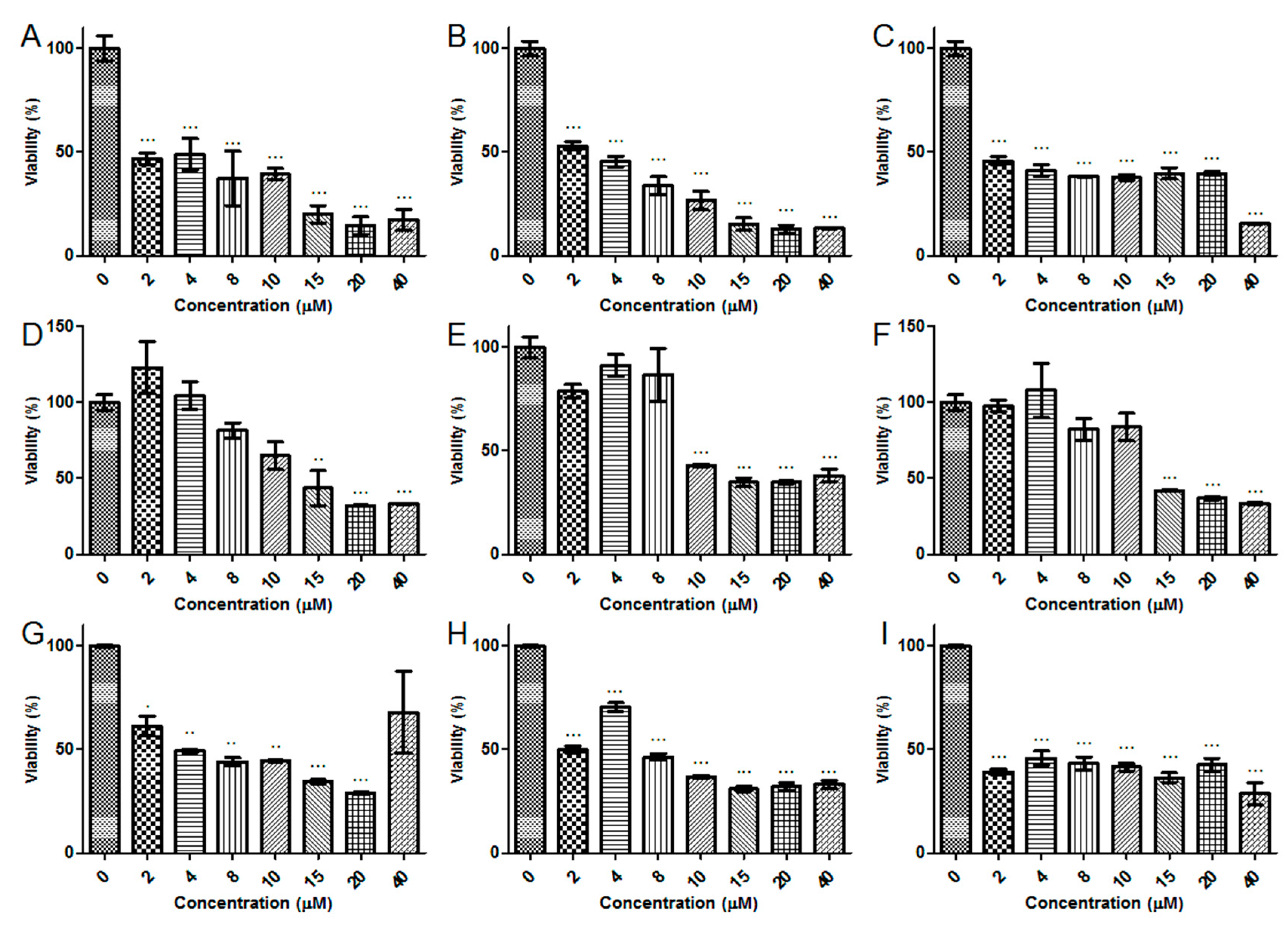
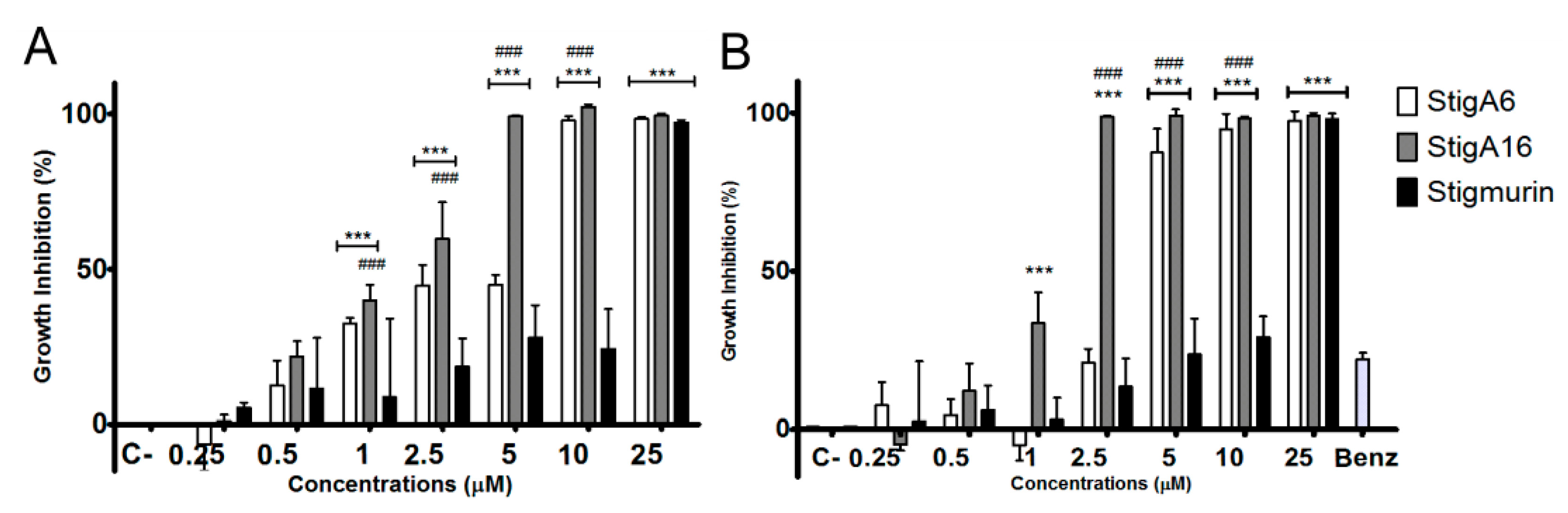
2.5. Antiproliferative Activity
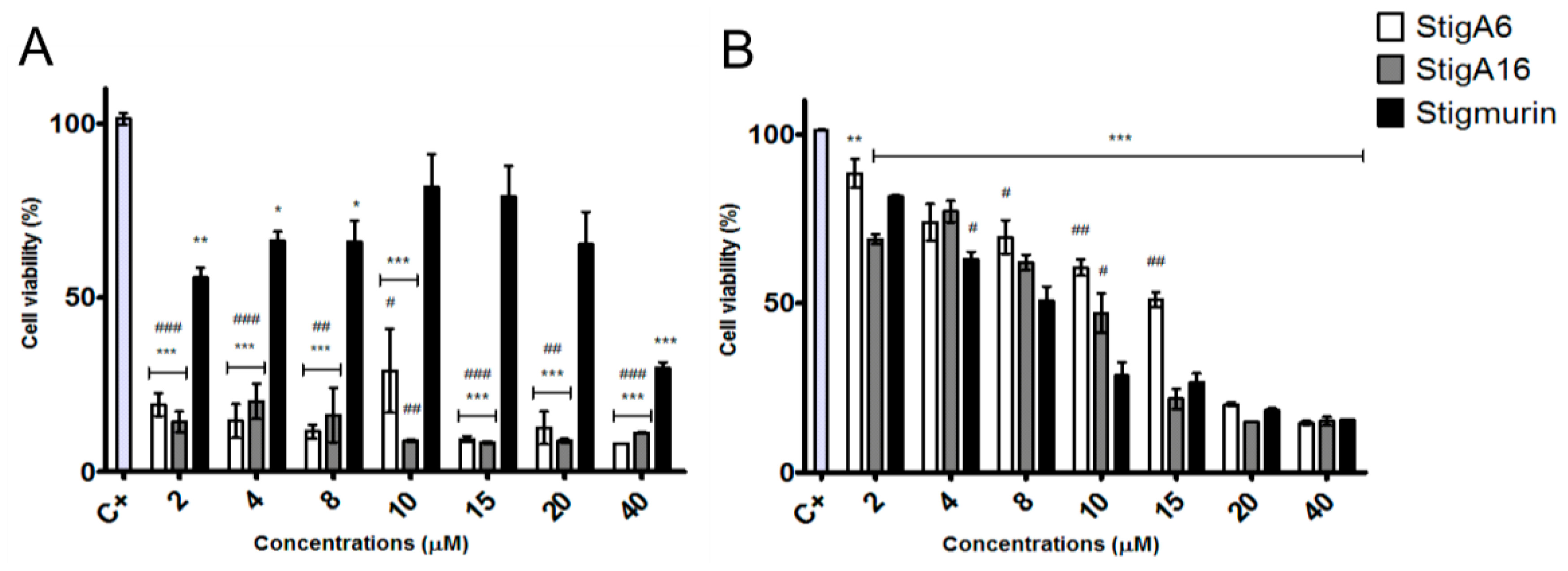
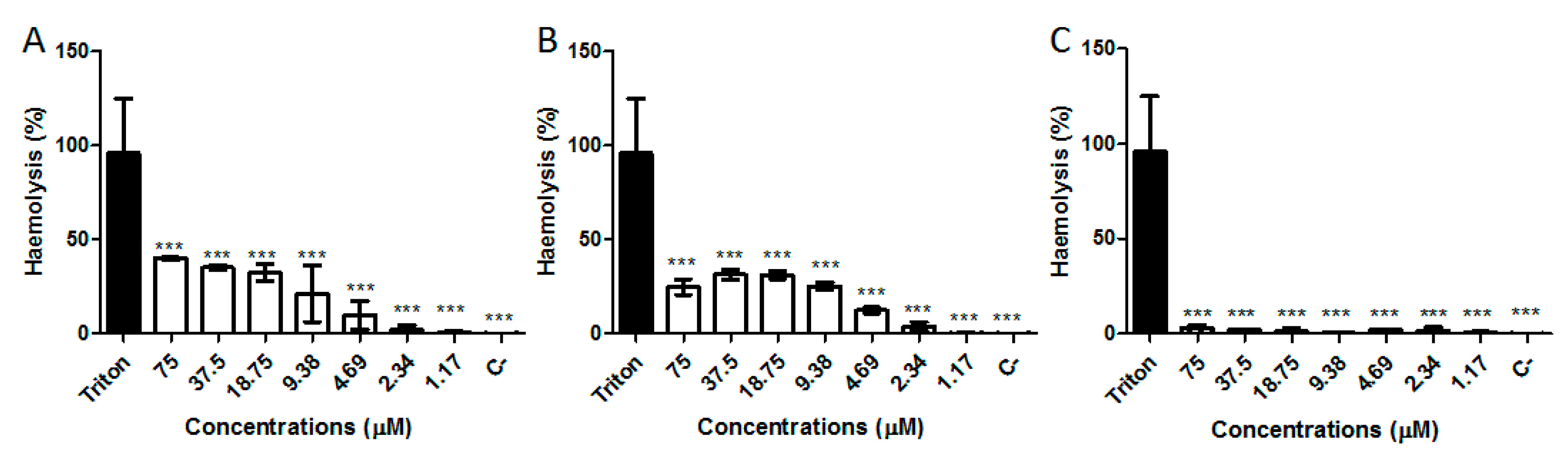
2.6. Hemolytic Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Peptide Synthesis
5.2. In Silico Structural Analysis
5.3. Analysis of Secondary Structure and Stability by Circular Dichroism
5.4. Antimicrobial Activity
5.5. Antiparasitic Activity
5.6. Antiproliferative Activity
5.7. Hemolytic Activity
5.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
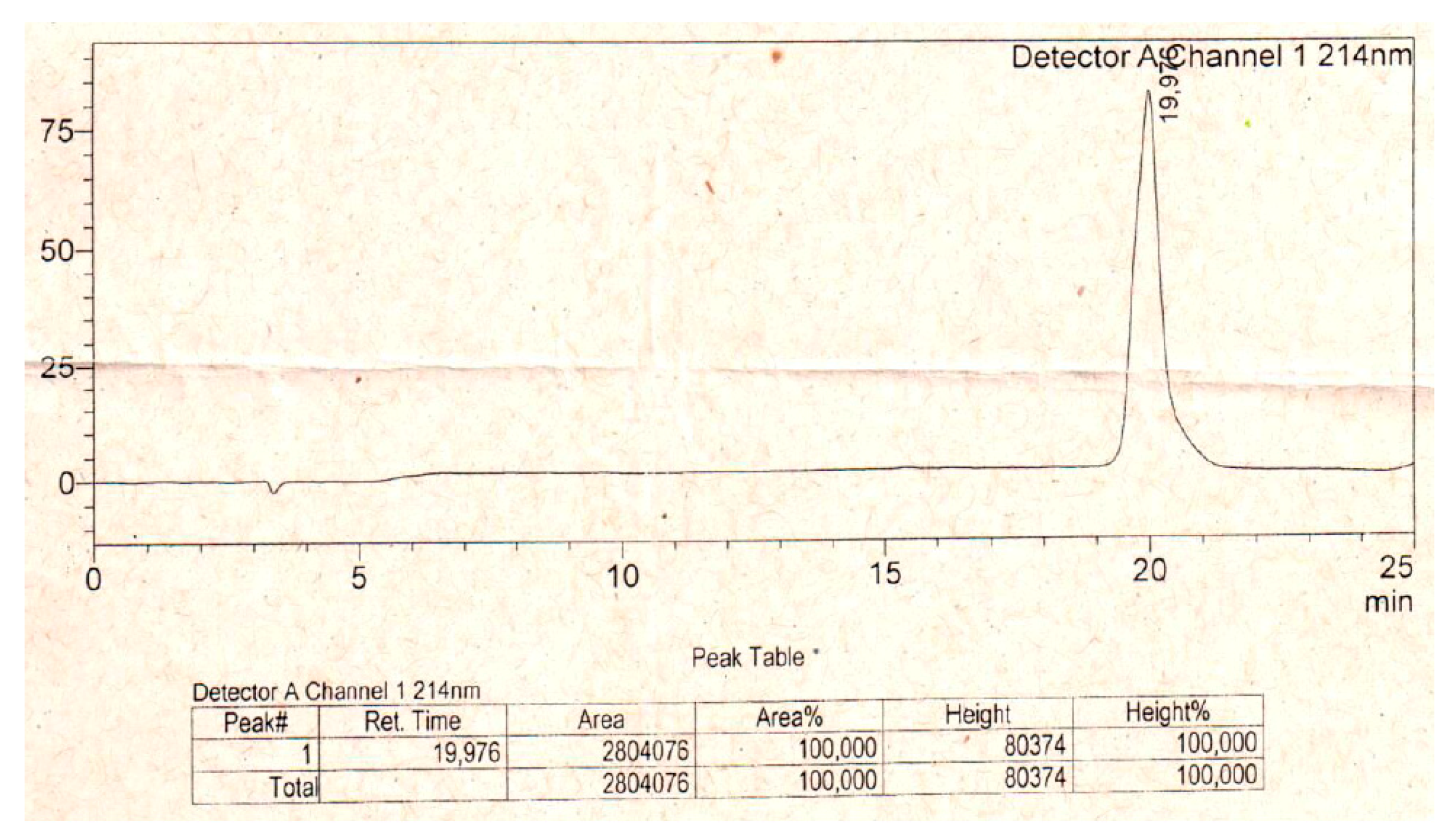
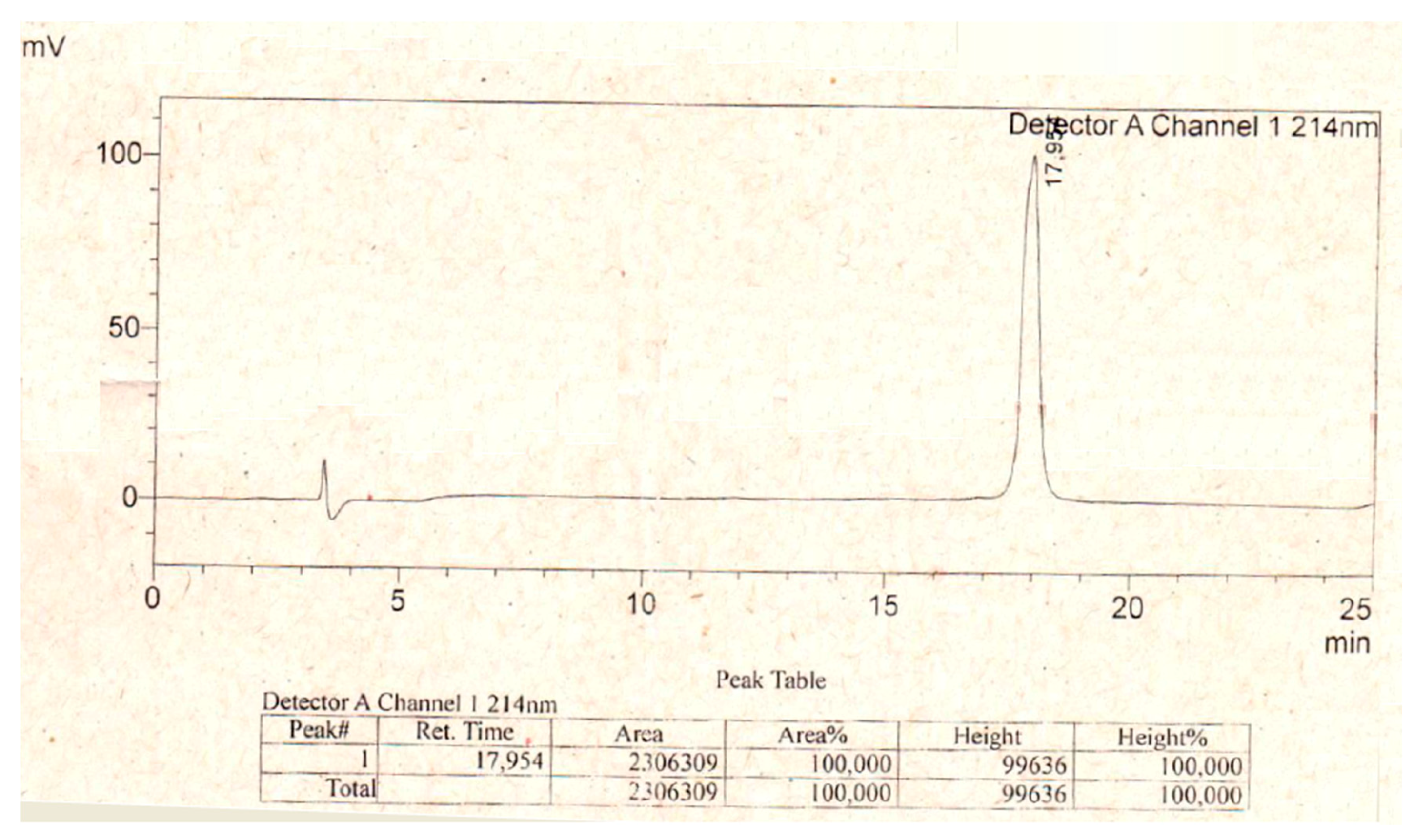

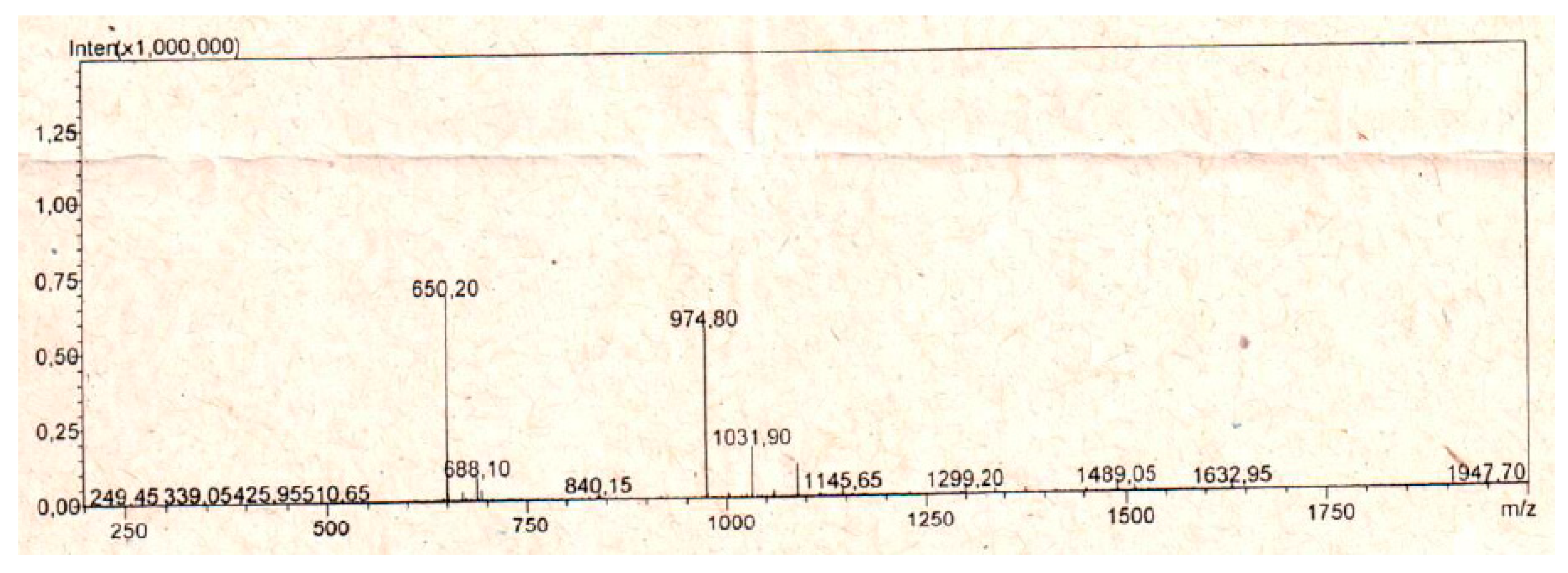
References
- Jeram, A.J. Phylogeny, classification and evolution of Silurian and Devonian scorpions. In Proceedings of the 17th European Colloquium of Arachnology, Edinburgh, UK, 14–18 July 1997; Selden, P.A., Ed.; The British Arachnological Society: Burnham Beeches, UK, 1997; pp. 17–31. [Google Scholar]
- Waddington, J.; Rudkin, D.M.; Dunlop, J.A. A new mid-Silurian aquatic scorpion—One step closer to land? Biol. Lett. 2015, 11, 20140815. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Albalas, Q. Scorpion venom peptides with no disulfide bridges: A review. Peptides 2014, 51, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.D.; Scortecci, K.C.; Kobashi, L.S.; Agnez-Lima, L.F.; Medeiros, S.R.B.; Silva-Junior, A.A.; Junqueira-de-Azevedo Ide, L.; Fernandes-Pedrosa Mde, F. Profiling the resting venom gland of the scorpion Tityus stigmurus through a transcriptomic survey. BMC Genom. 2012, 13, 362. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Hou, X.; Wang, L.; Zhang, Y.; Xi, X.; Wang, H.; Zhou, M.; Duan, J.; Wei, M.; Chen, T.; et al. AaeAP1 and AaeAP2: Novel antimicrobial peptides from the venom of the scorpion, Androctonus aeneas: Structural characterisation, molecular cloning of biosynthetic precursor-encoding cDNAs and engineering of analogues with enhanced antimicrobial and anticancer activities. Toxins 2015, 7, 219–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmaiah Narayana, J.; Chen, J.-Y. Antimicrobial peptides: Possible anti-infective agents. Peptides 2015, 72, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.M.; Fusté, E.; Rabanal, F.; Vinuesa, T.; Viñas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Toke, O. Antimicrobial peptides: New candidates in the fight against bacterial infections. Biopolymers 2005, 80, 717–735. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, N.; Matsuzaki, K. Polar angle as a determinant of amphipathic alpha-helix-lipid interactions: A model peptide study. Biophys. J. 2000, 79, 2075–2083. [Google Scholar] [CrossRef]
- Velasco-Bolom, J.-L.; Corzo, G.; Garduño-Juárez, R. Molecular dynamics simulation of the membrane binding and disruption mechanisms by antimicrobial scorpion venom-derived peptides. J. Biomol. Struct. Dyn. 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.; Snesrud, E.; Maybank, R.; Corey, B.; Ong, A.C.; Clifford, R.; Hinkle, M.; Whitman, T.; Lesho, E.; Schaecher, K.E. Escherichia coli Harboring mcr-1 and blaCTX-M on a Novel IncF Plasmid: First Report of mcr-1 in the United States. Antimicrob. Agents Chemother. 2016, 60, 4420–4421. [Google Scholar] [CrossRef] [PubMed]
