Oral and Intravenous Fumonisin Exposure in Pigs—A Single-Dose Treatment Experiment Evaluating Toxicokinetics and Detoxification
Abstract
:1. Introduction
2. Results
2.1. Toxicokinetic Study
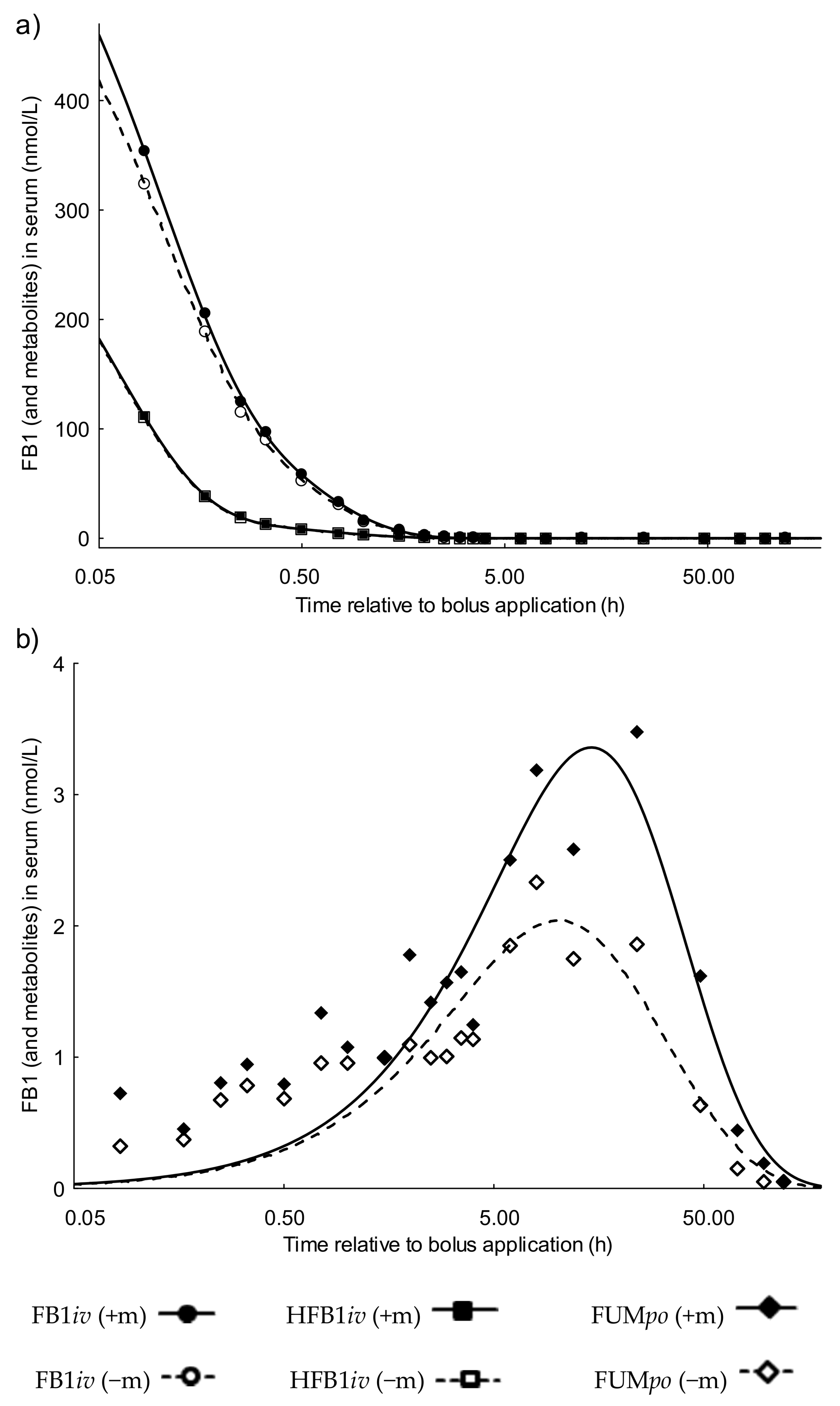
2.1.1. Serum Toxicokinetics of the FB1 Group Dosed Intravenously (FB1iv)
2.1.2. Serum Toxicokinetics of the Hydrolyzed FB1 Group Dosed Intravenously (HFB1iv)
2.1.3. Serum Toxicokinetics of the Fumonisin Group Dosed Orally (FUMpo)
2.1.4. Bioavailability
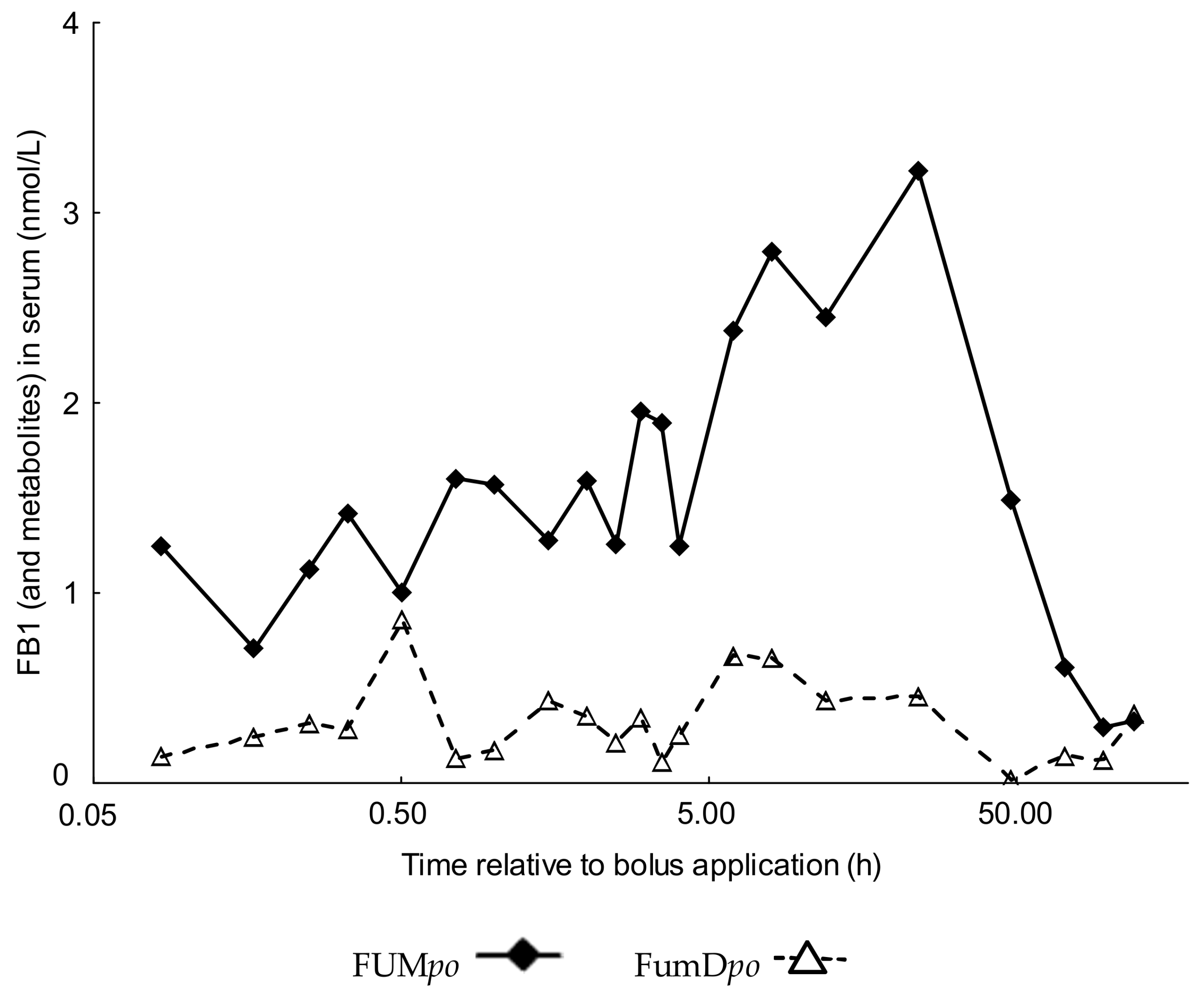
2.2. Comparative Fumonisin Kinetics after Oral Administration in the Absence (FUMpo) and Presence of Dietary Fumonisin Esterase (FumDpo)
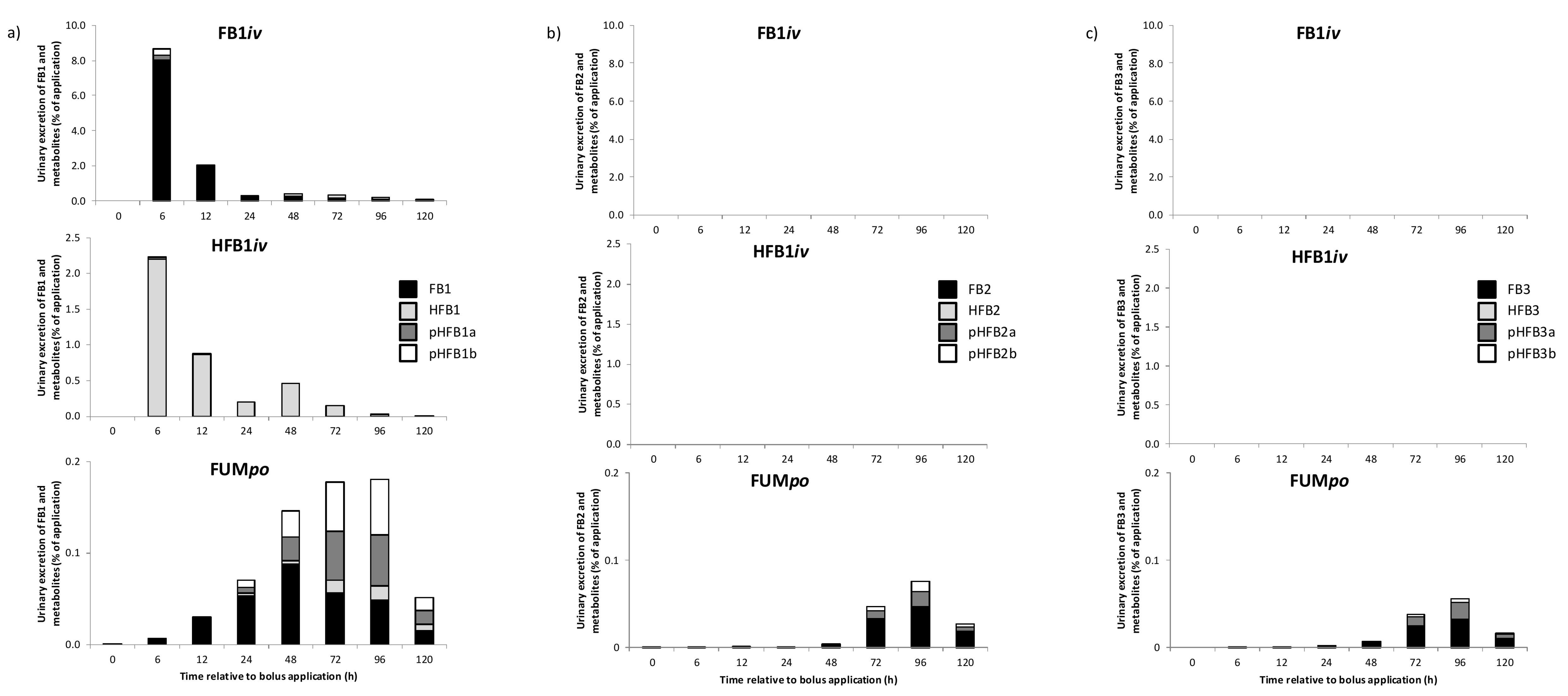
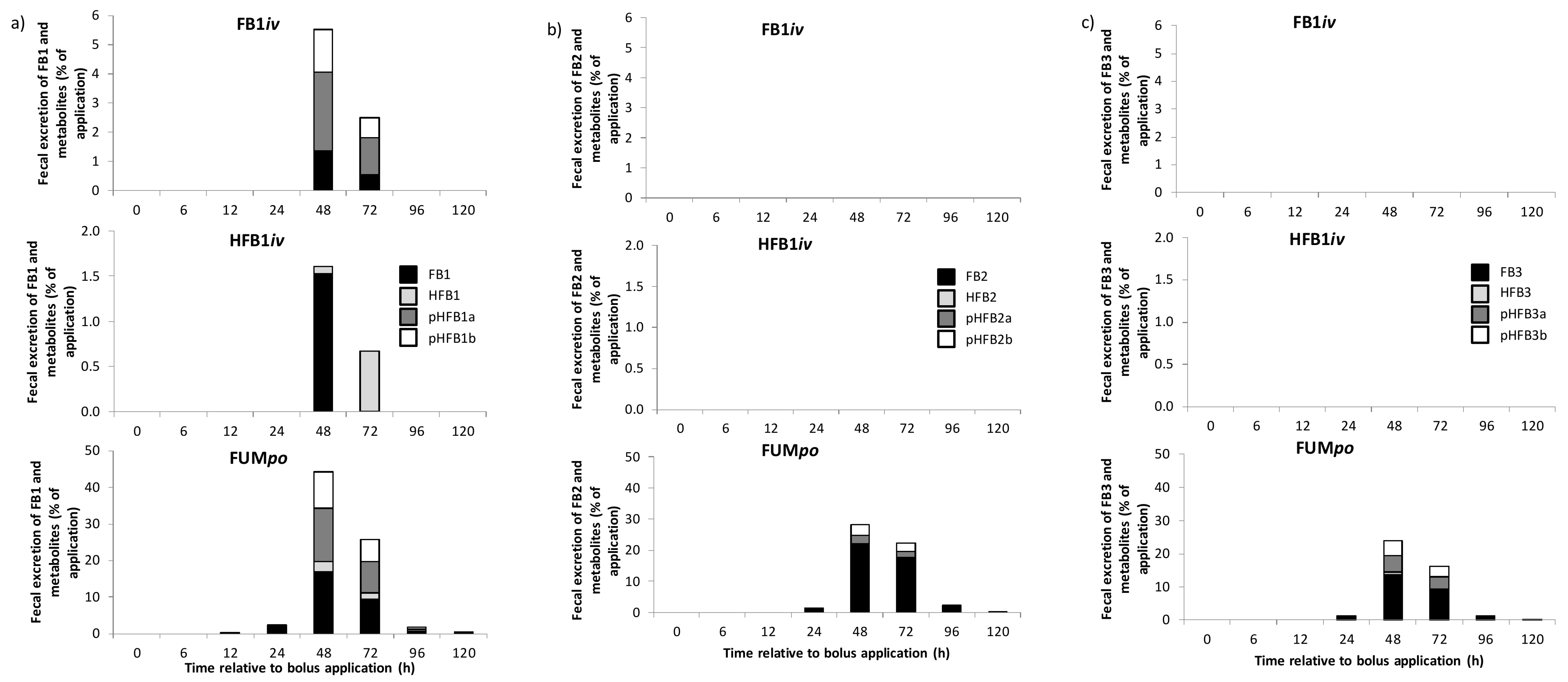
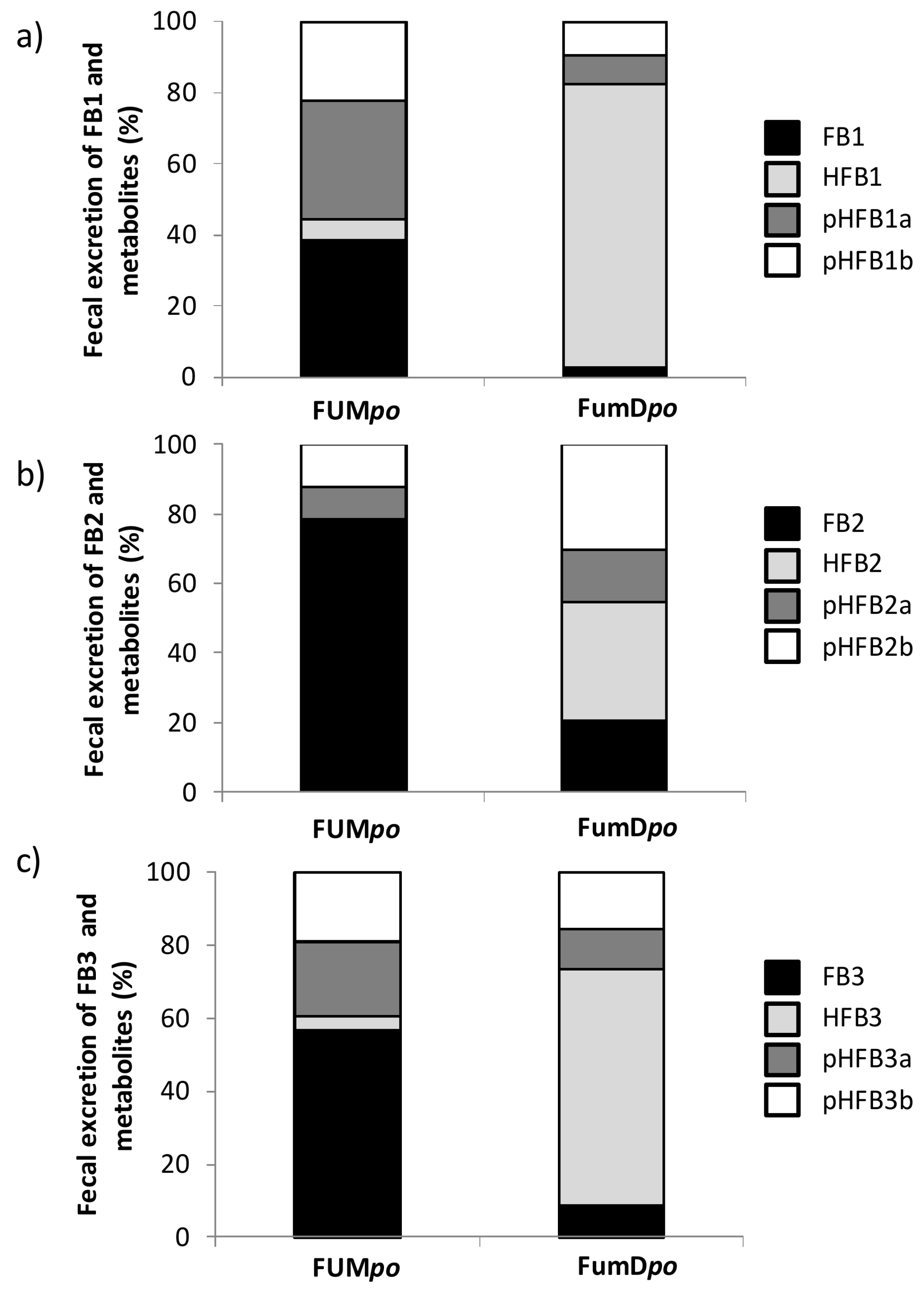
2.3. Urinary and Fecal Excretion and Metabolite Profiles
3. Discussion
4. Materials and Methods
4.1. Animals, Housing and Diet
4.2. Toxicokinetic Study
4.3. Mycotoxin Analysis
4.4. Calculations and Statistics
4.4.1. Serum Kinetics of the iv Groups
4.4.2. Serum Kinetics of the Oral Groups
4.4.3. Bioavailability
4.4.4. Urine and Feces Balance Calculations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kabak, B.; Dobson, A.D.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, W.C.; Jaskiewicz, K.; Marasas, W.F.; Thiel, P.G.; Horak, R.M.; Vleggaar, R.; Kriek, N.P. Fumonisins—Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar] [PubMed]
- Marasas, W.F. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109 (Suppl. 2), 239–243. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A.; Smith, G.W.; Haschek, W.M. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007, 137, 299–325. [Google Scholar] [CrossRef]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Marasas, W.F.; Kellerman, T.S.; Gelderblom, W.C.; Coetzer, J.A.; Thiel, P.G.; van der Lugt, J.J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 1988, 55, 197–203. [Google Scholar] [PubMed]
- Gelderblom, W.C.A.; Kriek, N.P.J.; Marasas, W.F.O.; Thiel, P.G. Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis 1991, 12, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.R.; Colvin, B.M.; Greene, J.T.; Newman, L.E.; Cole, J.R., Jr. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J. Vet. Diagn. Investig. 