Effect of Red Ginseng on Genotoxicity and Health-Related Quality of Life after Adjuvant Chemotherapy in Patients with Epithelial Ovarian Cancer: A Randomized, Double Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Toxicity
2.4. Health-Related Quality of Life
2.5. Survival
2.6. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
3.2. Toxicity
3.3. Health-Related Quality of Life
3.4. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suh, D.H.; Kim, M.; Kim, K.; Kim, H.J.; Lee, K.H.; Kim, J.W. Major clinical research advances in gynecologic cancer in 2016: 10-year special edition. J. Gynecol. Oncol. 2017, 28, 45. [Google Scholar] [CrossRef] [PubMed]
- Koensgen, D.; Oskay-Oezcelik, G.; Katsares, I.; Walle, U.; Klapp, C.; Mustea, A.; Stengel, D.; Porzsolt, F.; Lichtenegger, W.; Sehouli, J. Nord-Ostdeutschen Gesellschaft fur Gynakologische Onkologie working group “Quality of life”. Development of the Berlin Symptom Checklist Ovary (BSCL-O) for the measurement of quality of life of patients with primary and recurrent ovarian cancer: Results of a phase I and II study. Support. Care Cancer 2010, 18, 931–942. [Google Scholar] [PubMed]
- Von Gruenigen, V.E.; Huang, H.Q.; Gil, K.M.; Frasure, H.E.; Armstrong, D.K.; Wenzel, L.B. The association between quality of life domains and overall survival in ovarian cancer patients during adjuvant chemotherapy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2012, 124, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.S.; Bacon, M.; Tu, D.; Butler, L.; Bezjak, A.; Stuart, G.C. The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecol. Oncol. 2008, 108, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Plotti, F.; Scaletta, G.; Aloisi, A.; Luvero, D.; Capriglione, S.; Miranda, A.; Montera, R.; De Cicco Nardone, C.; Terranova, C.; Angioli, R. Quality of Life in Platinum-Sensitive Recurrent Ovarian Cancer: Chemotherapy Versus Surgery Plus Chemotherapy. Ann. Surg. Oncol. 2015, 22, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Yardley, J.H.; Donehower, R.C.; Boitnott, J.K. Taxol toxicity. Epithelial necrosis in the gastrointestinal tract associated with polymerized microtubule accumulation and mitotic arrest. Cancer 1989, 63, 1944–1950. [Google Scholar] [CrossRef]
- Vlassopoulos, A.; Lean, M.E.; Combet, E. Oxidative stress, protein glycation and nutrition—Interactions relevant to health and disease throughout the lifecycle. Proc. Nutr. Soc. 2014, 73, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Bai, X.Y.; Wang, C.H. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am. J. Chin. Med. 2014, 42, 543–559. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, J.; Xu, Y.; Zhang, C.; Wang, H.; He, Y.; Wang, T.; Yuan, D. Cardioprotective effects of saponins from Panax japonicus on acute myocardial ischemia against oxidative stress-triggered damage and cardiac cell death in rats. J. Ethnopharmacol. 2012, 140, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.; Ekholm Pettersson, F.; Inganas, M.; Paulie, S.; Gronvik, K.O. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine 2005, 23, 5411–5419. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hahm, D.H.; Yang, D.C.; Kim, J.H.; Lee, H.J.; Shim, I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J. Pharmacol. Sci. 2005, 97, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Newmark, H.; Zhou, R. Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp. Neurol. 2002, 173, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ahn, K.; Oh, T.H.; Nah, S.Y.; Rhim, H. Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem. Biophys. Res. Commun. 2002, 296, 247–254. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Wargovich, M.J. Inflammation, cancer, and targets of ginseng. J. Nutr. 2007, 137, 183S–185S. [Google Scholar] [PubMed]
- Konkimalla, V.B.; Efferth, T. Evidence-based Chinese medicine for cancer therapy. J. Ethnopharmacol. 2008, 116, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Seo, S.K.; Choi, Y.M.; Jeon, Y.E.; Lim, K.J.; Cho, S.H.; Choi, Y.S.; Lee, B.S. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: A double-blind randomized controlled trial. Menopause 2012, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, C.Y.; Lee, S.J. Effects of sun ginseng on subjective quality of life in cancer patients: A double-blind, placebo-controlled pilot trial. J. Clin. Pharm. Ther. 2006, 31, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Decordier, I.; Papine, A.; Vande Loock, K.; Plas, G.; Soussaline, F.; Kirsch-Volders, M. Automated image analysis of micronuclei by IMSTAR for biomonitoring. Mutagenesis 2011, 26, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Park, Y.S.; Lee, E.S.; Bang, S.M.; Heo, D.S.; Park, S.Y.; You, C.H.; West, K. Validation of the Korean version of the EORTC QLQ-C30. Qual. Life Res. 2004, 13, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Wang, X.S.; Lee, J.S.; Roh, J.W.; Lee, C.G.; Lee, W.S.; Lee, K.S.; Bang, S.M.; Mendoza, T.R.; Cleeland, C.S. Validation study of the korean version of the brief fatigue inventory. J. Pain Symptom. Manag. 2005, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Mendoza, T.R.; Heo, D.S.; Yoo, T.; Heo, B.Y.; Park, H.A.; Shin, H.C.; Wang, X.S.; Cleeland, C.S. Development of a cancer pain assessment tool in Korea: A validation study of a Korean version of the brief pain inventory. Oncology 2004, 66, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Nam, S.H.; Koh, S.; Hong, Y.S.; Lee, K.H.; Shin, S.W.; Hui, D.; Park, K.W.; Yoon, S.Y.; Won, J.Y.; et al. Validation of the Edmonton Symptom Assessment System in Korean patients with cancer. J. Pain Symptom. Manag. 2013, 46, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Martin, S.A.; Sesti, A.M.; Spritzer, K.L. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005, 6, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Power Analysis and Sample Size (PASS) 15 Software. Available online: https://www.ncss.com/software/pass/ (accessed on 26 February 2012).