- Melo, E.T.; Estrela, A.B.; Santos, E.C.G.; Machado, P.R.L.; Farias, K.J.S.; Torres, T.M.; Carvalho, E.; Lima, J.P.M.S.; Silva-Júnior, A.A.; Barbosa, E.G.; et al. Structural characterization of a novel peptide with antimicrobial activity from the venom gland of the scorpion Tityus stigmurus: Stigmurin. Peptides 2015, 68, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Daniele-Silva, A.; Machado, R.J.A.; Monteiro, N.K.V.; Estrela, A.B.; Santos, E.C.G.; Carvalho, E.; Araújo Júnior, R.F.; Melo-Silveira, R.F.; Rocha, H.A.O.; Silva-Júnior, A.A.; et al. Stigmurin and TsAP-2 from Tityus stigmurus scorpion venom: Assessment of structure and therapeutic potential in experimental sepsis. Toxicon 2016, 121, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Tarazi, S.; Abu-Alhaijaa, A.; Altall, Y.; Alshar’i, N.; Bodoor, K.; Al-Balas, Q. Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1. Pharmaceuticals 2014, 7, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Salud Bea, R.; Ascuitto, M.R.; de Johnson, L.E.L. Synthesis of analogs of peptides from Buthus martensii scorpion venom with potential antibiotic activity. Peptides 2015, 68, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Salud Bea, R.; Petraglia, A.F.; Ascuitto, M.R.; Buck, Q.M. Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides. Antibiotics 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Zhou, M.; Wang, L.; Chen, T.; Walker, B.; Shaw, C. Antimicrobial/cytolytic peptides from the venom of the North African scorpion, Androctonus amoreuxi: Biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides 2012, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, C.; Du, Q.; Wei, R.