1990, 2, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.E.; Bouhet, S.; Pascale, F.; Bailly, J.D.; Miller, J.D.; Pinton, P.; Oswald, I.P. Mycotoxin fumonisin B1 alters the cytokine profile and decreases the vaccinal antibody titer in pigs. Toxicol. Sci. 2005, 84, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Marasas, W.F. Fumonisins: History, world-wide occurrence and impact. Adv. Exp. Med. Biol. 1996, 392, 1–17. [Google Scholar] [PubMed]
- EFSA. 2006/576/EG Empfehlung der Kommission vom 17. August 2006 Betreffend das Vorhandensein von Deoxynivalenol, Zearalenon, Ochratroxin A, T-2- und HT-2-Toxin Sowie von Fumonisinen in zur Verfütterung an Tiere Bestimmten Erzeugnissen; EFSA: Parma, Italy, 2006. [Google Scholar]
- EFSA. Opinion of the Scientific Panel on Contaminants in Food Chain on a Request from the Commission Related to Fumonisins as Undesirable Substances in Animal Feed; EFSA: Parma, Italy, 2005; Volume 235, pp. 1–32. [Google Scholar]
- Dilkin, P.; Direito, G.; Simas, M.M.; Mallmann, C.A.; Correa, B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticillioides culture material in weaned piglets. Chem.-Biol. Interact. 2010, 185, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T. The fumonisin paradox: A review of research on oral bioavailability of fumonisin B1, a mycotoxin produced by Fusarium moniliforme. J. Toxicol. Toxin Rev. 2000, 19, 161–187. [Google Scholar] [CrossRef]
- Haschek, W.M.; Motelin, G.; Ness, D.K.; Harlin, K.S.; Hall, W.F.; Vesonder, R.F.; Peterson, R.E.; Beasley, V.R. Characterization of fumonisin toxicity in orally and intravenously dosed swine. Mycopathologia 1992, 117, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H., Jr. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [PubMed]
- Merrill, A.H., Jr.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ. Health Perspect. 2001, 109 (Suppl. 2), 283–289. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Enongene, E.; Voss, K.A.; Norred, W.P.; Meredith, F.I.; Sharma, R.P.; Spitsbergen, J.; Williams, D.E.; Carlson, D.B.; Merrill, A.H., Jr. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 2001, 109 (Suppl. 2), 301–308. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Torres, O.; Matute, J.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Mitchell, T.; Voss, K.A.; Maddox, J.R.; Gelineau-van Waes, J.B. Evidence for fumonisin inhibition of ceramide synthase in humans consuming maize-based foods and living in high exposure communities in Guatemala. Mol. Nutr. Food Res. 2015, 59, 2209–2224. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, D.; Schwartz, H.; Hametner, C.; Schatzmayr, G.; Haltrich, D.; Moll, W.D. Enzyme characteristics of aminotransferase FumI of Sphingopyxis sp. MTA144 for deamination of hydrolyzed fumonisin B1. Appl. Microbiol. Biotechnol. 2011, 91, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Bracarense, A.P.; Schwartz, H.E.; Trumel, C.; Cossalter, A.M.; Schatzmayr, G.; Kolf-Clauw, M.; Moll, W.D.; Oswald, I.P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012, 83, 1465–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masching, S.; Naehrer, K.; Schwartz-Zimmermann, H.E.; Sarandan, M.; Schaumberger, S.; Dohnal, I.; Nagl, V.; Schatzmayr, D. Gastrointestinal degradation of fumonisin B1 by carboxylesterase fumd prevents fumonisin induced alteration of sphingolipid metabolism in turkey and swine. Toxins 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Hahn, I.; Nagl, V.; Schwartz-Zimmermann, H.E.; Varga, E.; Schwarz, C.; Slavik, V.; Reisinger, N.; Malachova, A.; Cirlini, M.; Generotti, S.; et al. Effects of orally administered fumonisin B1, partially hydrolysed FB1, hydrolysed FB1 and N-(1-deoxy-d-fructos-1-yl)FB1 on the sphingolipid metabolism in rats. Food Chem. Toxicol. 2015, 76, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Fodor, J.; Balogh, K.; Weber, M.; Miklos, M.; Kametler, L.; Posa, R.; Mamet, R.; Bauer, J.; Horn, P.; Kovacs, F.; et al. Absorption, distribution and elimination of fumonisin B1 metabolites in weaned piglets. Food Add. Contam. Part A 2008, 25, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Trenholm, H.L.; Rotter, B.A.; Miller, J.D.; Savard, M.E.; Yeung, J.M.; Scott, P.M. Biological fate of fumonisin B1 in food-producing animals. Adv. Exp. Med. Biol. 1996, 392, 265–278. [Google Scholar] [PubMed]
- Prelusky, D.B.; Trenholm, H.L.; Savard, M.E. Pharmacokinetic fate of 14C-labelled fumonisin B1 in swine. Nat. Toxins 1994, 2, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Larranaga, M.R.; Anadon, A.; Diaz, M.J.; Fernandez-Cruz, M.L.; Martinez, M.A.; Frejo, M.T.; Martinez, M.; Fernandez, R.; Anton, R.M.; Morales, M.E.; et al. Toxicokinetics and oral bioavailability of fumonisin B1. Vet. Hum. Toxicol. 1999, 41, 357–362. [Google Scholar] [PubMed]