- Iarmarcovai, G.; Ceppi, M.; Botta, A.; Orsiere, T.; Bonassi, S. Micronuclei frequency in peripheral blood lymphocytes of cancer patients: A meta-analysis. Mutat. Res. 2008, 659, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Q.; Liu, K.; Li, G.; Zheng, R. Ginsenoside Rh(2) enhances antitumour activity and decreases genotoxic effect of cyclophosphamide. Basic Clin. Pharmacol. Toxicol. 2006, 98, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Anderson, S.; Du, W.; He, T.C.; Yuan, C.S. Red ginseng and cancer treatment. Chin. J. Nat. Med. 2016, 14, 7–16. [Google Scholar] [PubMed]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng. Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.Y.; Kang, H.J.; Kim, O.Y.; Lee, J.H. Beneficial effects of Korean red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: A randomized, double-blind, placebo-controlled trial. Nutr. J. 2012, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Wu, C.F.; Duan, L.; Yang, J.Y. Protective effects of total saponins from stem and leaf of Panax ginsengagainst cyclophosphamide-induced genotoxicity and apoptosis in mouse bone marrow cells and peripheral lymphocyte cells. Food Chem. Toxicol. 2008, 46, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Johnke, R.M.; Allison, R.R.; O’Brien, K.F.; Dobbs, L.J., Jr. Radioprotective potential of ginseng. Mutagenesis 2005, 20, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.; Bacon, M.; Carey, M.; Zee, B.; Tu, D.; Bezjak, A. Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. J. Clin. Oncol. 2004, 22, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, T.H.; Choi, T.Y.; Lee, M.S. Ginseng for health care: A systematic review of randomized controlled trials in Korean literature. PLoS ONE 2013, 8, e59978. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; King, R.W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 2005, 437, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Znaor, A.; Ceppi, M.; Lando, C.; Chang, W.P.; Holland, N.; Kirsch-Volders, M.; Zeiger, E.; Ban, S.; Barale, R.; et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 2007, 28, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lee, H.S.; Hwang, M.W.; Jin, M. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints: Single blind, randomized clinical trial of efficacy and safety. BMC Complement. Altern. Med. 2014, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Park, K.I.; Kim, J.W.; Yun, Y.J.; Kim, S.H.; Lee, C.H.; Park, J.W.; Lee, J.M. Efficacy and safety of Korean red ginseng for cold hypersensitivity in the hands and feet: A randomized, double-blind, placebo-controlled trial. J. Ethnopharmacol. 2014, 158, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Reddy, A.; Tannir, N.M.; Chisholm, G.B.; Lee, R.T.; Lopez, G.; Escalante, C.P.; Manzullo, E.F.; Frisbee Hume, S.; Williams, J.L.; et al. High-Dose Asian Ginseng (Panax Ginseng) for Cancer-Related Fatigue: A Preliminary Report. Integr. Cancer Ther. 2015, 14, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.L.; Liu, H.; Dakhil, S.R.; Linquist, B.; Sloan, J.A.; Nichols, C.R.; McGinn, T.W.; Stella, P.J.; Seeger, G.R.; Sood, A.; et al. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: A randomized, double-blind trial, N07C2. J. Natl. Cancer Inst. 2013, 105, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.L.; Soori, G.S.; Bauer, B.A.; Sloan, J.A.; Johnson, P.A.; Figueras, C.; Duane, S.; Mattar, B.; Liu, H.; Atherton, P.J.; et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: A randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support. Care Cancer 2010, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kioukia-Fougia, N.; Georgiadis, N.; Tsarouhas, K.; Vasilaki, F.; Fragkiadaki, P.; Meimeti, E.; Tsitsimpikou, C. Synthetic and natural nutritional supplements: Health “allies” or risk to public health? Recent Pat. Inflamm. Allergy Drug Discov. 2016. Epub ahead of print. [Google Scholar] [CrossRef]
- Lee, C.K.; Park, K.K.; Chung, A.S.; Chung, W.Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem. Toxicol. 2012, 50, 2565–2574. [Google Scholar] [PubMed]
- Suh, S.O.; Kroh, M.K.; Kim, N.R.; Joh, Y.G.; Cho, M.Y. Effects of red ginseng upon postoperative immunity and survival in patients with stage III gastric cancer. Am. J. Chin. Med. 2002, 30, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Li, Z.D.; Gao, F.; Zhang, Y.; Sun, H.; Li, P.P. Effects of combined Chinese drugs and chemotherapy in treating advanced non-small cell lung cancer. Chin. J. Integr. Med. 2009, 15, 415–419. [Google Scholar] [CrossRef] [PubMed]
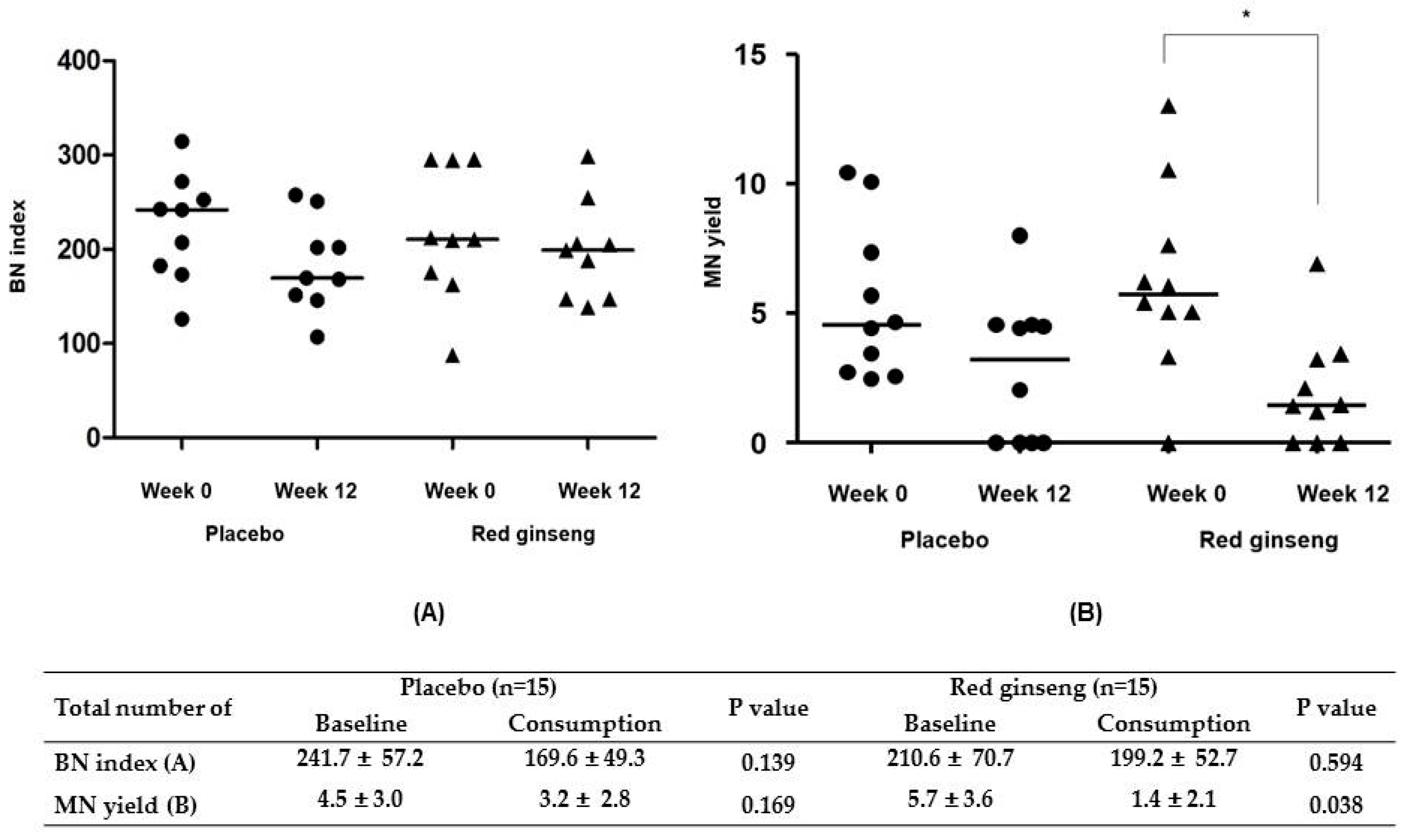

| Characteristics | Placebo (n = 15, %) | Red Ginseng (n = 15, %) | p Value |
|---|---|---|---|
| Age (year) * | 52.9 ± 10.1 | 55.9 ± 12.1 | 0.276 |
| Height (year) * | 154.8 ± 4.1 | 155.4 ± 7.8 | 0.986 |
| Weight (kg) * | 56.2 ± 8.7 | 58.3 ± 8.7 | 0.363 |
| BMI (kg/m2) * | 23.5 ± 4.0 | 24.1 ± 2.8 | 0.309 |
| Compliance (%) * | 93.9 ± 6.7 | 94.9 ± 6.8 | 0.599 |
| FIGO stage | 1.000 | ||
| I–II | 5 (33.3) | 4 (26.7) | |
| III–IV | 10 (66.7) | 11 (72.3) | |
| Histology | 0.272 | ||
| Serous | 9 (60) | 10 (66.7) | |
| Non-serous | 6 (40) | 5 (33.3) |
| Laboratory Markers | Placebo (n = 15) | p Value | Red Ginseng (n = 15) | p Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |||
| WBC (cells/μL) | 4285.7 ± 1944.7 | 5670.7 ± 2136.3 | 0.008 | 4068.8 ± 1304.4 | 5051.9 ± 1205.4 | 0.008 |
| Neutrophil (cells/μL) | 1989.1 ± 1560.3 | 2920.0 ± 1629.3 | 0.030 | 2057.6 ± 933.1 | 2804.1 ± 954.9 | 0.030 |
| Lymphocyte (cells/μL) | 1660.5 ± 663.5 | 2116.0 ± 769.0 | 0.778 | 1544.9 ± 538.4 | 1679.2 ± 597.1 | 0.163 |
| Hemoglobin | 10.2 ± 1.1 | 12.1 ± 0.8 | 0.001 | 10.4 ± 1.0 | 12.4 ± 0.9 | <0.001 |
| Platelet | 181.6 ± 86.1 | 214.8 ± 57.5 | 0.116 | 214.9 ± 113.9 | 209.8 ± 54.9 | 0.605 |
| AST | 28.6 ± 11.5 | 28.4 ± 14.6 | 0.834 | 20.1 ± 7.5 | 27.4 ± 21.5 | 0.010 |
| ALT | 32.5 ± 20.9 | 29.4 ± 23.3 | 0.683 | 20.2 ± 17.8 | 29.7 ± 36.8 | 0.009 |
| Bilirubin | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.237 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.070 |