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: Evaluation of their antimicrobial and anticancer activities. Biochimie 2013, 95, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Mandard, N.; Sy, D.; Maufrais, C.; Bonmatin, J.M.; Bulet, P.; Hetru, C.; Vovelle, F. Androctonin, a novel antimicrobial peptide from scorpion Androctonus australis: Solution structure and molecular dynamics simulations in the presence of a lipid monolayer. J. Biomol. Struct. Dyn. 1999, 17, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Torres-Larios, A.; Gurrola, G.B.; Zamudio, F.Z.; Possani, L.D. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur. J. Biochem. 2000, 267, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, Y.; Dai, C.; Zhao, R.; Li, S.; Wu, Y.; Cao, Z.; Li, W. Imcroporin, a new cationic antimicrobial peptide from the venom of the scorpion Isometrus maculates. Antimicrob. Agents Chemother. 2009, 53, 3472–3477. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.B.; Aufderhorst-Roberts, A.; Palma, M.S.; Connell, S.D.; Ruggiero Neto, J.; Beales, P.A. PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties. Biophys. J. 2015, 109, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Yasuda, A.; Naoki, H.; Corzo, G.; Andriantsiferana, M.; Nakajima, T. IsCT, a novel cytotoxic linear peptide from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2001, 286, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shin, S.Y.; Kim, K.; Lim, S.S.; Hahm, K.-S.; Kim, Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem. Biophys. Res. Commun. 2004, 323, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Meng, L.; Zhang, Q.; Cao, L.; Li, W.; Wu, Y.; Cao, Z. Hp1404, a new antimicrobial peptide from the scorpion Heterometrus petersii. PLoS ONE 2014, 9, e97539. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Dai, C.; Li, Z.; Fan, Z.; Song, Y.; Wu, Y.; Cao, Z.; Li, W. Antibacterial activity and mechanism of a scorpion venom peptide derivative in vitro and in vivo. PLoS ONE 2012, 7, e40135. [Google Scholar] [CrossRef] [PubMed]
- Ehret-Sabatier, L.; Loew, D.; Goyffon, M.; Fehlbaum, P.; Hoffmann, J.A.; van Dorsselaer, A.; Bulet, P. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J. Biol. Chem. 1996, 271, 29537–29544. [Google Scholar] [CrossRef] [PubMed]
- Moerman, L.; Bosteels, S.; Noppe, W.; Willems, J.; Clynen, E.; Schoofs, L.; Thevissen, K.; Tytgat, J.; Van Eldere, J.; Van Der Walt, J.; et al. Antibacterial and antifungal properties of alpha-helical, cationic peptides in the venom of scorpions from southern Africa. Eur. J. Biochem. 2002, 269, 4799–4810. [Google Scholar] [CrossRef] [PubMed]
- Guilhelmelli, F.; Vilela, N.; Smidt, K.S.; de Oliveira, M.A.; da Cunha Morales Álvares, A.; Rigonatto, M.C.L.; da Silva Costa, P.H.; Tavares, A.H.; de Freitas, S.M.; Nicola, A.M.; et al. Activity of Scorpion Venom-Derived Antifungal Peptides against Planktonic Cells of Candida spp. and Cryptococcus neoformans and Candida albicans Biofilms. Front. Microbiol. 2016, 7, 1844. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Silva, S.; Op den Camp, H.J.M.; Velasco, E.; Alvarez, M.; Alfonzo, M.J.M.; Jorquera, A.; De Sousa, L.; Delgado, O. In vitro leishmanicidal activity of Tityus discrepans scorpion venom. Parasitol. Res. 2006, 99, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Flores-Solis, D.; Toledano, Y.; Rodríguez-Lima, O.; Cano-Sánchez, P.; Ramírez-Cordero, B.E.; Landa, A.; Rodríguez de la Vega, R.C.; Del Rio-Portilla, F. Solution structure and antiparasitic activity of scorpine-like peptides from Hoffmannihadrurus gertschi. FEBS Lett. 2016, 590, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Conde, R.; Zamudio, F.Z.; Rodríguez, M.H.; Possani, L.D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–168. [Google Scholar] [CrossRef]
- Bandeira, L.D.; Perdigão, M.C.; Justino, B.I.C.; Pessoa Bezerra de Menezes, R.R.P.; Lima, S.T.; Borges, F.C.; Morlighem, J.-É.R.L.; Rádis-Baptista, G.; Costa, M.A.M. The dinoponeratoxin peptides from the giant ant Dinoponera quadriceps display in vitro antitrypanosomal activity. Biol. Chem. 2017, 2, 187–196. [Google Scholar] [CrossRef]
- Muelas-Serrano, S.; Nogal-Ruiz, J.J.; Gómez-Barrio, A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2000, 86, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2017. [Google Scholar] [CrossRef] [PubMed]
- Luna-Ramirez, K.; Tonk, M.; Rahnamaeian, M.; Vilcinskas, A. Bioactivity of Natural and Engineered Antimicrobial Peptides from Venom of the Scorpions Urodacus yaschenkoi and U. manicatus. Toxins 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, N.; Venyaminov, S.Y.; Woody, R.W. Estimation of the number of alpha-helical and beta-strand segments in proteins using circular dichroism spectroscopy. Protein Sci. Publ. Protein Soc. 1999, 8, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Van Stokkum, I.H.; Spoelder, H.J.; Bloemendal, M.; van Grondelle, R.; Groen, F.C. Estimation of protein secondary structure and error analysis from circular dichroism spectra. Anal. Biochem. 1990, 191, 110–118. [Google Scholar] [CrossRef]