- Fodor, J.; Meyer, K.; Riedlberger, M.; Bauer, J.; Horn, P.; Kovacs, F.; Kovacs, M. Distribution and elimination of fumonisin analogues in weaned piglets after oral administration of fusarium verticillioides fungal culture. Food Add. Contam. 2006, 23, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Bouhet, S.; Oswald, I.P. The intestine as a possible target for fumonisin toxicity. Mol. Nutr. Food Res. 2007, 51, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Szabo-Fodor, J.; Kametler, L.; Posa, R.; Mamet, R.; Rajli, V.; Bauer, J.; Horn, P.; Kovacs, F.; Kovacs, M. Kinetics of fumonisin B1 in pigs and persistence in tissues after ingestion of a diet containing a high fumonisin concentration. Cereal Res. Commun. 2008, 36, 331–336. [Google Scholar] [CrossRef]
- Shephard, G.S.; Thiel, P.G.; Sydenham, E.W.; Savard, M.E. Fate of a single dose of 14C-labelled fumonisin B1 in vervet monkeys. Nat. Toxins 1995, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Fodor, J.; Meyer, K.; Gottschalk, C.; Mamet, R.; Kametler, L.; Bauer, J.; Horn, P.; Kovacs, F.; Kovacs, M. In vitro microbial metabolism of fumonisin B1. Food Add. Contam. 2007, 24, 416–420. [Google Scholar] [CrossRef] [PubMed]
- GfE. Empfehlungen zur Energie- und Nährstoffversorgung von Schweinen; Ausschuss für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie; DLG-Verlag: Frankfurt, Germany, 2006. [Google Scholar]
- Tesch, T.; Bannert, E.; Kluess, J.; Frahm, J.; Kersten, S.; Breves, G.; Renner, L.; Kahlert, S.; Rothkötter, H.J.; Dänicke, S. Does dietary deoxynivalenol modulate the acute phase reaction in endotoxaemic pigs?—Lessons from clinical signs, white blood cell counts, and TNF-alpha. Toxins 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Schwartz-Zimmermann, H.E.; Gruber-Dorninger, C.; Dohnal, I.; Aleschko, M.; Schatzmayr, G.; Moll, W.D.; Applegate, T.J. Enzymatic hydrolysis of fumonisins in the gastrointestinal tract of broiler chickens. Poult. Sci. 2017, 96, 4342–4351. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Sulyok, M.; Fruhmann, P.; Berthiller, F.; Schuhmacher, R.; Hametner, C.; Adam, G.; Frohlich, J.; Krska, R. Assessment of human deoxynivalenol exposure using an LC-MS/MS based biomarker method. Toxicol. Lett. 2012, 211, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Riviere, J.E. Comparative Pharmacokinetics: Principles, Techniques, and Applications, 1st ed.; Iowa State University Press/AMES: Ames, IA, USA, 1999. [Google Scholar]