| ALP | 67.6 ± 22.5 | 68.4 ± 27.9 | 0.925 | 71.3 ± 15.1 | 72.8 ± 13.4 | 0.679 |
| BUN | 14.4 ± 5.2 | 14.9 ± 4.9 | 0.717 | 12.8 ± 2.7 | 12.9 ± 4.1 | 1.000 |
| Creatinine | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.363 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.111 |
| Adverse Events | Grade | Placebo (n = 15, %) | Red Ginseng (n = 15, %) | p Value |
|---|---|---|---|---|
| Nausea | 1 | 2 (13.3) | 1 (6.7) | 1.000 |
| Insomnia | 1 | 1 (6.7) | 1 (6.7) | 1.000 |
| Palpitation | 1 | 0 (0) | 1 (6.7) | 1.000 |
| Headache | 1 | 2 (13.3) | 1 (6.7) | 1.000 |
| Urticaria | 1 | 1 (6.7) | 1 (6.7) | 1.000 |
| Total No. of patients | 3 (20) | 2 (13.3) | 1.000 | |
| Outcomes | Placebo (n = 15) | p Value | Red Ginseng (n = 15) | p Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |||
| European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ)-C30 | ||||||
| Functional scale | ||||||
| Emotional | 37.5 ± 10.9 | 38.7 ± 13.9 | 0.702 | 44.9 ± 15.3 | 37.5 ± 12.7 | 0.027 |
| Symptom scale | ||||||
| Fatigue | 53.7 ± 11.8 | 45.8 ± 13.9 | 0.131 | 58.8 ± 19.2 | 46.1 ± 16.2 | 0.012 |
| Nausea and vomiting | 30.5 ± 6.4 | 27.3 ± 5.0 | 0.157 | 37.5 ± 15.3 | 27.2 ± 6.6 | 0.004 |
| Dyspnea | 40.6 ± 22.1 | 31.3 ± 14.4 | 0.161 | 47.1 ± 17.4 | 35.3 ± 17.8 | 0.021 |
| Brief Fatigue Inventory (BFI) | ||||||
| Severity | ||||||
| Worst fatigue | 4.25 ± 2.27 | 3.62 ± 2.71 | 0.658 | 5.59 ± 3.20 | 4.00 ± 3.32 | 0.026 |
| Interference | 19.25 ± 10.33 | 11.38 ± 11.84 | 0.084 | 23.24 ± 17.79 | 14.29 ± 17.59 | 0.014 |
| Brief Pain Inventory (BPI) | ||||||
| Pain interference | ||||||
| Enjoyment of life | 3.0 ± 3.6 | 1.8 ± 3.0 | 0.138 | 1.8 ±. 2.3 | 0.4 ± 0.7 | 0.035 |
| Hospital Anxiety and Depression Scale (HADS) | ||||||
| Anxiety | 7.8 ± 2.4 | 8.4 ± 1.5 | 0.119 | 9.1 ± 2.6 | 8.0 ± 2.6 | 0.015 |
| Sleep Scale from the Medical Outcome Study (MOS-SS) | ||||||
| Daytime somnolence | 74.5 ± 14.8 | 77.5 ± 16.8 | 0.342 | 74.7 ± 17.1 | 83.0 ± 8.9 | 0.043 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Kim, M.-K.; Lee, M.; Kwon, B.-S.; Suh, D.H.; Song, Y.S. Effect of Red Ginseng on Genotoxicity and Health-Related Quality of Life after Adjuvant Chemotherapy in Patients with Epithelial Ovarian Cancer: A Randomized, Double Blind, Placebo-Controlled Trial. Nutrients 2017, 9, 772. https://doi.org/10.3390/nu9070772
Kim HS, Kim M-K, Lee M, Kwon B-S, Suh DH, Song YS. Effect of Red Ginseng on Genotoxicity and Health-Related Quality of Life after Adjuvant Chemotherapy in Patients with Epithelial Ovarian Cancer: A Randomized, Double Blind, Placebo-Controlled Trial. Nutrients. 2017; 9(7):772. https://doi.org/10.3390/nu9070772
Chicago/Turabian StyleKim, Hee Seung, Mi-Kyung Kim, Maria Lee, Byung-Su Kwon, Dong Hoon Suh, and Yong Sang Song. 2017. "Effect of Red Ginseng on Genotoxicity and Health-Related Quality of Life after Adjuvant Chemotherapy in Patients with Epithelial Ovarian Cancer: A Randomized, Double Blind, Placebo-Controlled Trial" Nutrients 9, no. 7: 772. https://doi.org/10.3390/nu9070772