- Menezes, Y.A.S.; Félix-Silva, J.; da Silva-Júnior, A.A.; Rebecchi, I.M.M.; de Oliveira, A.S.; Uchoa, A.F.; Fernandes-Pedrosa Mde, F. Protein-rich fraction of Cnidoscolus urens (L.) Arthur leaves: Enzymatic characterization and procoagulant and fibrinogenolytic activities. Molecules 2014, 19, 3552–3569. [Google Scholar] [CrossRef] [PubMed]

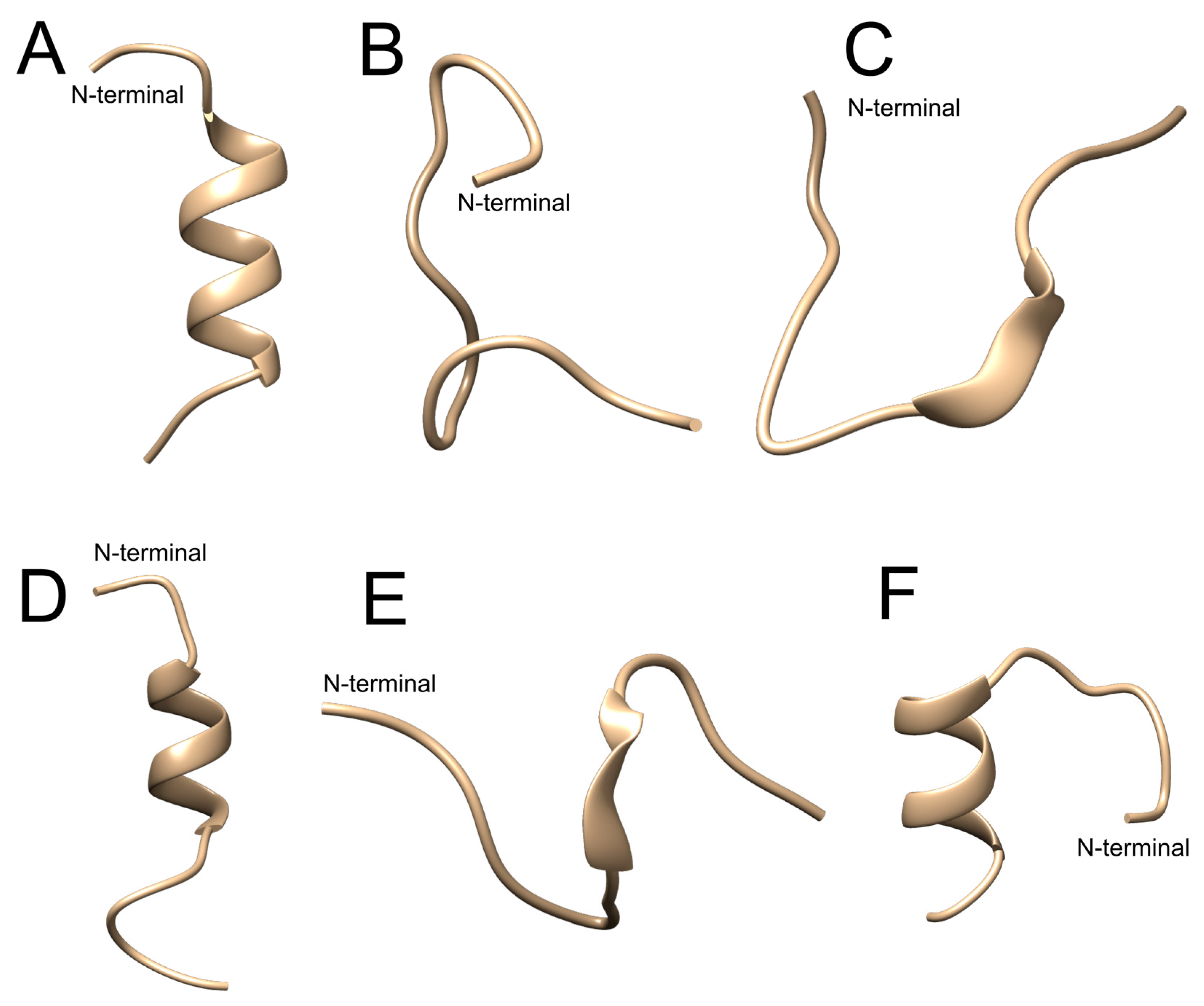
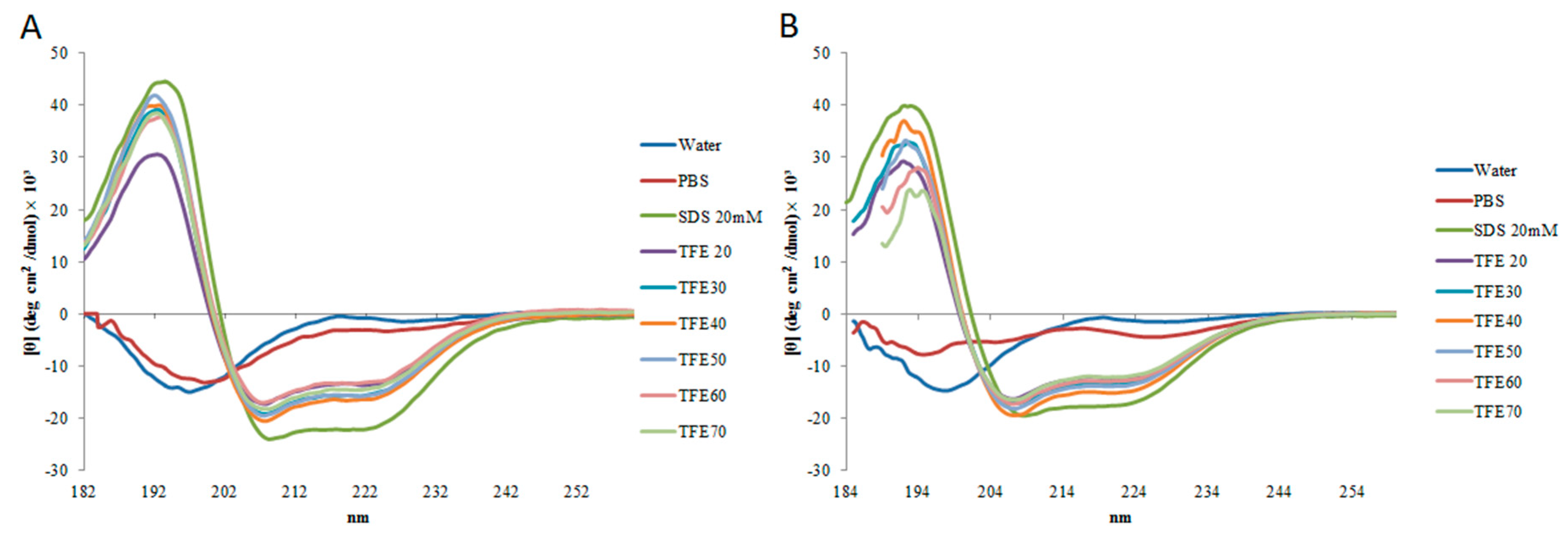
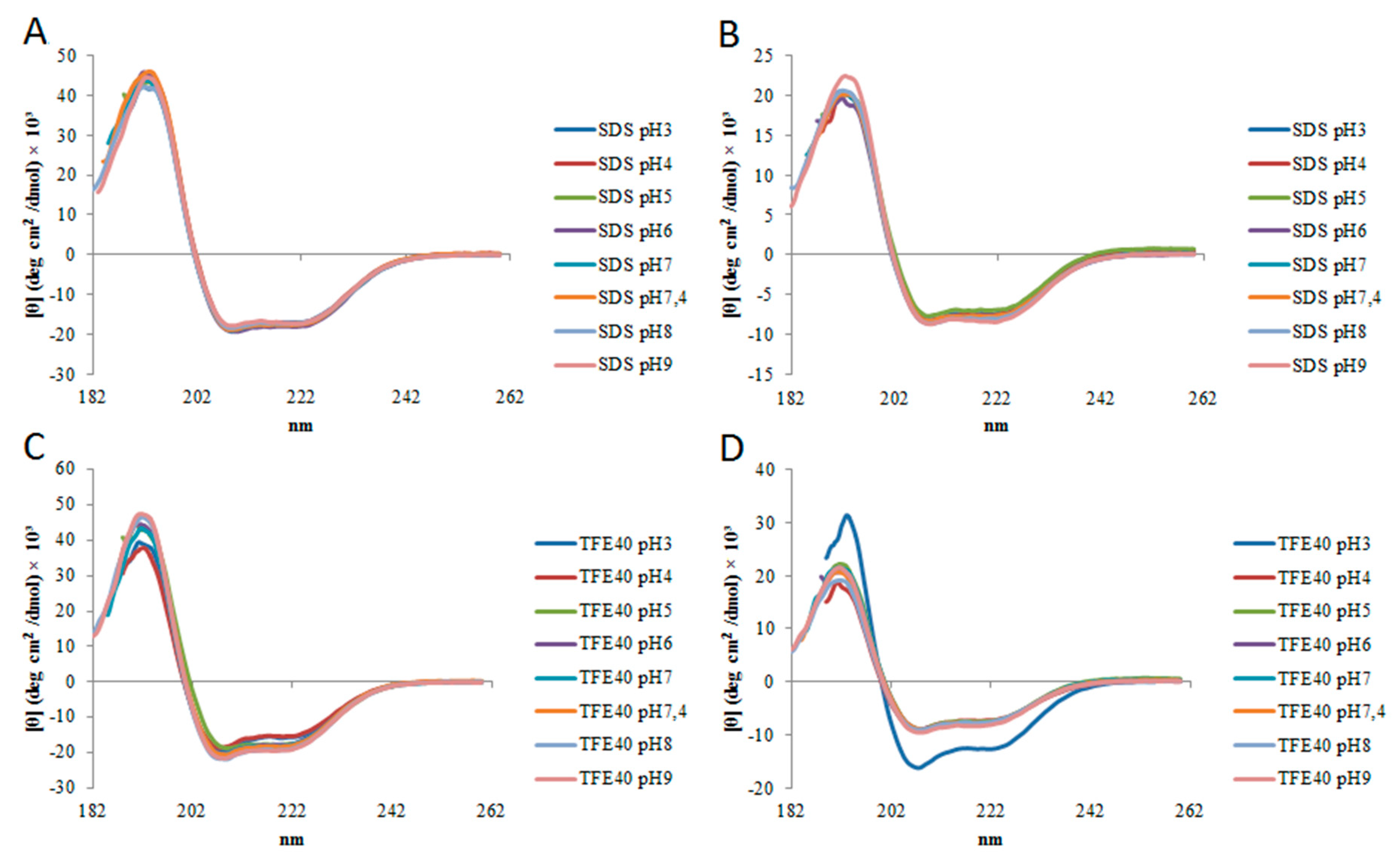
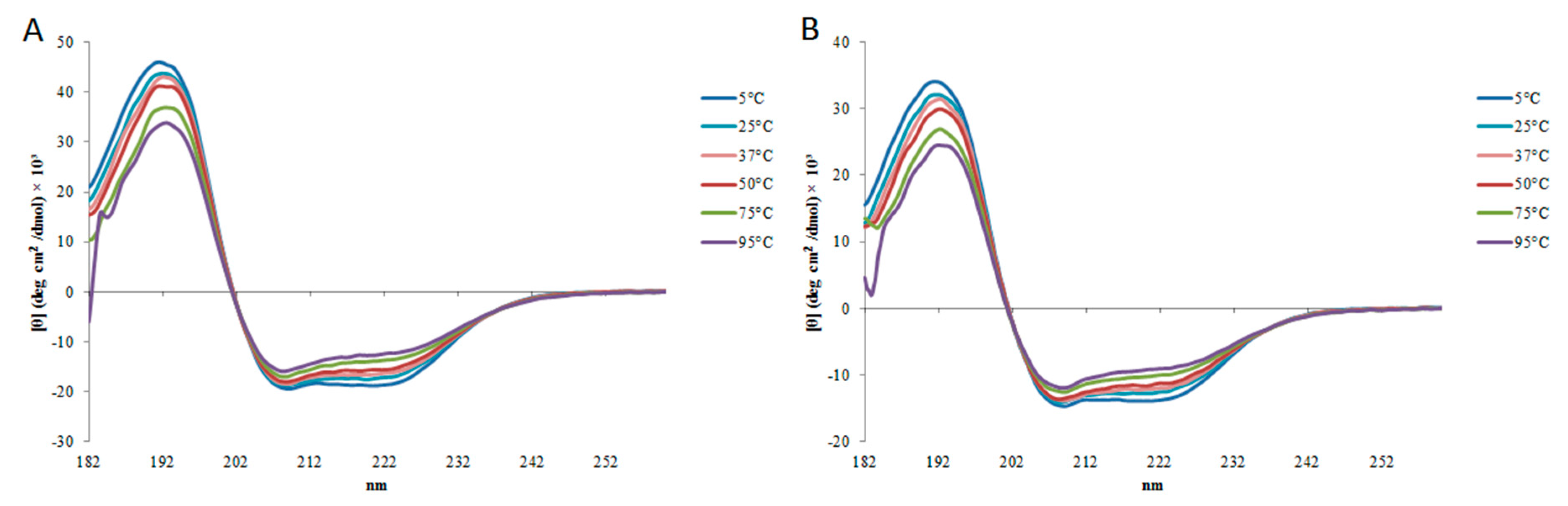
| StigA6 | StigA16 | |||||
|---|---|---|---|---|---|---|
| α-Helix (%) | β-Sheet (%) | Random (%) | α-Helix (%) | β-Sheet (%) | Random (%) | |
| Water | 5.65 ± 4.8 | 14.1 ± 4.5 | 78 ± 12.5 | 1.45 ± 0.7 | 13.75 ± 1.06 | 82.45 ± 1.4 |
| PBS | 4.55 ± 0.7 | 9.6 ± 0.5 | 86.05 ± 0.4 | 8.6 ± 8 | 15.2 ± 0.9 | 76.9 ± 10 |
| SDS 20 mM | 66.6 ± 2.1 | 2.15 ± 0.2 | 32.05 ± 2.8 | 58.8 ± 0.8 | 5.25 ± 0.2 | 35.55 ± 1.7 |
| TFE 20% | 47.7 ± 1.5 | 9.4 ± 3.1 | 42.6 ± 2.1 | 49.9 ± 2.5 | 10.45 ± 4.5 | 45.1 ± 1.2 |
| TFE 30% | 56.7 ± 3.3 | 7.15 ± 0.3 | 36.1 ± 3.1 | 50.75 ± 1.2 | 9.6 ± 3.2 | 39.85 ± 1.6 |
| TFE 40% | 58.4 ± 2.8 | 5.55 ± 0.6 | 35.5 ± 2.9 | 55.85 ± 0.4 | 5.55 ± 0.9 | 39.05 ± 0.2 |
| TFE 50% | 65.8 ± 13.4 | 4.2 ± 4.9 | 30.3 ± 8.9 | 52.5 ± 1.4 | 7.55 ± 1.3 | 40.1 ± 0.1 |
| TFE 60% | 52.95 ± 4.3 | 7.55 ± 2.4 | 39.8 ± 2.1 | 49.6 ± 0.6 | 5.55 0.2 | 46 ± 2.2 |
| TFE 70% | 54.3 ± 3.1 | 7.85 ± 2 | 38.15 ± 1.6 | 49.75 | 4.8 ± 0.7 | 45.7 ± 0.8 |
| Strains | StigA6 (µM) | StigA16 (µM) | Stigmurin (µM) |
|---|---|---|---|
| Gram-negative bacteria | |||