- Garrett, E.R. The bateman function revisited: A critical reevaluation of the quantitative expressions to characterize concentrations in the one compartment body model as a function of time with first-order invasion and first-order elimination. J. Pharmacokinet. Biopharm. 1994, 22, 103–128. [Google Scholar] [CrossRef] [PubMed]
| FB1−m | ||||||||
| Animal | 4 | 5 | 15 | 18 | 23 | 29 | Mean | SD |
| BW (kg) | 31.0 | 33.0 | 35.0 | 35.4 | 32.3 | 35.0 | 33.6 | 1.8 |
| FB1 (nmol·kg·BW−1) | 139 | 139 | 139 | 139 | 139 | 139 | 139 | 0 |
| A (nmol·L−1) | 410.0 | 200.3 | 238.7 | 766.9 | 1022.9 | 573.7 | 535.4 | 319.0 |
| α (h−1) | 14.9 | 5.6 | 7.4 | 16.4 | 12.2 | 8.1 | 10.8 | 4.4 |
| B (nmol·L−1) | 94.8 | 7.4 | 10.3 | 194.0 | 440.7 | 119.9 | 144.5 | 161.3 |
| β (h−1) | 2.8 | 0.6 | 0.8 | 2.2 | 2.4 | 1.5 | 1.7 | 0.9 |
| t1/2α (h) | 0.05 | 0.12 | 0.09 | 0.04 | 0.06 | 0.09 | 0.08 | 0.03 |
| t1/2β (h) | 0.25 | 1.26 | 0.83 | 0.32 | 0.29 | 0.47 | 0.57 | 0.40 |
| Vd (L·kg·BW−1) | 0.8 | 5.1 | 3.7 | 0.5 | 0.2 | 0.6 | 1.8 | 2.1 |
| Cl (L·kg·BW−1·h−1) | 2.3 | 2.8 | 3.1 | 1.0 | 0.5 | 0.9 | 1.8 | 1.1 |
| AUC (nmol·L−1·h) | 61 | 49 | 45 | 135 | 266 | 152 | 118 | 86 |
| RSD (nmol·L−1) | 1.9 | 1.4 | 1.7 | 3.1 | 3.6 | 5.8 | 2.9 | 1.7 |
| r2 | 0.998 | 0.999 | 0.997 | 0.998 | 0.999 | 0.998 | 0.998 | 0.001 |
| FB1+m | ||||||||
| A (nmol·L−1) | 483.1 | 214.9 | 260.4 | 843.1 | 1122.1 | 636.0 | 593 | 349 |
| α (h−1) | 16.0 | 6.0 | 7.8 | 15.9 | 12.1 | 8.1 | 11.0 | 4.3 |
| B (nmol·L−1) | 98.1 | 8.7 | 10.6 | 205.9 | 481.1 | 135.7 | 156.7 | 175.9 |
| β (h−1) | 2.9 | 0.6 | 0.7 | 2.1 | 2.4 | 1.5 | 1.7 | 0.9 |
| t1/2α (h) | 0.04 | 0.12 | 0.09 | 0.04 | 0.06 | 0.09 | 0.07 | 0.03 |
| t1/2β (h) | 0.24 | 1.22 | 0.94 | 0.33 | 0.29 | 0.46 | 0.58 | 0.40 |
| Vd (L·kg·BW−1) | 0.8 | 4.8 | 3.9 | 0.4 | 0.2 | 0.6 | 1.8 | 2.0 |
| Cl (L·kg·BW−1·h−1) | 2.2 | 2.7 | 2.9 | 0.9 | 0.5 | 0.8 | 1.7 | 1.1 |
| AUC (nmol·L−1·h) | 64 | 51 | 48 | 150 | 293 | 169 | 129 | 96 |
| AUCFB1−m% (%) | 95 | 96 | 93 | 90 | 91 | 90 | 93 | 3 |
| RSD (nMol·L−1) | 2.0 | 1.4 | 1.8 | 3.6 | 4.3 | 6.0 | 3.2 | 1.8 |
| r2 | 0.998 | 0.998 | 0.997 | 0.999 | 0.999 | 0.997 | 0.998 | 0.001 |
| HFB1−m | |||||||
| Animal | 1 | 8 | 13 | 21 | 28 | Mean | SD |
| BW (kg) | 34.9 | 35.6 | 33.5 | 34.0 | 32.0 | 34.0 | 1.4 |
| HFB1 (nmol·kg·BW−1) | 139 | 139 | 139 | 139 | 139 | 139 | 0 |
| A (nmol·L−1) | 69.5 | 297.3 | 205.2 | 122.8 | 724.1 | 283.8 | 260.8 |
| α (h−1) | 10.8 | 20.5 | 14.6 | 10.7 | 15.9 | 14.5 | 4.1 |
| B (nmol·L−1) | 8.8 | 1.0 | 1.2 | 42.2 | 48.6 | 20 | 23 |
| β (h−1) | 1.2 | 0.4 | 0.4 | 2.9 | 1.5 | 1.3 | 1.0 |
| t1/2α (h) | 0.06 | 0.03 | 0.05 | 0.07 | 0.04 | 0.05 | 0.02 |
| t1/2β (h) | 0.58 | 1.82 | 1.92 | 0.24 | 0.47 | 1.01 | 0.80 |
| Vd (L·kg·BW−1) | 8.5 | 21.3 | 22.4 | 1.8 | 1.2 | 11.0 | 10.3 |
| Cl (L·kg·BW−1·h−1) | 10.1 | 8.1 | 8.1 | 5.4 | 1.8 | 6.7 | 3.2 |
| AUC (nmol·L−1·h) | 14 | 17 | 17 | 26 | 78 | 30 | 27 |
| RSD (nmol·L−1) | 0.1 | 0.7 | 1.6 | 2.8 | 1.7 | 1.4 | 1.0 |
| r2 | 0.999 | 0.998 | 0.987 | 0.983 | 0.999 | 0.993 | 0.008 |
| HFB1+m | |||||||
| A (nmol·L−1) | 69.8 | 299.1 | 205.8 | 138.8 | 724.5 | 287.6 | 258.5 |
| α (h−1) | 11.2 | 20.6 | 14.7 | 7.4 | 15.6 | 13.9 | 4.9 |
| B (nmol·L−1) | 9.6 | 1.1 | 1.2 | 8.9 | 51.3 | 14.4 | 21.0 |
| β (h−1) | 1.2 | 0.4 | 0.3 | 0.9 | 1.5 | 0.9 | 0.5 |
| t1/2α (h) | 0.06 | 0.03 | 0.05 | 0.09 | 0.05 | 0.06 | 0.02 |
| t1/2β (h) | 0.56 | 1.81 | 2.37 | 0.80 | 0.45 | 1.20 | 0.85 |
| Vd (L·kg·BW−1) | 8.0 | 21.0 | 26.2 | 5.5 | 1.1 | 12.4 | 10.7 |
| Cl (L·kg·BW−1·h−1) | 9.9 | 8.0 | 7.7 | 4.8 | 1.7 | 6.4 | 3.2 |
| AUC (nmol·L−1·h) | 14 | 17 | 18 | 29 | 80 | 32 | 28 |
| AUCHFB1−m% (%) | 98.6 | 98.9 | 95.3 | 89.6 | 98.1 | 96.1 | 3.9 |
| RSD (nmol·L−1) | 0.1 | 0.8 | 1.6 | 3.0 | 2.0 | 1.5 | 1.1 |
| r2 | 0.999 | 0.995 | 0.987 | 0.981 | 0.998 | 0.992 | 0.008 |
| FB1−m | |||||||
| Animal | 2 | 14 | 17 | 25 | Mean | SD | |
| BW (kg) | 31.6 | 37.5 | 35.5 | 32.0 | 34.2 | 2.8 | |
| FB1 (nmol·kg·BW−1) | 3683 | 3103 | 3278 | 3637 | 3425 | 281 | |
| ka (h−1) | 0.15 | 2.22 | 0.25 | 0.08 | 0.68 | 1.03 | |
| ke (h−1) | 0.03 | 0.02 | 0.04 | 0.08 | 0.04 | 0.03 | |
| C0 (nmol·L−1) | 3.6 | 1.3 | 3.3 | 5.5 | 3.4 | 1.7 | |
| cmax (nmol·L−1) | 2.5 | 1.3 | 2.4 | 2.0 | 2.1 | 0.5 | |
| tmax (h) | 13.9 | 2.2 | 8.7 | 13.1 | 9.5 | 5.4 | |
| t1/2ka (h) | 4.6 | 0.3 | 2.7 | 9.1 | 4.2 | 3.7 | |
| t1/2ke (h) | 26.0 | 38.5 | 17.7 | 9.1 | 22.8 | 12.5 | |
| Vd (L·kg·BW−1) | 7.3 | 10.1 | 6.5 | 10.7 | 8.7 | 2.1 | |
| Cl (L·kg·BW−1·h−1) | 0.2 | 0.2 | 0.3 | 0.8 | 0.4 | 0.3 | |
| AUC (nmol·L−1·h) | 135 | 73 | 85 | 71 | 91 | 30 | |
| FAUC (%) | 4.3 | 2.8 | 3.1 | 2.3 | 3.1 | 0.8 | |