| Escherichia coli (ATCC 25922) | 4.69 | 2.34 | >150 |
| Enterobacter cloacae (ATCC 13047) | 18.75 | 9.38 | >150 |
| Pseudomonas aeruginosa (ATCC 27853) | 9.38 | 1.17 | >150 |
| Gram-positive bacteria | |||
| Staphylococcus aureus (ATCC 29213) | 2.34 | 2.34 | 9.38 |
| Staphylococcus epidermidis (ATCC 122225) | 1.17 | 9.38 | 9.38 |
| Enterococcus faecalis (ATCC 4028) | 1.17 | 1.17 | >150 |
| Yeasts | |||
| Candida albicans (ATCC 90028) | 9.38 | 4.69 | 37.5 |
| Candida krusei (ATCC 6258) | 37.5 | 9.38 | >150 |
| Candida glabrata (ATCC 90030) | 18.75 | 9.38 | >150 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parente, A.M.S.; Daniele-Silva, A.; Furtado, A.A.; Melo, M.A.; Lacerda, A.F.; Queiroz, M.; Moreno, C.; Santos, E.; Rocha, H.A.O.; Barbosa, E.G.; et al. Analogs of the Scorpion Venom Peptide Stigmurin: Structural Assessment, Toxicity, and Increased Antimicrobial Activity. Toxins 2018, 10, 161. https://doi.org/10.3390/toxins10040161
Parente AMS, Daniele-Silva A, Furtado AA, Melo MA, Lacerda AF, Queiroz M, Moreno C, Santos E, Rocha HAO, Barbosa EG, et al. Analogs of the Scorpion Venom Peptide Stigmurin: Structural Assessment, Toxicity, and Increased Antimicrobial Activity. Toxins. 2018; 10(4):161. https://doi.org/10.3390/toxins10040161
Chicago/Turabian StyleParente, Adriana M. S., Alessandra Daniele-Silva, Allanny A. Furtado, Menilla A. Melo, Ariane F. Lacerda, Moacir Queiroz, Cláudia Moreno, Elizabeth Santos, Hugo A. O. Rocha, Euzébio G. Barbosa, and et al. 2018. "Analogs of the Scorpion Venom Peptide Stigmurin: Structural Assessment, Toxicity, and Increased Antimicrobial Activity" Toxins 10, no. 4: 161. https://doi.org/10.3390/toxins10040161