| F (%) | 0.007 | 0.004 | 0.007 | 0.016 | 0.009 | 0.005 | |
| MRT (h) | 33.6 | 28.4 | 21.2 | 18.5 | 25.4 | 6.9 | |
| RSD (nmol·L−1) | 0.5 | 0.3 | 0.4 | 0.4 | 0.4 | 0.1 | |
| r2 | 0.684 | 0.655 | 0.764 | 0.672 | 0.694 | 0.048 | |
| FB1+m | |||||||
| ka (h−1) | 0.11 | 0.26 | 0.11 | 0.07 | 0.14 | 0.08 | |
| ke (h−1) | 0.03 | 0.02 | 0.04 | 0.07 | 0.04 | 0.02 | |
| c0 (nmol·L−1) | 9.0 | 2.8 | 5.9 | 5.8 | 5.9 | 2.5 | |
| cmax (nmol·L−1) | 5.7 | 2.3 | 3.2 | 2.1 | 3.3 | 1.7 | |
| tmax (h) | 16.6 | 10.6 | 13.6 | 13.7 | 13.6 | 2.5 | |
| t1/2ka (h) | 6.2 | 2.7 | 6.1 | 9.5 | 6.1 | 2.8 | |
| t1/2ke (h) | 25.3 | 32.4 | 15.5 | 9.5 | 20.7 | 10.2 | |
| Vd (L·kg·BW−1) | 1.7 | 7.4 | 10.3 | 11.3 | 7.7 | 4.3 | |
| Cl (L·kg·BW−1·h−1) | 0.05 | 0.16 | 0.46 | 0.83 | 0.4 | 0.3 | |
| AUC (nmol·L−1·h) | 328 | 132 | 132 | 80 | 168 | 109 | |
| AUCFUM-m% (%) | 41.3 | 55.0 | 64.4 | 89.5 | 62.6 | 20.3 | |
| FAUC (%) | 9.6 | 4.6 | 4.3 | 2.4 | 5.2 | 3.1 | |
| F | 0.004 | 0.007 | 0.019 | 0.018 | 0.012 | 0.008 | |
| MRT (h) | 34.7 | 31.5 | 25.0 | 23.2 | 28.6 | 5.4 | |
| RSD (nmol·L−1) | 1.2 | 0.5 | 0.5 | 0.5 | 0.7 | 0.4 | |
| r2 | 0.464 | 0.604 | 0.783 | 0.655 | 0.627 | 0.132 | |
| FB1−m | FB1+m | |||||
|---|---|---|---|---|---|---|
| FUMpo | FumDpo | p-Value | FUMpo | FumDpo | p-Value | |
| AUC (nMol·L−1·h) | 91.0 (12) | 9.0 (15.8) | <0.01 | 168.0 (45) | 27.7 (20.0) | 0.01 |
| FAUC (%); absolute | 3.1 (0.4) | 0.3 (0.6) | <0.01 | 5.2 (1.3) | 0.8 (0.6) | 0.01 |
| Urinary Excretion % | Fecal Excretion % | |||
|---|---|---|---|---|
| FB1−m | FB1+m | FB1−m | FB1+m | |
| FB1iv | 10.56 a (8.33–13.44) | 11.80 a (10.33–14.35) | 1.88 a (0.00–3.76) | 8.57 a (0.00–13.53) |
| HFB1iv | <LOD | 3.95 b (0.67–6.48) | 0.00 a (0.00–1.71) | 0.40 b (0.00–4.04) |
| FUMpo | 0.24 c (0.12–0.56) | 0.56 c (0.26–1.56) | 25.05 b (8.51–55.51) | 72.92 c (52.49–93.66) |
| FumDpo | 0.012 b (0.001–0.061) | 0.234 c (0.045–1.256) | 1.34 a,c (0.22–2.26) | 58.81 c (51.34–80.05) |
| Group | Fumonisin Application | Dose (nmol·kg·BW−1) | Fumonisin Esterase (U/kg feed) | n |
|---|---|---|---|---|
| CON | - | - | - | 6 (+1 ‡) |
| FB1iv | 100 µg FB1/kg BW iv bolus | 139 FB1 | - | 6 |
| HFB1iv | 56.2 µg HFB1/kg BW iv bolus | 139 HFB1 | - | 6 |
| FUMpo | culture material on top of morning ration, po bolus (calculated: 120 mg FB1 + 48 mg FB2 + 14 mg FB3/kg diet) | 3377 FB1 † 1367 FB2 † 584 FB3 † | - | 6 |
| FumDpo | culture material on top of morning ration, po bolus (calculated: 120 mg FB1 + 48 mg FB2 + 14 mg FB3/kg diet) | 3321 FB1 † 1344 FB2 † 575 FB3 † | 240 | 6 |
| FB1 | HFB1 | pHFB1a | pHFB1b | FB2 | HFB2 | pHFB2a | pHFB2b | FB3 | HFB3 | pHFB3a | pHFB3b | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | 58 | - | - | - | 58 | - | - | - | 58 | - | - | - | |
| Feed 1 | LOD (ng/g) | 20 | - | - | - | 20 | - | - | - | 20 | - | - | - |
| LOQ (ng/g) | - | - | - | - | - | - | - | - | - | - | |||
| Recovery (%) | 99 | 105 | 94 | 93 | 91 | - | - | - | 95 | - | - | - | |
| Serum | LOD (ng/mL) | 0.10 | 0.10 | 0.05 | 0.09 | 0.27 | - | - | - | 0.22 | - | - | - |
| LOQ (ng/mL) | 0.30 | 0.30 | 0.15 | 0.27 | 0.80 | - | - | - | 0.66 | - | - | - | |
| Recovery (%) | 103 | 104 | 101 | 94 | 106 | 101 | 101 | 95 | 105 | 93 | 109 | 103 | |
| Urine | LOD (ng/mL) | 0.90 | 1.4 | 0.27 | 0.36 | 0.90 | 0.27 | 0.18 | 0.27 | 0.18 | 0.36 | 0.18 | 0.18 |
| LOQ (ng/mL) | 3.0 | 4.5 | 0.90 | 1.2 | 3.0 | 0.90 | 0.60 | 0.90 | 0.60 | 1.2 | 0.60 | 0.60 | |
| Recovery (%) | 105 | 104 | 101 | 101 | 105 | 103 | 98 | 99 | 110 | 102 | 97 | 106 | |
| Feces | LOD (ng/g) | 35 | 131 | 25 | 33 | 185 | 217 | 16 | 10 | 121 | 82 | 10 | 10 |
| LOQ (ng/g) | 116 | 437 | 83 | 108 | 618 | 725 | 52 | 21 | 405 | 274 | 23 | 32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schertz, H.; Kluess, J.; Frahm, J.; Schatzmayr, D.; Dohnal, I.; Bichl, G.; Schwartz-Zimmermann, H.; Breves, G.; Dänicke, S. Oral and Intravenous Fumonisin Exposure in Pigs—A Single-Dose Treatment Experiment Evaluating Toxicokinetics and Detoxification. Toxins 2018, 10, 150. https://doi.org/10.3390/toxins10040150
Schertz H, Kluess J, Frahm J, Schatzmayr D, Dohnal I, Bichl G, Schwartz-Zimmermann H, Breves G, Dänicke S. Oral and Intravenous Fumonisin Exposure in Pigs—A Single-Dose Treatment Experiment Evaluating Toxicokinetics and Detoxification. Toxins. 2018; 10(4):150. https://doi.org/10.3390/toxins10040150
Chicago/Turabian StyleSchertz, Hanna, Jeannette Kluess, Jana Frahm, Dian Schatzmayr, Ilse Dohnal, Gerlinde Bichl, Heidi Schwartz-Zimmermann, Gerhard Breves, and Sven Dänicke. 2018. "Oral and Intravenous Fumonisin Exposure in Pigs—A Single-Dose Treatment Experiment Evaluating Toxicokinetics and Detoxification" Toxins 10, no. 4: 150. https://doi.org/10.3390/toxins10040150





